Abstract
The evolution of climatic niche specialization has important implications for many topics in ecology, evolution and conservation. The climatic niche reflects the set of temperature and precipitation conditions where a species can occur. Thus, specialization to a limited set of climatic conditions can be important for understanding patterns of biogeography, species richness, community structure, allopatric speciation, spread of invasive species and responses to climate change. Nevertheless, the factors that determine climatic niche width (level of specialization) remain poorly explored. Here, we test whether species that occur in more extreme climates are more highly specialized for those conditions, and whether there are trade-offs between niche widths on different climatic niche axes (e.g. do species that tolerate a broad range of temperatures tolerate only a limited range of precipitation regimes?). We test these hypotheses in amphibians, using phylogenetic comparative methods and global-scale datasets, including 2712 species with both climatic and phylogenetic data. Our results do not support either hypothesis. Rather than finding narrower niches in more extreme environments, niches tend to be narrower on one end of a climatic gradient but wider on the other. We also find that temperature and precipitation niche breadths are positively related, rather than showing trade-offs. Finally, our results suggest that most amphibian species occur in relatively warm and dry environments and have relatively narrow climatic niche widths on both of these axes. Thus, they may be especially imperilled by anthropogenic climate change.
Keywords: amphibians, climate, evolution, niche, phylogeny, specialization
1. Introduction
Climatic niche specialization is a very specific topic, but one that has surprisingly far-reaching implications. Every terrestrial species has a climatic niche, a set of temperature and precipitation conditions where it occurs [1–3]. The climatic niche is critically important because it may determine where that species occurs (either alone or in combination with other abiotic and biotic factors) and how it will respond to changes in climate over time. But these patterns of geographical distribution and response to climate change depend not only on the climatic niche but also on specialization in that niche (i.e. climatic niche width). If species were not specialized for a limited set of climatic conditions, every species could potentially occur almost anywhere (at least within a continent or island) and anthropogenic climate change would not be problematic for species persistence. But most species do appear to be specialized for a limited set of climatic conditions. For example, few species occur continuously from the poles to the Equator, or from sea level to above treeline within a region, regardless of the specific mechanisms that determine their geographical ranges. Given this, climatic niche specialization appears to have important implications for numerous topics in biogeography, ecology, evolution and conservation. There is now evidence that climatic niche specialization, coupled with climatic niche conservatism (climatic niches remaining similar over time within and among species; reviewed in [4]), can play a role in determining large-scale patterns of biogeography [5,6], patterns of species richness along gradients in latitude [7–10], elevation [11,12] and aridity [13], geographical patterns of community structure [14], allopatric speciation [15–18], patterns of geographical spread in invasive species [19–21] and may determine the responses of species to anthropogenic climate change [22–24]. Of course, climatic specialization is also important when there is climatic divergence, and the combination of climatic niche specialization and divergence may drive other patterns, such as parapatric speciation along environmental gradients and clade diversification [18,25,26]. For example, there would be no ecological speciation along environmental gradients if species were able to occur everywhere along the gradient and were not specialized for a more limited set of conditions.
What determines how specialized the climatic niche is in a given species? This question has remained relatively unexplored, especially in comparison to the burgeoning literature that addresses the consequences of specialization, conservatism and divergence in the climatic niche (see references above). Previous studies suggest that several different factors may influence the width of the climatic niche, but some of the most important factors are closely intertwined: these are seasonality, latitude and the specific aspect of the niche that is being considered (i.e. temperature, precipitation). In general, temperature seasonality increases with latitude, but precipitation seasonality decreases with latitude [27,28]. Analyses in three species-rich clades of vertebrates (hylid frogs, plethodontid salamanders and phrynosomatid lizards) suggest that this within-locality seasonality is the major driver of species-level niche width for both temperature and precipitation, rather than variation in climatic conditions across the species range [29]. Nevertheless, climatic variation between localities does have a significant (albeit smaller) impact on climatic niche width of species, potentially reflecting the role of local adaptation on the species niche width [29].
A question that is especially poorly explored is whether species that occur in more extreme climatic conditions on a given niche axis (i.e. have extreme niche positions) are more highly specialized for those conditions (figure 1a). For example, does adapting to more extreme or stressful conditions require a trade-off that limits the ability of those species to occur across a broad range of conditions? Or are species that can tolerate more extreme conditions able to do so simply because they can tolerate a broader range of conditions than other species (figure 1b)? Or is there no relationship between the niche width of species and their position on an environmental gradient (figure 1c)? Or does it depend on which end of the gradient is being considered? For example, are species that occur under colder temperatures able to tolerate a broad range of conditions while species that occur in warmer conditions have only a limited range of tolerances (figure 1d)?
Figure 1.
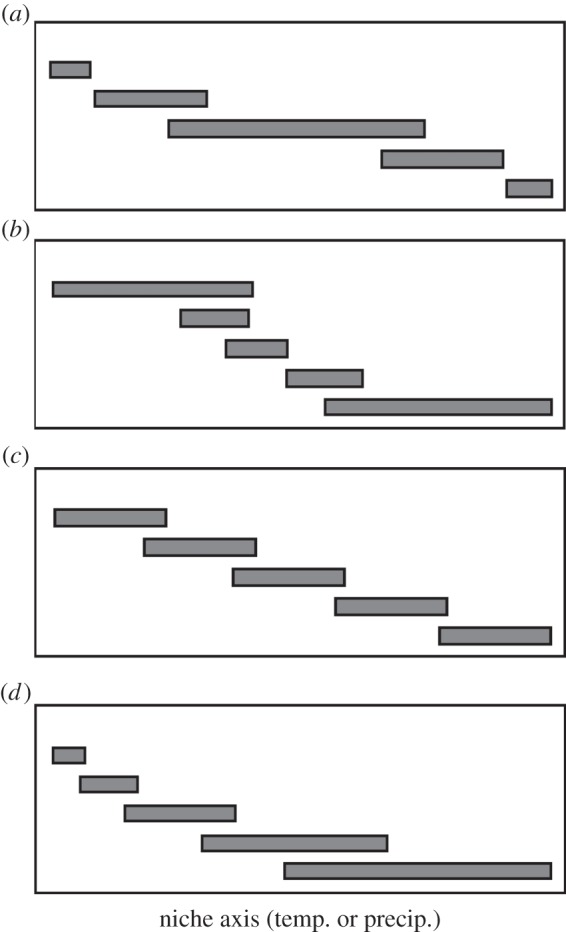
Hypothetical examples illustrating different possible relationships between climatic niche breadth and niche position (adapted from [13], their fig. 1). Each grey bar represents the range of values where a species occurs on a climatic niche axis (temperature, precipitation). In (a), species that occur in the most extreme positions on the niche axis have narrower niche widths (i.e. they are more specialized for these extreme conditions). In (b), species that occur in more extreme conditions occur under a broader range of conditions than other species in the clade, and therefore have wider niche widths. In (c), species have similar niche widths regardless of position on this niche axis. In (d), species that occur on one end of the gradient are more specialized, whereas species that occur on the other end are more generalized (i.e. have broader niche widths).
