Abstract
Invertebrate lineages tend to originate and become extinct at a higher rate in onshore than in offshore habitats over long temporal durations (more than 10 Myr), but it remains unclear whether this pattern scales down to durations of stages (less than 5 Myr) or even sequences (less than 0.5 Myr). We assess whether onshore–offshore gradients in long-term turnover between the tropical Eocene and the warm-temperate Plio-Pleistocene can be extrapolated from gradients in short-term turnover, using abundances of molluscan species from bulk samples in the northeast Atlantic Province. We find that temporal turnover of metacommunities does not significantly decline with depth over short durations (less than 5 Myr), but significantly declines with depth between the Eocene and Plio-Pleistocene (approx. 50 Myr). This decline is determined by a higher onshore extinction of Eocene genera and families, by a higher onshore variability in abundances of genera and families, and by an onshore expansion of genera and families that were frequent offshore in the Eocene. Onshore–offshore decline in turnover thus emerges only over long temporal durations. We suggest that this emergence is triggered by abrupt and spatially extensive climatic or oceanographic perturbations that occurred between the Eocene and Plio-Pleistocene. Plio-Pleistocene metacommunities show a high proportion of bathymetric generalists, in contrast to Eocene metacommunities. Accordingly, the net cooling and weaker thermal gradients may have allowed offshore specialists to expand into onshore habitats and maintain their presence in offshore habitats.
Keywords: macroevolution, macroecology, stasis, temporal turnover, niche conservatism, Mollusca
1. Introduction
The ecological and evolutionary dynamics of marine metacommunities generate a variety of outcomes at 10 kyr to 10 Myr scales, ranging from temporally stable metacommunities that exhibit stasis [1–5], up to temporally variable species combinations that do not have analogues in present-day ecosystems, especially in the Pleistocene [6–8]. Although differences in temporal scale can contribute to different degrees of stasis [9], some variation observed in marine environments can be also caused by onshore–offshore gradients in demographic and evolutionary rates (or by other types of environmental gradients, [10–13]). Extinction rates of Palaeozoic genera [14,15] and origination rates of Triassic–Jurassic genera [16] tend to be higher onshore (i.e. above storm-wave base), and superfamilies and orders exhibit a higher number of originations onshore than offshore at temporal scales that exceed the duration of geological stages [17,18]. These observations imply that temporal metacommunity turnover declines from onshore to offshore. The turnover along onshore–offshore gradients can be further modulated by latitude, which represents a major correlate of evolutionary rates [19,20], because thermal gradients are steeper and bathymetric ranges smaller in the tropics [21]. Some studies imply that the onshore–offshore decline in turnover reflects a decline in the frequency of major evolutionary innovations but does not necessarily scale down to species-level gradients (frequency of species originations does not peak onshore [22]). Therefore, estimates of temporal turnover measured over intervals varying in the duration at multiple taxonomic levels and partitioned according to depth and latitude are needed to understand temporal dynamics of metacommunities. It is also necessary to identify the components of turnover that generate such onshore–offshore gradients (e.g. variability in population sizes, extinction rates and origination rates [23]).
It can be expected that onshore metacommunities should be more volatile than their offshore counterparts because onshore habitats (i) are environmentally steeper along a latitudinal gradient [24] and (ii) exhibit higher temporal environmental variability (e.g. greater annual temperature range at each latitude [25]). Nonetheless, these two factors can select for larger niche breadth [26–29] and thus seem to generate an increase in the proportion of eurytopic species in onshore, environmentally steeper habitats with higher seasonality and higher frequency of storm disturbances [30,31]. The simple expectation about the higher volatility of onshore metacommunities thus can be reversed by selection for eurytopic strategies that can increase resistance or resilience to temporal environmental fluctuations in variable (onshore) habitats [32–35] or by fluctuating selection that protects genetic variance [36].
