Abstract
A striking example of plant/pollinator trait matching is found between Andean species of Passiflora with 6–14-cm-long nectar tubes and the sword-billed hummingbird, Ensifera ensifera, with up to 11-cm-long bills. Because of the position of their anthers and stigmas, and self-incompatibility, these passionflower species depend on E. ensifera for pollination. Field observations show that the bird and plant distribution match completely and that scarcity of Ensifera results in reduced passionflower seed set. We here use nuclear and plastid DNA sequences to investigate how often and when these mutualisms evolved and under which conditions, if ever, they were lost. The phylogeny includes 26 (70%) of the 37 extremely long-tubed species, 13 (68%) of the 19 species with tubes too short for Ensifera and four of the seven bat-pollinated species for a total of 43 (69%) of all species in Passiflora supersection Tacsonia (plus 11 outgroups). We time-calibrated the phylogeny to infer the speed of any pollinator switching. Results show that Tacsonia is monophyletic and that its stem group dates to 10.7 Ma, matching the divergence at 11.6 Ma of E. ensifera from its short-billed sister species. Whether pollination by short-billed hummingbirds or by Ensifera is the ancestral condition cannot be securely inferred, but extremely long-tubed flowers exclusively pollinated by Ensifera evolved early during the radiation of the Tacsonia clade. There is also evidence of several losses of Ensifera dependence, involving shifts to bat pollination and shorter billed birds. Besides being extremely asymmetric—a single bird species coevolving with a speciose plant clade—the Ensifera/Passiflora system is a prime example of a specialized pollinator not driving plant speciation, but instead being the precondition for the maintenance of isolated populations (through reliable seed set) that then underwent allopatric speciation.
Keywords: coevolution, asymmetric coevolution, hummingbirds, molecular clock, Passiflora, phylogenetics
1. Introduction
Few evolutionary transitions in plant reproductive systems are irreversible, a conclusion now widely accepted based on changes in floral syndromes, sexual systems or self-pollination inferred on molecular phylogenies [1]. Among the exceptions may be the transition to hummingbird pollination. In a recent review of the topic of evolutionary reversibility, Barrett suggested that a directional bias in favour of transitions to, but not away from, pollination by hummingbirds may be due to the efficiency of these pollinators [2], the nature of genetic mutations in floral pigments that may make it difficult to return from red to blue or yellow colours [3] or the acquisition of thin long nectar tubes, difficult to modify [4]. Studies of floral trait change in the best-investigated North American systems, Aquilegia and Penstemon sensu lato, imply several shifts between moth, bee and hummingbird pollination, with a unidirectional trend towards long-tubed (hawkmoth- or hummingbird-pollinated) flowers in Aquilegia but not Penstemon [4,5]. This indicates trait reversibility over a few million years, the time frame for North American hummingbird/plant interactions [6]. But what about more extreme floral adaptations, such as those among many-centimetre-long flowers pollinated by long-billed Andean hummingbirds? How long did it take for them to evolve, and is there a unidirectional trend from short flowers to long flowers as in the North American Aquilegia system?
To study these questions, we focused on the Passifloraceae, which are among the most species-rich groups with hummingbird-pollinated species. The largest genus is Passiflora, with 560 species of which 95% occur in tropical Central and South America, almost half (250 species) in subgenus Passiflora [7]. Within this subgenus, there is a group of species with floral tubes ranging from a few to 14 cm long (figure 1). These passionflowers are grouped into supersection Tacsonia, which comprises 62–64 species, all restricted to the high Andes at 1700 to approximately 4000 m ([8–11]; figure 2b). Passiflora supersection Tacsonia is characterized by several morphological traits, suggesting that the group might be monophyletic [9], although this has not really been tested. The best-sampled phylogeny so far included only seven Tacsonia species, which formed a clade [12]. While most species of the supersection have hummingbird-adapted flowers, the longest tubed-flowers are restricted to 37 species pollinated by the sword-billed hummingbird, Ensifera ensifera, whereas the 19 species with shorter tubed red flowers (hypanthium 1–3 cm long) are pollinated by shorter billed hummingbirds [13]. Bats pollinate another seven species that have greenish or white flowers [11,14]. Like most Passiflora, Tacsonia species are self-incompatible and depend on cross-pollination to set seed [10].
Figure 1.