To our knowledge, only one previous study has addressed the relationship between niche position and niche specialization with climatic data [13], despite considerable interest in related topics such as the evolution of thermal tolerances [30–32] and the evolution of niche breadth and specialization in general [33,34]. Wiens et al. [13] tested whether species that occur in deserts are specialized for a more limited range of precipitation regimes, relative to species that occur in more mesic environments. The study focused on a group of lizards (Phrynosomatidae) that occur across nearly all terrestrial habitats in North and Central America, with relatively high species richness in desert regions. They found that precipitation niche widths tend to be narrower in species occurring in drier environments (depending somewhat on how this is quantified), but contrary to the expectation of niches generally narrowing in more extreme climates (figure 1a), niches are wider in phrynosomatid species occurring in more mesic environments (as in figure 1d). However, it remains unclear whether similar patterns apply to temperature and to other taxonomic groups.
Another surprisingly unexplored question is whether there are trade-offs between niche widths on different climatic niche axes. For example, does being able to tolerate a broad range of temperatures also entail an inability to tolerate a broad range of precipitation regimes? The term trade-off usually refers to a negative correlation between two aspects of performance or fitness [33,35]. Under the trade-off hypothesis, we would expect to see a negative relationship between species niche widths on two or more major axes of the climatic niche (e.g. temperature versus precipitation). Such a trade-off might be expected for at least two major reasons. First, trade-offs are considered to be an important factor driving ecological specialization in general [33,36,37]. Therefore, it seems logical that this idea should also apply to the climatic niche, one of the most fundamental ecological characteristics of a species. Second, previous work suggests that there might be apparent trade-offs because of different latitudinal patterns of seasonality on the two major climatic niche axes [28,29]. Specifically, tropical species appear to have narrow niche widths for temperature and wide niche widths for precipitation, whereas temperate species appear to have wide niche widths for temperature and narrow niche widths for precipitation. Given these expectations, there should be a strong negative relationship between temperature niche breadths (TNB) and precipitation niche breadths (PNB) among a set of species that occur across different latitudes (figure 2), as would be expected given trade-offs between niche breadths on these two axes.
Figure 2.
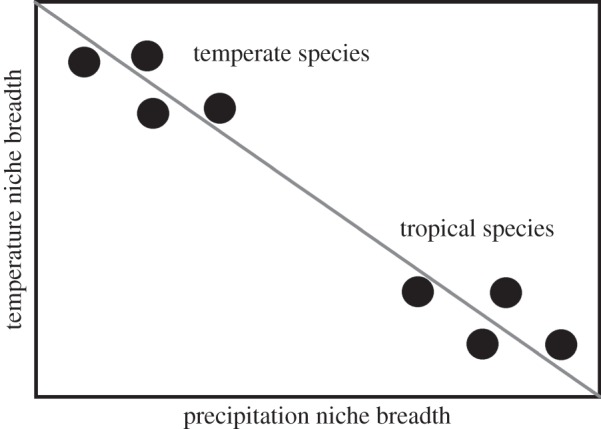
Hypothetical example illustrating why an apparent trade-off may arise between temperature and precipitation niche breadths (TNB and PNB) in a broadly distributed clade. In temperate regions, species are expected to have broad TNB and narrow PNB, whereas in species in tropical regions are expected to have narrow TNB and broad PNB. Note that this relationship between different climatic niche widths is expected given observed patterns of within-locality seasonality in tropical regions versus temperate regions [15,17,27–29], but has not been tested directly with climatic data among species.
There are also reasons that these trade-offs might not actually be present, however. First, apart from these latitudinal patterns of seasonality, we do not know of specific mechanistic reasons why this trade-off should occur for temperature and precipitation variables (e.g. involving physiology). Second, species with large geographical ranges might occur across many different temperature and precipitation regimes, whereas narrowly distributed species might occur under a limited range of conditions on both axes [38,39]. This latter pattern would raise the question of why some species are able to tolerate such a broad range of conditions (and others seemingly not), and if the narrowly distributed species are actually confined to their limited range of conditions by their climatic tolerances. In summary, there are strong a priori reasons to predict a trade-off between different aspects of climatic niche width, and also some reasons why such a trade-off might not be expected. Importantly, to our knowledge, no previous studies have tested this trade-off hypothesis with explicit climatic data.
Here, we test both of these hypotheses (niche position versus specialization and trade-offs in specialization on different niche axes) using phylogenetic comparative methods at a global scale in amphibians. Amphibians offer a useful model system for several reasons. First, there are detailed range maps available for most species [40], from which climatic data can be obtained using GIS-based methods and global environmental data layers [41]. Second, a time-calibrated phylogeny is available for the group that includes approximately 40% of the described species [10,42], with representatives of nearly all families and most genera. This allows testing these hypotheses in a phylogenetic framework. Third, amphibians are broadly distributed across latitudes and climates, from the Arctic Circle to the equatorial tropics, and from the wettest rainforests to some of the driest deserts on Earth (but like most other groups of organisms, they have their highest species richness in wet tropical regions [10,43]). Perhaps because of these reasons, amphibians are increasingly used as a model system in large-scale macroecological research [10,43–46].
2. Material and methods
(a). Climatic data
Climatic data for each species were obtained from species range maps, and range maps were obtained from the IUCN database [40] for most described amphibian species (6307 of 7212; [47]). The IUCN range maps for each species are developed through consultation with a set of experts on that taxon and are based on known occurrences for that species, which are then used to develop one or more polygons encompassing these known sites. These polygons are then used to depict the species' range.