Here we assess for the first time to our knowledge, whether the onshore–offshore gradients in temporal turnover can be observed at short time-scales and how they scale up to long time-scales. A key issue is whether an onshore–offshore gradient in long-term turnover simply follows from incremental additions of short-term turnovers of small magnitude or whether it is driven by more abrupt turnovers of higher magnitude. We focus on a time interval that: (i) encompasses climatic and palaeogeographical changes that may be needed to cause significant macroevolutionary changes, and (ii) is sufficiently long so that it naturally separates time-scales of recurrent, orbitally forced oscillations (10–100 thousand years, [26]) from environmental perturbations occurring over million-year time-scales. Environmental conditions generated by such perturbations are effectively outside of evolutionary history of individual species (lifetimes of bivalve species typically exceed 5 Myr [37]) and can have disproportionate consequences for evolutionary trajectories of onshore and offshore lineages. We therefore focus on turnover along onshore–offshore gradients between the tropical Eocene and warm-temperate Plio-Pleistocene in the northeast Atlantic and Mediterranean, using a unique field-based dataset with molluscs, representing a highly diverse clade and a proxy of the evolution of marine ectotherms [20]. This region underwent significant climatic and palaeogeographical changes during the Oligocene, Miocene and Pliocene, resulting in the loss of coral reefs and mangroves [38]. Although we expect that such intervening changes must accentuate long-term turnover relative to within-stage turnover at any depth along an onshore–offshore gradient, it remains unclear whether metacommunities along onshore–offshore gradients respond differently to such changes.
First, we measure temporal turnover in molluscan marine metacommunities along onshore–offshore transects: (i) at short (within-stage) time-scales within the Ypresian (Lower Eocene), within the Lutetian (Middle Eocene), and within the Piacenzian–Gelasian (Plio-Pleistocene) (less than 5 Myr), and (ii) at long (between-epoch) time-scales between the Lower-Middle Eocene and the Plio-Pleistocene (more than 5 Myr), and assess whether a gradient in turnover is related to gradients in variability in abundance, extinction and origination. Second, we evaluate onshore–offshore shifts in the abundance of genera and families that persisted from the Eocene to the Plio-Pleistocene and onshore–offshore gradients in bathymetric breadth.
2. Material and methods
We evaluate onshore–offshore gradients in temporal turnover of molluscan species, genera and families (bivalves, gastropods, scaphopods) within the Eocene tropical successions (30–35° N) and within the Plio-Pleistocene warm-temperate successions (40–43° N) in the northeast Atlantic Province. We measure the turnover between these epochs at the genus level because they do not share any species in common. We also evaluate turnover in families to ensure that the findings do not depend on genus-level classification, which remains in flux. The fossil assemblages are represented by bulk samples collected at bed resolution and sieved with 1 mm mesh size. We assign the assemblages to four depths using sedimentological criteria, including two onshore habitats (peritidal and nearshore) and two offshore habitats close to and below storm-wave base (inner shelf and outer shelf, see the electronic supplementary material). The Eocene is represented by the Ypresian deposits of the Pyrenean Foreland (93 assemblages, [39]) and the Aquitaine Basin (four assemblages), and by new bulk samples collected in the Lutetian deposits of the Paris Basin (nine assemblages). Ypresian assemblages correspond to fully tropical conditions with corals, large foraminifers and mangrove habitats [39]. Lutetian assemblages capture slightly lower temperatures [40] but still reflect tropical conditions with corals and large foraminifers (with mean temperatures not falling below 17–18°C). The Plio-Pleistocene is represented by 35 bulk samples collected in the Piacenzian siliciclastic deposits of Lower Arno basins and 13 bulk samples from the Piacenzian–Gelasian of Piedmont-Padan basins, on two opposite sides of the Northern Apennines. These deposits bracket the mid Pliocene warm interval and cooling after the Piacenzian/Gelasian boundary [41]. The Eocene is represented by the total of 47 410 individuals, 625 species and 149 families. The Plio-Pleistocene is represented by the total of 109 771 individuals, 445 species and 108 families.
We use Bray–Curtis dissimilarity to quantify temporal turnover in species, genus and family abundance [42], using square-root transformed proportional abundance data, and plot dissimilarities on a logit scale because they effectively represent proportions [43]. We measure dissimilarity at each of the four depths: (i) at local scale of individual assemblages, and (ii) at regional scale by pooling a constant number of assemblages per sequence, per stage and per epoch at each depth.