Representative species of Passiflora supersection Tacsonia. (a) Passiflora tarminiana, Peru, dependent on E. ensifera for pollination; (b) Passiflora ampullacea, Ecuador, dependent on E. ensifera for pollination. (c) Passiflora peduncularis, Peru, pollinated by bats. (d) Passiflora unipetala, Bellavista Cloud Forest Reserve, Pichincha, Ecuador, being visited by Anoura fistulata. Photo credits: figure (a) by P. M. Joergensen, (b) by G. Onore, (c) by T. Boza and (d) by N. Muchhala.
Figure 2.
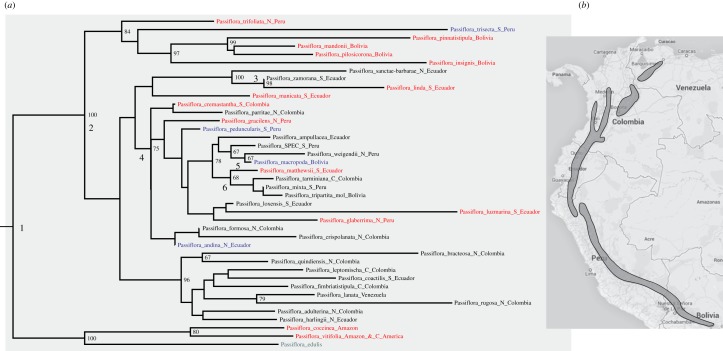
(a) Phylogeny for 37 species of Passiflora supersection Tacsonia based on 2867 aligned nucleotides of plastid and nuclear DNA markers. Species names in black refer to Passiflora species with nectar tubes 6–14 cm long and thus dependent on E. ensifera for pollination; names in red refer to species with nectar tubes less than 6 cm and pollinated by short-billed hummingbirds; in blue are species pollinated by bats; in green species pollinated by bees. Electronic supplementary material, table S2, provides the basis for each species’ scoring. (b) Distribution map of E. ensifera from Birdlife International (2014) (www.birdlife.org). The distribution of supersection Tacsonia (in grey) completely matches that of Ensifera (in black). (Online version in colour.)
The long-tubed Tacsonia flowers exactly match the up to 11-cm-long bill of the sword-billed hummingbird (figure 3a), a common Andean species that occurs between 1400 and 4000 m.a.s.l. (figure 2b shows its geographical range). This bird species is the only pollinator capable of depositing pollen grains on the stigmas of these passionflowers while drinking nectar [15,16]. Northern Ensifera males have bills 10.4 cm long, females 11.2 cm long; birds from the southern part of the range have slightly shorter bills [17]. The morphological fit between the bird's bill length and the flower tubes and stamen and stigma positions, together with the overlap between Ensifera and the combined geographical ranges of the long-tubed passionflower species, make this relationship a clear case of plant/pollinator coevolution. Like many hummingbirds, E. ensifera is a trap-liner, regularly revisiting individual plants or flowers, which in the case of Tacsonia last 4–5 days. A dated hummingbird phylogeny shows that E. ensifera diverged from its short-billed sister species, Pterophanes cyanopterus, approximately 11.6 Ma [6].
Figure 3.
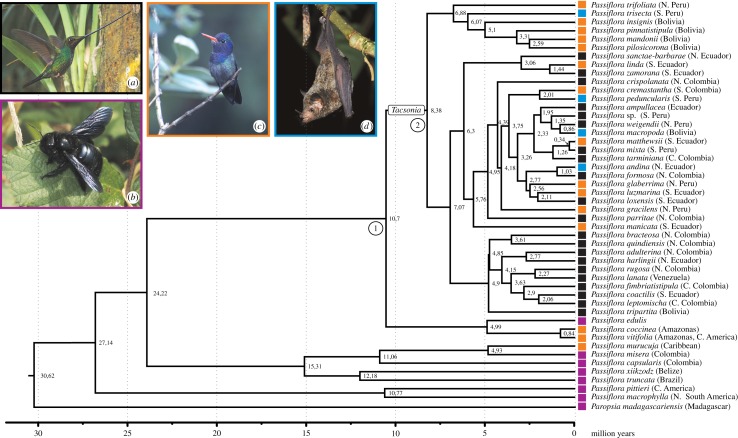
Molecular clock tree for 37 species of Passiflora supersection Tacsonia, with species colour-coded by pollinator type: bee, bat, short-billed hummingbird and E. ensifera. Electronic supplementary material, table S2, provides the basis for each species' scoring. The photos show (a) E. ensifera, (b) Xylocopa carpenter bee on a leaf of Rubus, (c) short-billed hummingbird (Hylocharis cyanus) and (d) the bat Glossophaga commissarisi, which pollinates species of Tacsonia. Electronic supplementary material, figure S2, shows the 95% confidence intervals around divergence times, figure S3 a parsimony-based ancestral state reconstruction and figure S4 a likelihood-based reconstruction of pollination syndromes. Photo credits: (a) J. C. Boone, (b) H. Mouret, (c) D. Sanchez and (d) K. Schneeberger (all from Wikipedia.org).