To obtain climatic data, we used climatic rasters from the WorldClim database [41], using the package raster v. 2.1–49 [48] in R v. 3.0.2 [49]. The WorldClim database consists of 19 climatic variables based on averages of monthly temperature and precipitation data from 1950 to 2000. Data are taken from thousands of weather stations all over the world and are then spatially interpolated to locations between weather stations. The climatic data used had a spatial resolution of approximately 5 km2 (2.5 min), and a similar scale has been used in other macroecological studies of amphibians [44,46]. We overlaid the climatic rasters on the distribution map of each species and extracted the climatic values for each grid cell in the range for each species, resulting in a data matrix for each species, using the R packages maptools v. 0.8–27 [50] and raster [48]. We retained only the 2712 species that were represented in the phylogeny (see below).
We acknowledge that climatic data from 1950 to 2000 may not reflect current global climate change. Furthermore, the locality data may not reflect recent distributional shifts caused by these climatic changes. Thus, both the climatic data and distributional data may reflect the world before these changes, rather than current climate and ranges in 2014. However, the latter would be almost impossible to estimate for thousands of species, and not necessarily more relevant to the questions being asked here.
Distributional and climatic data were taken from range maps rather than point localities. Both approaches have advantages and disadvantages. For example, range maps may not reflect details of the geographical range that are relevant for climate (e.g. a species shown as occurring in a mountain range may be absent from high elevations or low valleys). On the other hand, use of climatic data from point localities may be biased towards parts of the geographical range that are easier to access or collect in. However, the relatively coarse spatial scale of the climatic data may reduce potential differences between these two approaches. Finally, we note that obtaining climatic data from IUCN range maps is a relatively standard approach in large-scale macroecological studies in amphibians [10,44,46].
We focused on a limited set of variables to test our hypotheses (see below for details). Specifically, we focused on annual mean temperature (Bio1), maximum temperature of the warmest month (Bio5), minimum temperature of the coldest month (Bio6), annual precipitation (Bio12), precipitation of the wettest quarter (Bio16) and precipitation of the driest quarter (Bio17). For these variables, we focused on the mean value of Bio1 across grid cells in the range of each species, the maximum value of Bio5 (the hottest temperature experienced by the species, both across the year and across the species range), the minimum value of Bio6 (the lowest temperature across the year and range), and mean, maximum and minimum values for Bio12 across the species range. We also examined maximum values of Bio16 and minimum values of Bio17 across the range of each species. Values for each variable for each of the 2712 species analysed are summarized in the electronic supplementary material, appendix S1. Note that temperature variables are multiplied by 10 relative to the raw values.
Bio1 and Bio12 are standard variables for describing the overall climatic distribution of a species (e.g. tropical versus temperate, mesic versus arid; [43]), and the temperature extremes (Bio5, Bio6) are essential for describing TNB. Bio12 also provides the most intuitive and straightforward way to describe PNB across the species range [29]. We also included measures of quarterly precipitation extremes (Bio16, Bio17).
(b). Phylogenetic framework
We used a time-calibrated phylogeny [10] that includes 2871 amphibian species (approx. 40% of currently described species; [47]). The topology of the tree is the same as that of Pyron & Wiens [42]. This tree is based on a maximum-likelihood supermatrix analysis of nine nuclear and three mitochondrial genes and was time-calibrated using penalized likelihood [51] with secondary calibrations from a study using multiple fossil calibration points across amphibians [52]. Importantly, the topology and estimated ages of major clades are very similar to those from other recent studies [53–55]. The distribution ranges of some species in the phylogeny were not available in the IUCN database, so these 159 species were pruned from the tree using the R package geiger v. 1.99–3.1 [56]. We note that unsampled species can potentially influence some kinds of comparative analyses (e.g. estimating diversification rates), but should have little impact on analyses of trait correlation [57], especially given the strong phylogenetic signal in the climatic variables (see below). Similarly, we did not account for uncertainty in the phylogeny. The topology is relatively strongly supported [42] and largely congruent with most previous higher level phylogenetic analyses [53–55] and lower level taxonomy (e.g. most families and genera appear to be monophyletic). Furthermore, closely related species appear to share similar climatic niche values (high λ values for the variables studied, see below). Thus, any changes in estimated relationships among these closely related species should have limited impact on our analyses of relationships between climatic variables.
(c). Data analysis
We first tested the hypothesis that species in more extreme niche positions on a given climatic gradient have narrower niche widths on that gradient. We then tested whether there are trade-offs in niche breadth on the precipitation and temperature niche axes. We first calculated the niche breadth for each species for both temperature and precipitation. For TNB, we subtracted the minimum value of the minimum temperature of the coldest month (Bio6) across all 2.5 min grid cells in the species range from the maximum value of the maximum temperature of the warmest month (Bio5). This represents the range of macroclimatic temperatures experienced by each species across the year and across the species range and reflects the impacts of both seasonal and spatial variation [29]. For PNB, we first used an index based on subtracting the minimum values of Bio12 from the maximum values of Bio12 across the range of each species [29]. This measure reflects spatial variation in precipitation across the species range, but not seasonal variation. As an alternative index, we used the maximum value of wettest quarter precipitation (Bio16) across the species range minus the minimum value of driest quarter precipitation (Bio17), which reflects both seasonal and spatial variation. However, we note that seasonal extremes for precipitation are not as easy to interpret as for temperature. Presumably, quarterly values for precipitation are more relevant for occurrence in a given location than short-term monthly extremes, but yearly values may be more relevant than quarterly extremes for survival in a given habitat (e.g. deserts and rainforests are defined based on yearly rainfall values, not a few wet or dry months).
We then determined the niche position of each species for both temperature and precipitation. We first estimated the range of values across all 2712 species included in the analyses for a standard measure of temperature (annual mean temperature; Bio1) and of precipitation (annual precipitation; Bio12). Although these measures cannot reflect all relevant aspects of temperature or precipitation, they should reflect fundamental aspects of both (e.g. tropical versus temperate, high versus low elevation, arid versus mesic). For these analyses, we used the mean value for each variable (across the grid cells of the species range) to characterize its niche position. We then tested the relationships between our measures of within-species niche width (TNB and PNB) and the position of the species relative to others on that axis (TNB versus Bio1; PNB versus Bio12).