Short-term turnover at each depth is measured as: (i) turnover between composite depositional sequences (approx. 0.15–0.5 Myr sequences in the Eocene and approx. 0.25 Myr sequences in the Plio-Pleistocene, table 1), and (ii) within-stage turnover within the Ypresian, Lutetian and Piacenzian–Gelasian (less than 5 Myr). Long-term turnover is measured as turnover between the two epochs (between Lower-Middle Eocene and Plio-Pleistocene). The first measure of short-term turnover allows testing of whether turnover increases with temporal separation between sequences. The second measure of short-term turnover allows comparison with onshore–offshore gradients in long-term turnover, and this comparison is visualized by non-metric multi-dimensional scaling (NMDS) of assemblages coded by habitat, stage and epoch.
Table 1.
Time-environment table showing the number of assemblage samples at each depth for each depositional sequence, stage and epoch. (The numbers in parentheses show the number of samples with more than 50 specimens (sequences) and with more than 100 specimens (stages) that were used in size-standardized analyses. Four outer shelf samples in the Eocene did not allow the analysis of short-term turnover between sequences. Two samples from Eocene were not assigned to sequences and were thus used in analyses of stages only.)
| epoch (stage) | sequences | age midpoint (Myr) | duration (yr) | peritidal | nearshore | inner shelf | outer shelf |
|---|---|---|---|---|---|---|---|
| Pleistocene (Gelasian) | San Miniato S6 | 2.55 | ∼220 000 | 3 (3) | 2 (0) | 5 (5) | 0 |
| Pliocene (Piacenzian) | Ponte e Elsae S5 | 2.8 | ∼220 000 | 0 | 3 (3) | 6 (6) | 0 |
| Pliocene (Piacenzian) | Pietrafita S4 | 3.25 | ∼150 000 | 4 (4) | 5 (5) | 3 (3) | 4 (4) |
| Pliocene (Piacenzian) | Certaldo S3 | 3.45 | ∼200 000 | 0 | 0 | 1 (0) | 12 (4) |
| epoch (stage) | sequences | age midpoint (Myr) | duration (yr) | peritidal | nearshore | inner shelf | outer shelf |
| Eocene (Lutetian) | Paris Basin A8–A10 | ∼45 | ∼100 000 | 0 | 0 | 4 (4) | 0 |
| Eocene (Lutetian) | Paris Basin A6–A7 | ∼45 | ∼100 000 | 0 | 0 | 4 (4) | 0 |
| Eocene (Ypresian) | Castigaleu H–I | 49.45 | ∼280 000 | 4 (4) | 1 (1) | 1 (0) | 0 |
| Eocene (Ypresian) | Castigaleu F–G | 49.75 | ∼280 000 | 4 (4) | 5 (5) | 1 (1) | 0 |
| Eocene (Ypresian) | Castigaleu C–D | 50.15 | ∼280 000 | 0 | 17 (11) | 2 (0) | 0 |
| Eocene (Ypresian) | Castigaleu A–B | 50.45 | ∼280 000 | 0 | 15 (10) | 1 (1) | 0 |
| Eocene (Ypresian) | Figols—C | 50.9 | ∼500 000 | 17 (16) | 2 (2) | 0 | 0 |
| Eocene (Ypresian) | Figols—B | 51.5 | ∼500 000 | 9 (9) | 3 (3) | 0 | 0 |
| Eocene (Ypresian) | Figols—A | 52.1 | ∼500 000 | 9 (8) | 1 (0) | 0 | 0 |
| epoch | stage | age midpoint (Myr) | duration (yr) | peritidal | nearshore | inner shelf | outer shelf |
| Plio-Pleistocene | Piacenzian–Gelasian | 3.05 | ∼1 100 000 | 7 (7) | 10 (10) | 15 (14) | 16 (11) |
| Eocene | Lutetian (Middle) | 43.51 | ∼3 680 000 | 0 | 0 | 14 (9) | 0 |
| Eocene | Ypresian (Late) | 50.8 | ∼3 400 000 | 43 (24) | 45 (21) | 0 | 4 (4) |