Given that approximately 37 of the 62–64 species of Tacsonia are pollinated primarily or exclusively by the sword-billed hummingbird, while the remaining species are pollinated by short-billed hummingbirds or bats and assuming that the group is monophyletic, Tacsonia passionflowers make a suitable study system for addressing the question of specialization on, or de-specialization away from, a single pollinator species. Specialization on a single species entails the risk of interdependence, which may increase local or global extinction. Indeed, the scarcity of E. ensifera has been suggested as causing local extinction of Passiflora mixta, a member of supersection Tacsonia, in open landscapes in Ecuador [16]. Answering the question of increasing or decreasing specialization required a densely sampled phylogeny in which all pollination syndromes would be appropriately represented. Since we were interested in the evolutionary speed of any pollinator shifts, we applied a molecular clock model to the data to infer absolute divergence times for the Tacsonia passionflower clade.
2. Material and methods
(a). Plant material, DNA isolation, amplification and sequencing
The taxonomic names and authors, geographical origin, voucher information and place of deposition and GenBank accession numbers for all sequences produced for this study are listed in the electronic supplementary material, table S1. Approximately 0.2 g (dry weight) of leaf tissue was taken from 53 herbarium specimens of Tacsonia, representing 43 species from throughout the geographical and morphological range of the supersection. A total of 140 new sequences were deposited in GenBank. As outgroups, we used GenBank-downloaded sequences of 11 species from the Passiflora subgenera Decaloba, Astrophea and Passiflora (supersections Coccinea and Passiflora) based on Krosnick et al. [7]. As a more distant outgroup, we included Paropsia madagascariensis because its divergence time from other Passifloraceae has been estimated in another study [18] and could thus serve as a cross-validation point for our molecular clock dating (§2b).
DNA isolation relied on Nucleospin Plant II kits (Macherey-Nagel, Düren, Germany) and the manufacturer's protocol with the exception of incubation time, which was increased to 60 min. DNA concentrations were quantified using a NanoDrop 2000 microvolume spectrophotometer (Thermo Fisher Scientific). The plastid trnL-F spacer region was amplified using the Taberlet et al. [19] primers c and f and an annealing temperature of 52°C. For samples that did not amplify with this primer combination, we additionally used the internal primers d and e. Our second plastid marker was the ndhF gene amplified with primers 5.5F and 10.2R of Davis et al. [20] with the same annealing temperature. As nuclear markers, we used the internal transcribed spacer (ITS) region amplified with the primer pair 5 and 4 of White et al. [21], and the low-copy glutamine synthetase gene (ncpGS) with the primer pair 687 and 994 of Emshwiller & Doyle [22]. PCR products were cleaned and purified, and then sequenced on an ABI Prism 3130 Genetic Analyzer (Applied Biosystems) using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Inc., Warrington, UK). Chromatogram inspection and sequence assembly was done with CodonCode aligner (CodonCode Corporation), alignment with MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/), followed by visual inspection in Mesquite v. 2.75 [23]. All sequences were BLAST-searched in GenBank. Any ITS sequences with ambiguous base calls were removed from the final alignments to avoid using paralogous copies. For the ncpGS gene, which amplified in two distinct copies, one of a length of 545 and one of 657 aligned nucleotides, we only used the longer sequences.