We next tested explicitly whether species that have more extreme values on a given niche axis have narrower niche widths. To obtain a measure of whether species have extreme values for the group, we calculated the midpoint of mean species values on each axis and then calculated the absolute distance between each species and that midpoint. Specifically, the midpoint of temperature = (max. species value for mean Bio1 − min. value mean Bio1)/2 + (min. value for mean Bio1). For precipitation, the midpoint = (max. value mean Bio12 − min. value mean Bio12)/2 + (min. value mean Bio12). To obtain the distance of each species from the midpoint for temperature, we subtracted the species mean value of Bio1 from the midpoint of mean values across all species. Similarly, for precipitation, we subtracted the mean value of Bio12 from the midpoint of Bio12. Thus, species that are more distant from the overall midpoint of species values can be considered to have more extreme values on this axis.
Finally, we tested whether there are trade-offs between niche widths on the temperature and precipitation axes. Specifically, we tested for a negative relationship between PNB and TNB. We note that we only tested trade-offs in the colloquial sense, and not in the evolutionary sense [58]. A rigorous test of trade-offs among species would require explicitly showing that evolutionary decreases in niche width in one variable were associated with evolutionary increases in another [58]. However, this distinction should only be important if the initial hypothesis of a trade-off is supported by showing a negative relationship between PNB and TNB.
We tested these hypotheses in a phylogenetic framework using phylogenetic generalized least squares (PGLS; [59]). Prior to performing PGLS, we first found the best-fitting model of evolution for each variable. We compared the fit of Brownian motion, Ornstein Uhlenbeck, white noise and estimated lambda models using estimated likelihood values and the Akaike information criterion, using geiger [56]. These comparisons all showed that the estimated lambda model had the best fit (see the electronic supplementary material, table S1), with substantial phylogenetic signal in all climatic variables (λ = 0.66–0.92), concordant with many previous studies in amphibians [7,11,12,16,24,44]. The lambda model was therefore used for all PGLS analyses (i.e. branch lengths adjusted based on λ values estimated via maximum likelihood). PGLS analyses were conducted using the R package caper, v. 0.5 [60]. For comparison and ease of visualization, we also present results from ordinary least-squares regression (OLS; without accounting for phylogeny).
3. Results
Major results are summarized in table 1 and figure 3. We found that amphibian species occurring in warmer environments (i.e. higher mean values of annual mean temperature) tend to have narrower TNB (figure 3a), as expected given lower temperature seasonality in tropical latitudes (PGLS: r2 = 0.120; p < 0.001). For precipitation (figure 3b), species at the drier end of the gradient (i.e. lower mean annual precipitation) tend to have narrower niche widths (using spatial variation in Bio12 within species to measure niche widths; PGLS: r2 = 0.116; p < 0.001). We found a similar relationship using a measure of PNB that incorporates both seasonal and spatial variation within species (max. Bio16 − min. Bio17; PGLS: r2 = 0.099; p < 0.001). On both the temperature and precipitation niche axes, species do show a significant trend towards narrower niche widths in more extreme environments (table 1), but both of these cases are, nevertheless, driven by narrower niche widths on one end of the niche axis, rather than narrower niche widths on both (figure 3). Finally, we do not find evidence of trade-offs in niche breadths on the temperature and precipitation axes. Instead, there is a positive relationship (figure 3c) between TNB and PNB (PGLS: r2 = 0.202; p < 0.001).
Table 1.
Results of the main hypotheses tested in this study, from ordinary least-squares regression (OLS) and PGLS. TNB and PNB are temperature niche breadth and precipitation niche breadth, respectively. Values of r2 are adjusted for multiple variables.
| variables | r2 | p-value |
|---|---|---|
| OLS | ||
| TNB versus mean annual temperature | 0.240 | <2.2−16 |
| PNB versus annual precipitation | 0.144 | <2.2−16 |
| TNB versus distance from midpoint temperature | 0.152 | <2.2−16 |
| PNB versus distance from midpoint precipitation | 0.144 | <2.2−16 |
| TNB versus PNB | 0.066 | <2.2−16 |
| PGLS | ||
| TNB versus mean annual temperature | 0.120 | <2.2−16 |
| PNB versus annual precipitation | 0.116 | <2.2−16 |
| TNB versus distance from midpoint temperature | 0.049 | <2.2−16 |
| PNB versus distance from midpoint precipitation | 0.082 | <2.2−16 |
| TNB versus PNB | 0.202 | <2.2−16 |
Figure 3.
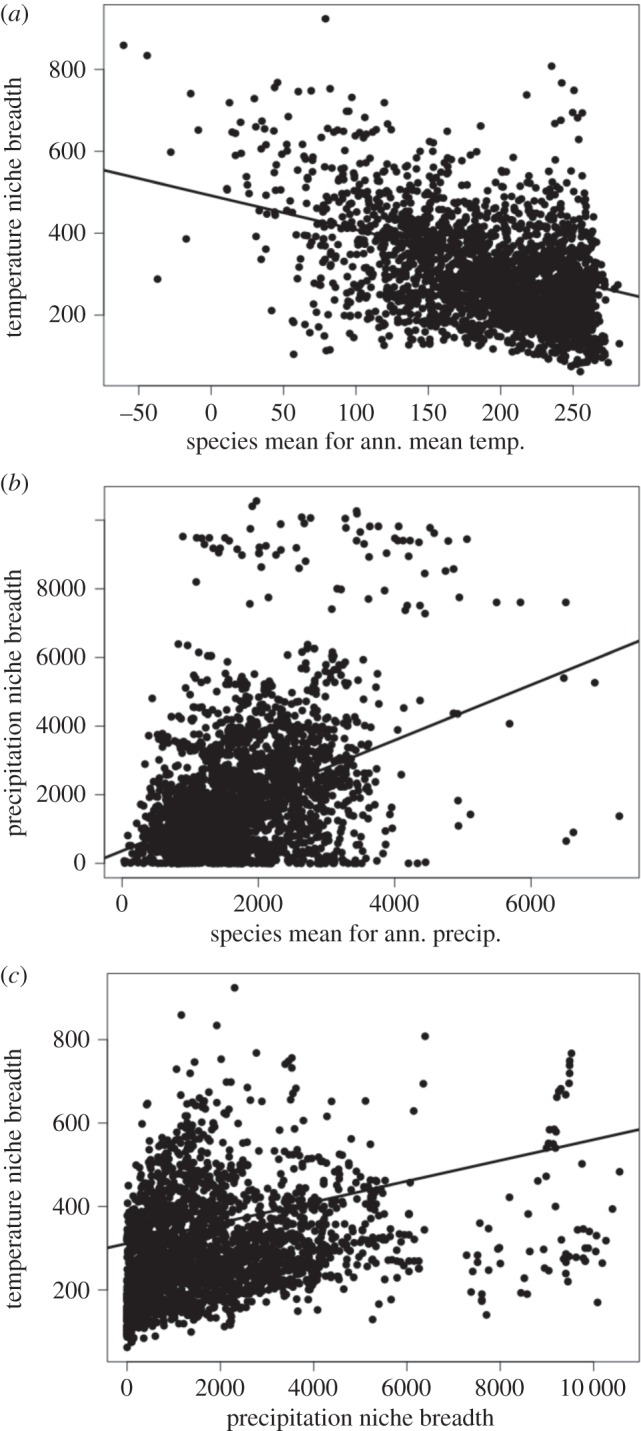
Results of the major hypotheses tested in this study, shown using the raw data for ease of interpretation. PGLS results are concordant and shown in table 1. Note that temperatures are multiplied by 10. (a) Negative relationship between species' TNB (Bio5−Bio6) and their mean values for annual mean temperature (Bio1). (b) Negative relationship between species' PNB (maximum Bio12−minimum Bio12) and their mean values for annual precipitation (Bio12). (c) Positive relationship between TNB and PNB.