Local-scale short-term turnover within each depth is based on an average of all pairwise dissimilarities between assemblages: (i) from two sequences (each with four assemblages standardized to n = 50 individuals), and (ii) from one stage (with four assemblages standardized to n = 100). Local-scale short-term turnover within each depth is based on an average of all pairwise dissimilarities between assemblages from two epochs (Lower-Middle Eocene and Plio-Pleistocene) (each with four assemblages standardized to n = 100). Regional-scale short-term and long-term turnover within each depth is based on a single dissimilarity: four local assemblages are randomly drawn from each sequence, stage and epoch, resampled (without replacement) to the same sample size (n) per assemblage and pooled into one regional-scale assemblage per sequence (n = 200), per stage (n = 400) and per epoch (n = 400). We test the significance of onshore–offshore difference in turnover with pairwise between-habitat tests of homogeneity in multivariate dispersions (HMD, [44]). Multivariate dispersion corresponds to mean Bray–Curtis dissimilarity between assemblages (from one stage in within-stage turnover and from two epochs in between-epoch turnover) and their centroid. HMD tests whether multivariate dispersions are larger onshore than offshore within stages and between epochs. To assess the contribution of species-level processes to temporal turnover in genus and family abundance, we further compute turnover where Bray–Curtis dissimilarity is based: (i) on per-genus and per-family median species (proportional) abundance, and (ii) on per-genus and per-family species richness. At regional scales, we also use Spearman's rank correlation to test the relation in species, genus and family rank abundances between sequences and between epochs, and the Mantel test to test whether Bray–Curtis dissimilarity increases and Spearman's rank correlation decreases with increasing temporal separation between sequences at regional scales, separately within onshore and offshore habitats.
In analyses of dissimilarities, rank correlations and the Mantel test, we double-standardize abundance data by random sampling of the same number of individuals per assemblage and by random sampling of the same number of assemblages per depth and per time interval. Standardizations are repeated 1000 times, generating means and 2.5th and 97.5th percentiles on summary statistics. The subsets of assemblages and individuals drawn in consecutive standardizations are not mutually exclusive. To assess significance of tests that are computed with standardized data, we thus compute a combined p-value with averaging approach rather than using the product of p-values as in Fisher's approach [45].