(b). Phylogenetic analyses and molecular clock dating
Phylogenetic analyses used maximum likelihood (ML) as implemented in RAxML v. 7.6.3 [24] and Bayesian inference as implemented in BEAST v. 1.8.0 [25]. For the ML analysis, we used all four markers (trnL-F, ndhF, ITS and ncpGS), whereas for the Bayesian analysis, we excluded ncpGS because the long copy of this nuclear region amplified in only 17 species. Tree searches were carried out on the CIPRES science gateway portal [26]. In the absence of topological conflict (defined as greater than 75% ML bootstrap support) between the plastid and nuclear trees, data partitions were concatenated. To increase bootstrap support, we repeated the ML analyses with the full dataset of 44 species of Tacsonia, 10 outgroups, and 3581 aligned nucleotides and a reduced set of 37 species of Tacsonia, three outgroups and 2867 aligned nucleotides. For the ML analyses, we used the GTR + G nucleotide substitution model (with four rate categories), but for the Bayesian runs, the slightly less parameter-rich HKY + G model (with four rate categories). To calibrate the genetic distances, we applied an ITS rate of 5.5 × 10−9 [27] to the ITS data matrix, without specifying a rate for the plastid data partition. We ran relaxed clock models using the uncorrelated lognormal model because the ucld.stdev value was more than 0.5, both with a Yule tree prior with gamma height distribution. MCMC chains were run for 40 million generations, sampling every 10 000th generation. Stationarity was checked in Tracer v. 1.5 [28], and output files were inspected in TreeAnnotator v. 1.8.0 (part of the BEAST package). The first 20% of trees were discarded as burn-in, and a posterior probability limit of 0.98 was set to retrieve a maximum clade credibility tree. All trees were viewed and annotated in FigTree v. 1.4.0 [29].
(c). Scoring of morphological flower traits and pollination syndromes
Morphological information for each species was taken from floras (cited in the electronic supplementary material, table S2), focusing on hypanthium length and flower colour. A character matrix was created in Mesquite, with hypanthium length divided into five categories (less than 1 cm, 1–2.9 cm, 3–5.9 cm, 6–9.99 cm and more than 10 cm). For each species, we searched for field observations on its pollinators, and species lacking direct pollinator observations were categorized based on flower colour and tube length, using the following criteria: (i) E. ensifera-dependent if flowers were pink, red or purple and hypanthium tubes more than 6 cm long; (ii) pollinated by other hummingbirds if flowers were pink, red or purple and tubes 1–5.9 cm long; (iii) pollinated by bats if flowers were greenish or white and/or there were actual observations of bat pollination; (iv) bee pollination, based on actual observations for some of the outgroup species (electronic supplementary material, table S2). Ancestral state reconstruction relied on maximum parsimony and likelihood optimization in Mesquite v. 2.75 [30], with the BEAST chronogram as the input tree and using the Mk-1 model of state transitions. As before, the tree was rooted on representatives of supersections Coccinea and Passiflora, based on Krosnick et al. [7].
3. Results
(a). Phylogeny and chronogram of Passiflora supersection Tacsonia
Because of variable success in PCR amplification for the nuclear and plastid regions, the individual alignments vary in the number of plant accessions (electronic supplementary material, table S1, shows all used sequences); especially the ncpGS alignments were highly incomplete, including only 17 sequences. The concatenated alignment comprised 44 Tacsonia species, 10 outgroups and 3581 nucleotides (electronic supplementary material, figure S1), but included many almost identical sequences. We therefore reduced the dataset to 37 Tacsonia, and just three outgrups (figure 2). The monophyly of Tacsonia is maximally supported (node 2 in figure 2), and there is statistical support for several nodes relevant to our study question, namely switches between Ensifera and short-billed hummingbirds. Switches between Ensifera and bats as pollinators occurred in the cloud forests of Bolivia, Peru and Ecuador, and one switch from short-billed hummingbirds to bats occurred in the group including Passiflora trisecta.
The molecular-clock chronogram is shown in figure 3, and a chronogram with 95% confidence intervals around the time estimates is shown as the electronic supplementary material, figure S2. The time tree contains slightly more outgroup species than figure 2 for the purpose of cross validation with results from a fossil-calibrated angiosperm-wide study [18]. In that study, the divergence between P. madagascariensis and Passiflora was dated to 28 (18–38) Ma, which is close to the 30.6 (20.2–40.4) Ma obtained in our study (electronic supplementary material, figure S2). The divergence of the Tacsonia clade from the most closely related Passiflora group occurred 10.7 (7.6–14.5) Ma, while the Tacsonia crown group dates to 8.4 (6.2–11.2) Ma.