4. Discussion
Specialization in the climatic niche is important for many topics in evolutionary biology, ecology and conservation, such as patterns of speciation, species richness and responses to anthropogenic climate change (see Introduction). In this paper, we test two fundamental hypotheses about patterns of specialization in the climatic niche using large-scale phylogenetic and climatic data from amphibians. First, we test whether species in more extreme environments tend to be more specialized for those environments. Second, we test whether there are trade-offs between niche breadths on different climatic niche axes (temperature, precipitation). Surprisingly, our results do not support either hypothesis. We find that for both temperature and precipitation, species tend to have narrower niches on one end of the niche axis (figure 1d), but not both (figure 1a). Thus, we do not support the idea that species necessarily become more highly specialized as they adapt to more extreme environments on a given niche axis, although this may happen in some cases (i.e. in warmer and drier environments in amphibians; figure 3). Furthermore, we find that species with wider TNB tend to have wider PNB, rather than finding trade-offs between niche breadths on these niche axes (figure 2). This is particularly surprising given the general importance of trade-offs in evolutionary ecology, and that such a trade-off was expected given patterns of latitudinal variation in PNB and TNB when considered separately (figure 2). In the paragraphs that follow, we discuss potential explanations for these patterns, possible sources of error, and implications for global climate change and amphibians.
(a). Niche width and niche position
There are several potential ways in which niche width might vary with niche position (figure 1). Our concordant results from temperature and precipitation suggest the same pattern in phrynosomatid lizards [13], in which precipitation niche widths are wider on one end and narrower on another (figure 1d). We do not support the idea that niches are generally narrower on both extremes (figure 1a). Even though the index that we used to reflect occurrence in extreme environments does show a significant association with niche breadths, this relationship seems to be driven by narrow niche widths at only one end of each axis. This pattern makes intuitive sense. Our measure of extreme niche position is based on the distance between each species' value and the midpoint for the group and is seemingly dominated (in each case) by the larger number of species at one end of each gradient. Thus, for temperature, the pattern is driven by the larger number of narrow-niched tropical species at the warmer end of the gradient (figure 3a), whereas for precipitation the pattern is driven by the many narrow-niched species at the drier end of the gradient (figure 3b).
Our results (and those from lizards [13]) raise the intriguing possibility that this pattern of narrowing niche widths on one end of a niche axis may be general. However, the mechanistic explanation for this pattern may depend on which niche axis is being considered, and perhaps the group of organisms in question. For temperature, the narrower niches in warmer climates may reflect the impacts of reduced temperature seasonality in the tropics [27–29]. It is important to remember, however, that it is possible for a single species to experience a broad range of annual mean temperatures in the tropics by occurring across a broad range of elevations. Thus, the pattern of species having relatively narrow temperature niche widths in the tropics is thought to be caused by the evolution of narrow niche widths in response to greater elevational temperature zonation [27] and not simply an artefact of their being in the tropics per se. By contrast, in temperate regions, a species could have a relatively broad TNB even if it occurred at only a single locality, because of the strong impact of within-locality seasonal variation on overall climatic niche widths for temperature [29].
We propose a somewhat different explanation for patterns of niche breadth and position along the precipitation gradient. For precipitation, we find a similar relationship between niche position and niche breadth regardless of whether our measure of niche breadth incorporates seasonality. We find that species at the drier end of the spectrum tend to have narrower niches than those at the wetter end. Examining spatial patterns of annual precipitation at the global scale, one factor that might contribute to this pattern is that most of the Earth's terrestrial surface is on the drier end of this spectrum (less than 2000 mm yr−1), and even many tropical rainforest regions have less than 4000 mm yr−1. Indeed, despite the well-known dependence of amphibians on water, almost all amphibian species have mean Bio12 values under 4000 mm yr−1, with a very large number in the driest quarter (less than 2000 mm yr−1). Only a few small, scattered areas are at the wettest half of the range (greater than 4000 mm yr−1), including parts of northwestern South America, the equatorial Andes, New Guinea and the southeastern Himalayas. Further, there are large changes in annual precipitation over small spatial scales near these areas. Thus, it may be possible for a single species (with moderate range size) at the wettest end of the spectrum to experience a broad range of precipitation regimes, whereas most other species can only experience a more limited range of drier regimes. Perhaps most importantly, it makes intuitive sense that amphibian species may require special adaptations to survive in drier areas and thus may be specialized for those conditions (e.g. burrowing behaviour, desiccation-resistant skin, rapid larval development [61,62]). But there may be no need for lineages adapted to wet tropical forests with 3000 mm yr−1 to have special adaptations to survive in even wetter tropical forests. Furthermore, for species that occur predominantly in drier climates, the range of precipitation regimes that they can experience (both spatially and seasonally) must be limited. Specifically, species that are desert specialists must have narrow precipitation niche widths, partly because they are specialists for those conditions, and also because deserts (by definition) include only a limited range of annual rainfall values (but note that species could occur predominantly in deserts but still have a geographical range that extends into much wetter conditions). Taken together, these factors may help explain why there are narrower niches under drier conditions and wider niches under wetter conditions. However, we recognize that the specific mechanistic explanations for these patterns may depend (to some extent) on the group of organisms being considered. Further study is needed on the generality of these patterns in other clades and their underlying mechanisms.
(b). Trade-offs in climatic niche width
Another surprising result of our study is that we do not support the idea that there are trade-offs in niche breadth on different climatic niche axes. We expected such trade-offs given their importance in evolutionary ecology in general and explaining specialization in particular [33]. Furthermore, the contrasting latitudinal patterns of seasonality in temperature and precipitation might be expected to generate a negative relationship between niche breadths on these two axes (figure 2), even if this negative relationship were not necessarily caused by trade-offs at the organismal level. Instead, we found a positive relationship between niche breadths for temperature and precipitation (figure 3c).
There are several potential explanations for this pattern. One explanation is that many species might cope with extreme conditions for both temperature and precipitation using similar mechanisms (e.g. inactivity during hot, dry and cold periods), whereas other species might require a narrow set of conditions on both axes. Alternately, species that are primarily confined to a given geographical area based on their tolerance to a limited range of conditions on one axis (e.g. precipitation) might therefore be exposed to a limited range of conditions on the other (e.g. temperature). As one intuitive example of the possible mechanisms that might underlie these patterns in amphibians, there are many derived reproductive modes in anurans that involve placing eggs out of water (e.g. in foam nests or in leaves overhanging ponds) and that seem to confine the lineages that have these modes to moist, tropical areas [55]. Conversely, lineages that simply place their eggs directly in water seemingly occur everywhere that anurans do, from rainforests to deserts [55]. Again, further studies will be needed to test the generality of this pattern and the specific mechanisms that underlie it (in amphibians and possibly other groups).
(c). Methodological issues
We note that there are several methodological issues that may have impacted our results, although we do not think that they should overturn them. First, our climatic data are at a relatively coarse spatial scale (approx. 5 km2). Although this might influence our estimates of climatic niche parameters for montane tropical species (where climate can change dramatically over small spatial scales; [27]), it should have much less impact for temperate species and tropical lowland species. Furthermore, it seems that this source of error should decrease the chances of our finding significant relationships, but not create significant relationships where none exist.
Second, we did not account for the effect of geographical range size on our estimates of climatic niche width. There is now some evidence that range size and climatic niche width are positively related [38,39]. However, the causal relationships are not so clear. If greater climatic niche width drives larger range sizes, then range size should not be a factor that confounds measurements of climatic niche width. On the other hand, if species only appear to have narrow niche widths because their range sizes are small, then this may be problematic. However, this will only happen if there are non-climatic factors that limit species range sizes and prevent them from reaching all parts of their geographical range that are within the climatic tolerances (see below). Previous analyses suggest that within-locality seasonal variability in climate is a major driver of climatic niche widths for both temperature and precipitation in amphibian species, rather than climatic variation across the species range [29]. This result strongly suggests that climatic niche widths should primarily drive range sizes rather than range sizes primarily driving climatic niche widths (or at least that range sizes do not primarily determine species niche widths, as they are determined more by variation within a locality rather than between them). Nevertheless, further analysis of this issue would be valuable.
Third, our measures of climatic niche width are based on the realized climatic niche width (the range of climatic conditions where the species actually occurs) and not the fundamental climatic niche width (the range of conditions that the species can tolerate physiologically [1,2]). There are various reasons why the realized climatic niche width might be smaller than the fundamental climatic niche width [2], such as non-climatic barriers to dispersal (water bodies, interactions with other species), absence of the full range of tolerable climatic conditions where the species occurs (e.g. for species on islands) or simply insufficient time to spread to all physiologically suitable locations within a region [46]. However, we do not see the fundamental climatic niche as being the only relevant or important measure of the climatic niche. We are interested in the actual climatic niches of species, not their hypothetical niches based on physiology alone. There is now evidence that species interactions (and the interactions of these species interactions with climate) may be important in setting species range limits [63] and in driving local extinctions and declines related to anthropogenic climate change [64], especially in vertebrates. At the same time, it is important to keep in mind that our estimates of climatic niche width should not be taken as proxies for physiological tolerances.
Fourth, we used relatively simple, univariate measures of the climatic niche, although these seemed more appropriate for the hypotheses of interest here than multivariate estimates. Fifth, even assuming that species ranges are set primarily by physiological tolerances to climate, there is no guarantee that the climatic variables used here are the ones that set their geographical ranges (and thereby determining the climatic niche widths for these variables). Finally, our results were conducted at a broad phylogenetic scale, and it is possible that more fine-scaled studies within different amphibian clades might find somewhat different patterns.
(d). Implications for conservation and global change
Our results on patterns of climatic niche specialization also have important conservation implications, in showing that amphibian species that occur in warmer and drier environments have narrower climatic niche widths for temperature and precipitation, respectively (figure 3). Importantly, as climates are predicted to become warmer and drier in many regions of the world [65], these species with narrow climatic niche widths are those that may be most likely to eventually have their entire (current) geographical ranges outside of their current climatic niches. Thus, these species may be forced to either: (i) track suitable climatic conditions over space through dispersal, (ii) acclimate or (iii) adapt to the changed conditions evolutionarily, or (failing these three options) go extinct [66,67]. Furthermore, analyses based on past rates of climatic niche evolution in amphibians suggest that adaptation may occur too slowly to keep pace with the projected rates of anthropogenic climate change [24], whereas niche tracking over space may be limited by human habitat modification, slow dispersal and many other factors. Unfortunately, our results suggest that most amphibian species already occur at the warmer and drier ends of the temperature and precipitation niche gradients and thus have relatively narrow climatic niche breadths for both temperature and precipitation (figure 3). Of course, the idea that amphibians are imperilled by climate change is hardly new [68], but analyses of general patterns of climatic niche widths offer an important perspective.
5. Conclusions
In this study, we use large-scale datasets for climate and phylogeny in amphibians to test fundamental hypotheses about the factors that influence the evolution of climatic niche widths. Our results reject our initial hypotheses that climatic niche widths generally become narrower at both of the extreme ends of a climatic gradient and that there are trade-offs between climatic niche widths for temperature and precipitation. The generality of these results should be tested in other groups of organisms, and their causes should be tested with more fine-scaled mechanistic studies. Our results also have potentially important implications for species survival under climate change.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the organizers (J. Vamosi, S. Renner and S. Armbruster) for inviting J.J.W. to contribute a paper to this special issue. We thank S. Renner, M. Olalla-Tárraga and an anonymous reviewer for helpful comments on the manuscript.