In figure 3, 37 species of Passiflora supersection Tacsonia are coded as to their pollinators, with the basis for each coding shown in the electronic supplementary material, table S2 (Material and methods). Ancestral state reconstruction under parsimony (electronic supplementary material, figure S3) or ML (electronic supplementary material, figure S4) suggests that the ancestral state in Tacsonia may have been pollination by short-billed hummingbirds (node 2 in figure 3), followed by pollination by Ensifera at the next node; that node, however, lacks statistical support (see figure 2). A problem is that our species sampling in supersections Coccinea and Passiflora, the outgroups, is too poor to reliably infer their ancestral pollination by either bees or short-billed hummingbirds, which in turn prevents reliably inference at the root of section Tacsonia. It is nevertheless clear from our data that the adaptation to Ensifera evolved during the early radiation of the Tacsonia clade. Moves away from Ensifera to pollination by short-billed hummingbirds and bats occurred several times (figure 3), even when considering only statistically supported nodes (figure 2).
4. Discussion
Our data show that Passiflora supersection Tacsonia is monophyletic, diverged from the remaining Passiflora approximately 10.7 Ma, and underwent radiation at 9–8 Ma, matching a major uplift phase of the Northern Andes [31]. Our species sampling in the most closely related groups, supersections Coccinea and Passiflora, is too poor to reliably infer whether the ancestral pollination mode in Tacsonia was bee pollination or pollination by short-billed hummingbirds, but there is unambiguous support for an early coevolution between species of Tacsonia and E. ensifera, today the exclusive pollinator of over half the species (37 of 62–64). The interaction could have begun approximately 11 Ma, when Ensifera diverged from its relatively short-billed sister species, P. cyanopterus (bill length: 2.9 cm; [32]), an event dated to 11.6 Ma [6].
All species of Tacsonia are restricted to cloud forests between 1700 and approximately 4000 m altitude, the habitat of the sword-billed hummingbird, and the geographical distributions of the plant and animal partners in this mutualism overlap completely. The radiation of crown group Tacsonia, however, was mainly linked to the colonization of the newly arising Andean cloud forest habitat, not pollinator shifts because there are large clades that are entirely Ensifera-pollinated (figure 3; electronic supplementary material, figures S3 and S4). The Tacsonia mutualism with Ensifera, a reliable pollinator because of its trap-lining behaviour and strong flight ability, probably enabled populations to persist, that is, be pollinated and set seed, even in isolated patches, but was not per se the driving selective factor in species divergence (because there are too few switches to/from Ensifera-pollination).
Studying the effects of deforestation on the P. mixta/E. ensifera system at two sites in the Ecuadorean Andes, Lindberg & Olesen [16] found that few plants in the deforested, open land were visited by E. ensifera resulting in a low fruit set. This indicates the dependence of long-tubed passionflowers on their sole effective pollinator [16] and demonstrates the risks that dependence on a single pollinator species must carry, especially in plants unable to reproduce by self-pollination. Indeed, habitat fragmentation has been linked to the local extinction of at least four species of Tacsonia ([8]; M. Schwerdtfeger 2000, personal communication cited in [16]). This situation must have created the conditions conducive to shifts back to pollination by shorter billed hummingbirds or bats. Such shifts necessarily were linked to a shortening of nectar tube lengths since no other pollinator in the high Andes at 1700 to approximately 4000 m has the proboscis or bill length required to take up and deposit pollen from Passiflora with nectar tubes longer than 6 cm.
Bat pollination appears to have evolved both from short-billed hummingbird pollination and from Ensifera-pollination (figure 3; electronic supplementary material, figure S3 and S4) and to have resulted in shorter nectar tubes and whitish-greenish flowers (figure 1d). Our sampling includes four of seven bat-pollinated species and lacks the bat-pollinated Passiflora colombiana, P. unipetala and P. weberbaueri. Based on its morphology, P. unipetala is closest to the likewise bat-pollinated P. andina ([11]; figure 2); we do not know the relationships of P. colombiana and P. weberbaueri.
In Aquilegia, flower tube length evolved unidirectionally from short to long, with two types of transitions, bumblebee to hummingbird and hummingbird to hawkmoth [4]. In Tacsonia, there is no such irreversibility in tube length even though the colour switches from red bird-pollinated flowers to pale bat-pollinated flowers in Tacsonia resemble those in Aquilegia, which repeatedly switched from red bird-pollinated flowers to pale moth-pollinated flowers.