Data accessibility
Data are available in the electronic supplementary material, appendix S1.
Funding statement
M.F.B. thanks the CAPES Foundation for funding to visit J.J.W. and work on this project.
References
- 1.Hutchinson GE. 1957. Concluding remarks. Cold Spring Harb. Symp. 22, 415–427. ( 10.1101/SQB.1957.022.01.039) [DOI] [Google Scholar]
- 2.Soberón J. 2007. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123. ( 10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- 3.Holt RD. 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl Acad. Sci. USA 106, 19 659–19 665. ( 10.1073/pnas.0905137106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiens JJ, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324. ( 10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 5.Crisp MD, et al. 2009. Phylogenetic biome conservatism on a global scale. Nature 458, 754–756. ( 10.1038/nature07764) [DOI] [PubMed] [Google Scholar]
- 6.Smith BT, Bryson RW, Houston D, Klicka J. 2012. An asymmetry in niche conservatism contributes to the latitudinal species diversity gradient in New World vertebrates. Ecol. Lett. 15, 1318–1325. ( 10.1111/j.1461-0248.2012.01855.x) [DOI] [PubMed] [Google Scholar]
- 7.Wiens JJ, Graham CH, Moen DS, Smith SA, Reeder TW. 2006. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am. Nat. 168, 579–596. ( 10.1086/507882) [DOI] [PubMed] [Google Scholar]
- 8.Rangel TF, Diniz-Filho JAF, Colwell RK. 2007. Species richness and evolutionary niche dynamics: a spatial pattern-oriented simulation experiment. Am. Nat. 274, 165–174. [DOI] [PubMed] [Google Scholar]
- 9.Buckley LB, et al. 2010. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B 277, 2131–2138. ( 10.1098/rspb.2010.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pyron RA, Wiens JJ. 2013. Large-scale phylogenetic analyses reveal the causes of high tropical amphibian diversity. Proc. R. Soc. B 280, 20131622 ( 10.1098/rspb.2013.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozak KH, Wiens JJ. 2010. Niche conservatism drives elevational diversity patterns in Appalachian salamanders. Am. Nat. 176, 40–54. ( 10.1086/653031) [DOI] [PubMed] [Google Scholar]
- 12.Hutter CR, Guayasamin JM, Wiens JJ. 2013. Explaining Andean megadiversity: the evolutionary and ecological causes of glassfrog elevational richness patterns. Ecol. Lett. 16, 1135–1144. ( 10.1111/ele.12148) [DOI] [PubMed] [Google Scholar]
- 13.Wiens JJ, Kozak KH, Silva N. 2013. Diversity and niche evolution along aridity gradients in North American lizards (Phrynosomatidae). Evolution 67, 1715–1728. ( 10.1111/evo.12053) [DOI] [PubMed] [Google Scholar]
- 14.Stephens PR, Wiens JJ. 2009. Bridging the gap between biogeography and community ecology: niche conservatism and community structure in emydid turtles. Mol. Ecol. 18, 4664–4679. ( 10.1111/j.1365-294X.2009.04378.x) [DOI] [PubMed] [Google Scholar]
- 15.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 16.Kozak KH, Wiens JJ. 2006. Does niche conservatism drive speciation? A case study in North American salamanders. Evolution 60, 2604–2621. ( 10.1111/j.0014-3820.2006.tb01893.x) [DOI] [PubMed] [Google Scholar]
- 17.Cadena CD, et al. 2012. Latitude, elevational climatic zonation and speciation in New World vertebrates. Proc. R. Soc. B 279, 194–201. ( 10.1098/rspb.2011.0720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hua X, Wiens JJ. 2013. How does climate influence speciation? Am. Nat. 182, 1–12. ( 10.1086/670690) [DOI] [PubMed] [Google Scholar]
- 19.Peterson AT. 2003. Predicting the geography of species’ invasions via ecological niche modeling. Q. Rev. Biol. 78, 419–433. ( 10.1086/378926) [DOI] [PubMed] [Google Scholar]
- 20.Wiens JJ, Graham CH. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539. ( 10.1146/annurev.ecolsys.36.102803.095431) [DOI] [Google Scholar]
- 21.Petitpierre B, Kueffer C, Broennimann O, Randin C, Daehler C, Guisan A. 2012. Climatic niche shifts are rare among terrestrial plant invaders. Science 335, 1344–1348. ( 10.1126/science.1215933) [DOI] [PubMed] [Google Scholar]
- 22.Deutsch C, Tewksbury JJ, Huey RB, Sheldon K, Ghalambor C, Haak D, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tingley MW, Monahan WB, Beissinger SR, Moritz C. 2009. Birds track their Grinnellian niche through a century of climate change. Proc. Natl Acad. Sci. USA 106, 19 637–19 643. ( 10.1073/pnas.0901562106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quintero I, Wiens JJ. 2013. Rates of projected climate change dramatically exceed past rates of climatic-niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103. ( 10.1111/ele.12144) [DOI] [PubMed] [Google Scholar]
- 25.Kozak KH, Wiens JJ. 2007. Climatic zonation drives latitudinal variation in speciation mechanisms. Proc. R. Soc. B 274, 2995–3003. ( 10.1098/rspb.2007.1106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak KH, Wiens JJ. 2010. Accelerated rates of climatic-niche evolution underlie rapid species diversification. Ecol. Lett. 13, 1378–1389. ( 10.1111/j.1461-0248.2010.01530.x) [DOI] [PubMed] [Google Scholar]
- 27.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 28.Vázquez DP, Stevens RD. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1–E19. ( 10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 29.Quintero I, Wiens JJ. 2013. What determines the climatic niche width of species? The role of spatial and temporal climatic variation in three vertebrate clades. Glob. Ecol. Biogeogr. 22, 422–432. ( 10.1111/geb.12001) [DOI] [Google Scholar]
- 30.Huey RB, Kingsolver JG. 1993. Evolutionary responses to extreme temperatures in ectotherms. Am. Nat. 141, S31–S46. [Google Scholar]
- 31.Lenski RE, Bennett AF. 1993. Evolutionary response of Escherichia coli to thermal stress. Am. Nat. 142, S47–S64. ( 10.1086/285522) [DOI] [PubMed] [Google Scholar]
- 32.Bennett AF, Lenski RE. 2007. An experimental test of evolutionary trade-offs during temperature adaptation. Proc. Natl Acad. Sci. USA 104, 8649–8654. ( 10.1073/pnas.0702117104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233. ( 10.1146/annurev.es.19.110188.001231) [DOI] [Google Scholar]
- 34.Whitlock MC. 1996. The Red Queen versus the Jack-of-All-Trades: evolutionary rates and the evolution of specialization. Am. Nat. 148, S65–S77. ( 10.1086/285902) [DOI] [Google Scholar]
- 35.Roff DA, Fairbairn DJ. 2007. The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447. ( 10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- 36.MacArthur RH. 1972. Geographical ecology. New York, NY: Harper and Row. [Google Scholar]
- 37.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Pither J. 2003. Climate tolerance and interspecific variation in geographic range size. Proc. R. Soc. Lond. B 102, 427–432. ( 10.1098/rspb.2002.2275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slayter RA, Hirst M, Sexton JP. 2013. Niche breadth predicts geographic range size: a general ecological pattern. Ecol. Lett. 16, 1104–1114. ( 10.1111/ele.12140) [DOI] [PubMed] [Google Scholar]
- 40.IUCN. 2012. IUCN red list of threatened species, v. 2012.1 See http://www.iucnredlist.org (Accessed August 2013).