5. Conclusion
As shown here, the Tacsonia clade of Passiflora diverged from its sister group around 10.7 Ma and acquired long corolla tubes early during its evolution as a result of coevolution with the sword-billed hummingbird, itself dated to 11.6 Myr. Among the interesting features of this coevolution is its asymmetry, involving the interaction of one species of animal with a large clade of plants. Its specialized and therefore dependable pollinator enables even small and isolated population of Tacsonia to set seed, a situation conducive to allopatric speciation. Ongoing work in our laboratory is focusing on the few other plant species adapted to Ensifera and on understanding the variation in bill length from the northern to the southern part of the bird's range [17].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank P. M. Jørgensen and M. Schwerdtfeger for advice on supersection Tacsonia; the herbaria in Munich (M and MSB), St Louis (MO), Vienna (W), Goettingen (GOET) and Quito (QCA) for leaf fragments; M. Silber for help with laboratory work; O. Perez for help with figure 1 and the handling editor S. Armbruster for his comments.
Data accessibility
All data were uploaded as the electronic supplementary material, tables S1 and S2, and all sequences have been submitted to GenBank. GenBank numbers are listed in electronic supplementary material, table S1.
References
- 1.Barrett SCH. 2013. The evolution of plant reproductive systems: how often are transitions irreversible? Proc. R. Soc. B 280, 20130913 ( 10.1098/rspb.2013.0913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson JD, Wilson P. 2008. Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence, and directionality. Int. J. Plant Sci. 169, 23–38. ( 10.1086/523361) [DOI] [Google Scholar]
- 3.Smith SD, Rausher MD. 2011. Gene loss and parallel evolution contribute to species difference in flower color. Mol. Biol. Evol. 28, 2799–2810. ( 10.1093/molbev/msr109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittall JB, Hodges SA. 2007. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–710. ( 10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- 5.Wilson P, Wolfe AD, Armbruster WS, Thomson JD. 2007. Constrained lability in floral evolution: counting convergent origins of hummingbird pollination in Penstemon and Keckiella. New Phytol. 176, 883–890. ( 10.1111/j.1469-8137.2007.02219.x) [DOI] [PubMed] [Google Scholar]
- 6.McGuire JA, Witt CC, Remsen JV, Corl A, Rabowsky D, Altshuler DL, Dudley R. 2014. Molecular phylogenetics and diversification of hummingbirds. Curr. Biol. 24, 1–7. ( 10.1016/j.cub.2014.03.016) [DOI] [PubMed] [Google Scholar]
- 7.Krosnick SE, Porter-Utley KE, MacDougal JM, Jørgensen PM, McDade LA. 2013. New insights into the evolution of Passiflora subgenus Decaloba (Passifloraceae): phylogenetic relationships and morphological synapomorphies. Syst. Bot. 38, 1–22. ( 10.1600/036364413X670359) [DOI] [Google Scholar]
- 8.Escobar LK. 1980. Interrelationships of the edible species of Passiflora centering around Passiflora mollissima (H.B.K.) Bailey, subgenus Tacsonia. PhD dissertation, University of Texas, Austin, TX, USA. [Google Scholar]
- 9.Escobar LK. 1988. Passifloraceae. In Flora de Colombia , vol. 10 (eds Pinto P, Lozano G.), pp. 1–139. Colombia: Instituto de Ciencias Naturales, Museo de Historia Natural, Universidad Nacional de Bogota. [Google Scholar]
- 10.Yockteng R, Coppens d'Eeckenbrugge G, Souza-Chies TT. 2011. Passiflora In Wild crop relatives: genomic and breeding resources tropical and subtropical fruits (ed. Kole C.). Berlin, Germany: Springer. [Google Scholar]
- 11.Jørgensen PM, Muchhala N, MacDougal JM. 2012. Passiflora unipetala, a new bat-pollinated species of Passiflora supersect. Tacsonia (Passifloraceae). Novon 22, 174–179. ( 10.3417/2011095) [DOI] [Google Scholar]