- 41.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 42.Pyron RA, Wiens JJ. 2011. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 61, 543–583. ( 10.1016/j.ympev.2011.06.012) [DOI] [PubMed] [Google Scholar]
- 43.Buckley LB, Jetz W. 2007. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B 274, 1167–1173. ( 10.1098/rspb.2006.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olalla-Tárraga M, et al. 2012. Climatic niche conservatism and the evolutionary dynamics in species’ range boundaries: congruence across mammals and amphibians globally. J. Biogeogr. 38, 2237–2247. ( 10.1111/j.1365-2699.2011.02570.x) [DOI] [Google Scholar]
- 45.Whitton FJS, Purvis A, Orme CDL, Olalla-Tárraga M. 2012. Understanding global patterns in amphibian geographic range size: does Rapoport rule? Glob. Ecol. Biogeogr. 21, 179–190. ( 10.1111/j.1466-8238.2011.00660.x) [DOI] [Google Scholar]
- 46.Munguía M, Rahbek C, Rangel TF, Diniz-Filho JAF, Araújo MB. 2012. Equilibrium of global amphibian species distributions with climate. PLoS ONE 7, e34420 ( 10.1371/journal.pone.0034420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AmphibiaWeb: information on amphibian biology and conservation (web application). 2013. Berkeley, CA: AmphibiaWeb See http://amphibiaweb.org/ (Accessed 8 December 8 2013).
- 48.Hijmans RJ. 2013. raster: raster: geographic data analysis and modeling. R package v. 2.1–49 See http://CRAN.R-project.org/package=raster.
- 49.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 50.Bivand R, Lewin-Koh N. 2013. maptools: Tools for reading and handling spatial objects. R package v. 0.8–27 See http://CRAN.R-project.org/package=maptools.
- 51.Sanderson MJ. 2002. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 19, 101–109. ( 10.1093/oxfordjournals.molbev.a003974) [DOI] [PubMed] [Google Scholar]
- 52.Wiens JJ. 2011. Re-evolution of lost mandibular teeth in frogs after more than 200 million years, and re-evaluating Dollo's law. Evolution 65, 1283–1296. ( 10.1111/j.1558-5646.2011.01221.x) [DOI] [PubMed] [Google Scholar]
- 53.Roelants K, Gower DJ, Wilkinson M, Loader SP, Biju SD, Guillaume K, Moriau L, Bossuyt F. 2007. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA 104, 887–892. ( 10.1073/pnas.0608378104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiens JJ. 2007. Global patterns of diversification and species richness in amphibians. Am. Nat. 170, S86–S106. ( 10.1086/519396) [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Mestre I, Pyron RA, Wiens JJ. 2012. Phylogenetic analyses reveal unexpected patterns in the evolution of reproductive modes in frogs. Evolution 66, 3687–3700. ( 10.1111/j.1558-5646.2012.01715.x) [DOI] [PubMed] [Google Scholar]
- 56.Harmon LJ, Weir J, Brock C, Glor RE, Challenger W. 2008. GEIGER: a statistical package for investigating evolutionary radiation in a comparative context. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 57.Ackerly DD. 2000. Taxon sampling, correlated evolution, and independent contrasts. Evolution 54, 1480–1492. ( 10.1111/j.0014-3820.2000.tb00694.x) [DOI] [PubMed] [Google Scholar]
- 58.Stephens PR, Wiens JJ. 2008. Testing for evolutionary trade-offs in a phylogenetic context: ecological diversification and locomotor performance in emydid turtles. J. Evol. Biol. 21, 77–928. [DOI] [PubMed] [Google Scholar]
- 59.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667. ( 10.1086/286013) [DOI] [Google Scholar]
- 60.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2012. caper: Comparative analyses of phylogenetics and evolution in R. R package v. 0.5 See http://CRAN.R-project.org/package=caper.
- 61.Bentley PJ. 1966. Adaptations of Amphibia to arid environments. Science 152, 619–623. ( 10.1126/science.152.3722.619) [DOI] [PubMed] [Google Scholar]
- 62.Navas CA, Antoniazzi MM, Jared C. 2004. A preliminary assessment of anuran physiological and morphological adaptation to the Caatinga, a Brazilian semi-arid environment. Int. Congr. Ser. 1275, 298–305. ( 10.1016/j.ics.2004.08.061) [DOI] [Google Scholar]
- 63.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690. [Google Scholar]
- 64.Cahill AE, et al. 2013. How does climate change cause extinction? Proc. R. Soc. B 280, 20121890 ( 10.1098/rspb.2012.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.IPCC 2007 Climate change 2007, synthesis report. In Contributions of working groups I, II and III to the fourth assessment report of the Intergovernmental Panel on Climage Change (eds Core Writing Team, Pachauri RK, Reisinger A.), pp. 104 Geneva, Switzerland: IPCC. [Google Scholar]
- 66.Holt RD. 1990. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315. ( 10.1016/0169-5347(90)90088-U) [DOI] [PubMed] [Google Scholar]
- 67.Visser ME. 2008. Keeping up with a warming world: assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hof C, Araujo MB, Jetz W, Rahbek C. 2011. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material, appendix S1.