- 12.Yockteng R, Nadot S. 2004. Infrageneric phylogenies: a comparison of chloroplast-expressed glutamine synthetase, cytosol-expressed glutamine synthetase and cpDNA maturase K in Passiflora. Mol. Phylogen. Evol. 31, 397–402. ( 10.1016/S1055-7903(03)00276-8) [DOI] [PubMed] [Google Scholar]
- 13.Escobar LK. 1992. Passiflora brachyantha (Passifloraceae), a new species from the Andes of southern Ecuador. Novon 2, 198–200. ( 10.2307/3391547) [DOI] [Google Scholar]
- 14.Killip EP. 1938. The American species of Passifloraceae. Bot. Ser. Field Mus. Nat. Hist. 19, 613. [Google Scholar]
- 15.Snow DW, Snow BK. 1980. Relationship between hummingbirds and flowers in the Andes of Colombia. Bull. Br. Mus. (Nat. Hist.) Zool. Ser. 38, 105–139. [Google Scholar]
- 16.Lindberg AB, Olesen JM. 2001. The fragility of extreme specialization: Passiflora mixta and its pollinating hummingbird Ensifera ensifera. Trop. Ecol. 17, 323–329. [Google Scholar]
- 17.Sánchez Osés C. 2003. Taxonomy, phylogeny, and biogeography of the Andean hummingbird genera Coeligena Lesson, 1832; Pterophanes Gould, 1849; Ensifera Lesson 1843; and Patagona Gray, 1840 (Aves: Trochiliformes). Dissertation, University of Bonn, Bonn, Germany: See http://hss.ulb.uni-bonn.de/2003/0273/0273.htm [Google Scholar]
- 18.Bell CD, Soltis DE, Soltis PS. 2010. The age and diversification of the angiosperms re-revisited. Am. J. Bot. 97, 1296–1303. ( 10.3732/ajb.0900346) [DOI] [PubMed] [Google Scholar]
- 19.Taberlet P, Gielly G, Pautou G, Bouvet J. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 17, 1105–1109. ( 10.1007/BF00037152) [DOI] [PubMed] [Google Scholar]
- 20.Davis CC, Anderson WR, Donoghue MJ. 2001. Phylogeny of Malpighiaceae: evidence from chloroplast ndhF and trnL-F nucleotide sequences. Am. J. Bot. 88, 1830–1846. ( 10.2307/3558360) [DOI] [PubMed] [Google Scholar]
- 21.White TJ, Burns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications (eds Innis M, Gelfand D, Sninsky J, White TJ.), pp. 315–322. San Diego, CA: Academic Press. [Google Scholar]
- 22.Emshwiller E, Doyle JJ. 1999. Chloroplast-expressed glutamine synthetase (ncpGS): potential utility for phylogenetic studies with an example from Oxalis (Oxalidaceae). Mol. Phyl. Evol. 12, 310–319. ( 10.1006/mpev.1999.0613) [DOI] [PubMed] [Google Scholar]
- 23.Maddison WP, Maddison DR.2011. Mesquite: a modular system for evolutionary analysis. Version 2.75. See http://mesquiteproject.org .
- 24.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. ( 10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 25.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7.5. Mol. Biol. Evol. 29, 1969–1973. ( 10.1093/molbev/mss075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proc. of the 9th Gateway Computing Environments Workshop (GCE), New Orleans, LA, 21 November, pp. 1–8. Piscataway, NJ: IEEE ( 10.1109/GCE.2010.5676129) [DOI]
- 27.Kay KM, Whittall JB, Hodges SA. 2006. A survey of nuclear ribosomal internal transcribed spacer substitution rates across angiosperms: an approximate molecular clock with life history effects. BMC Evol. Biol. 6, 36 ( 10.1186/1471-2148-6-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambaut A, Drummond AJ. 2007. Tracer v1.4 See http://beast.bio.ed.ac.uk/tracer.
- 29.Rambaut A. 2009. FigTree v1.4.0 See http://tree.bio.ed.ac.uk/software/figtree/.
- 30.Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis, v. 2.75 See http://mesquiteproject.org. [Google Scholar]
- 31.Garzione CN, Hoke GD, Libarkin JC, Withers S, MacFadden B, Eiler J, Ghosh P, Mulch A. 2008. Rise of the Andes. Science 320, 1304–1307. ( 10.1126/science.1148615) [DOI] [PubMed] [Google Scholar]
- 32.Colwell RK. 2000. Rensch's rule crosses the line: convergent allometry of sexual dimorphism in hummingbirds and flower mites. Am. Nat. 156, 495–510. ( 10.1086/303406) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data were uploaded as the electronic supplementary material, tables S1 and S2, and all sequences have been submitted to GenBank. GenBank numbers are listed in electronic supplementary material, table S1.