We quantify relative utilization of onshore habitats by genera and families as the proportion of a taxon's individuals found onshore (relative to the taxon's total abundance) to assess temporal changes in utilization of onshore–offshore gradients by genera and families that persist from the Eocene to Plio-Pleistocene. We measure a bathymetric breadth of individual species with Hurlbert's measure [46], which weights bathymetric range by abundance and by frequency of samples at each depth.
3. Results
(a). Onshore–offshore gradients in temporal turnover within stages
Spearman's rank correlations in proportional abundances between sequences are moderately high in the Eocene ( [species] = 0.36,
[species] = 0.36,  [genera] = 0.4,
[genera] = 0.4, 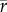 [families] = 0.55) and rather low in the Plio-Pleistocene (
[families] = 0.55) and rather low in the Plio-Pleistocene (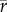 [species] = 0.17,
[species] = 0.17, 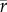 [genera] = 0.23,
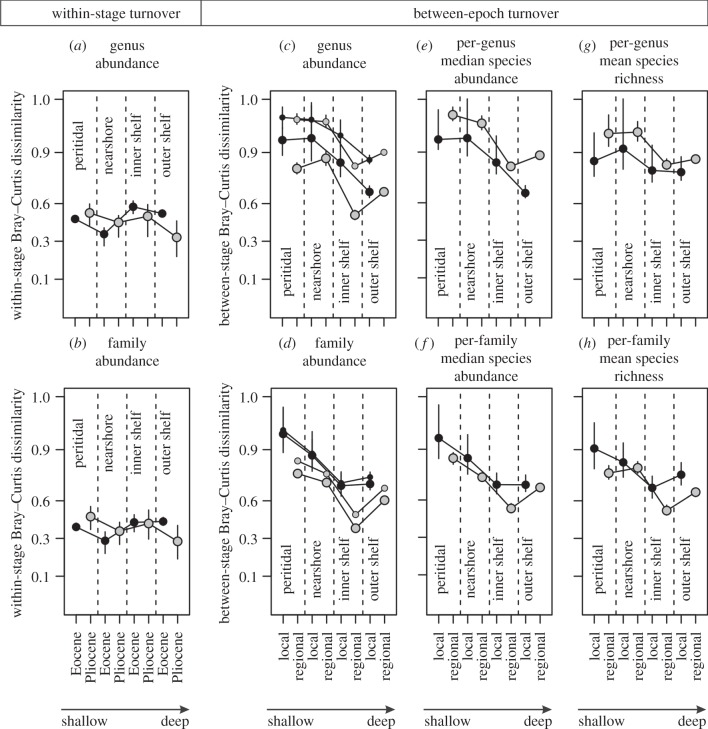
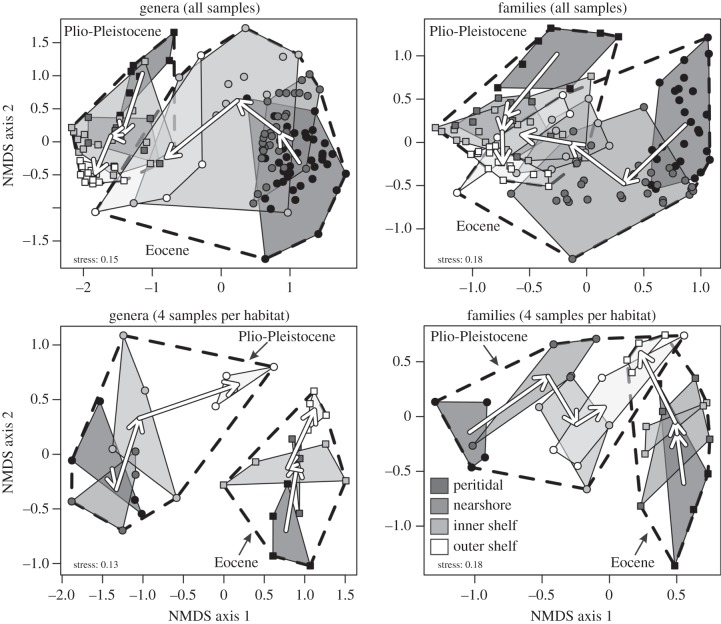
[genera] = 0.23, 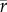 [families] = 0.34) (electronic supplementary material, table S1). Significant correlations between sequences occur in most Eocene comparisons (20–23 out of 29 comparisons at Bonferroni-corrected α = 0.0017) and are less frequent in the Plio-Pleistocene (3–5 out of 16 comparisons at Bonferroni-corrected α = 0.003). Spearman's rank correlations do not decline and Bray–Curtis dissimilarities do not increase with increasing separation between sequences (electronic supplementary material, figure S1 and table S2). Among-sequence dissimilarities are reduced at all depths when local assemblages at each depth are pooled (figure 1a,b). Multivariate dispersions of individual habitats along onshore–offshore gradients in a double-standardized NMDS do not visibly decline within the Eocene or within the Plio-Pleistocene (figure 2), and pairwise onshore–offshore differences in within-stage turnover are close to zero and never significantly positive (table 2 and figure 1a,b). The short-term turnover in abundance at genus and family level thus does not decline with depth within the Eocene or Plio-Pleistocene.
[families] = 0.34) (electronic supplementary material, table S1). Significant correlations between sequences occur in most Eocene comparisons (20–23 out of 29 comparisons at Bonferroni-corrected α = 0.0017) and are less frequent in the Plio-Pleistocene (3–5 out of 16 comparisons at Bonferroni-corrected α = 0.003). Spearman's rank correlations do not decline and Bray–Curtis dissimilarities do not increase with increasing separation between sequences (electronic supplementary material, figure S1 and table S2). Among-sequence dissimilarities are reduced at all depths when local assemblages at each depth are pooled (figure 1a,b). Multivariate dispersions of individual habitats along onshore–offshore gradients in a double-standardized NMDS do not visibly decline within the Eocene or within the Plio-Pleistocene (figure 2), and pairwise onshore–offshore differences in within-stage turnover are close to zero and never significantly positive (table 2 and figure 1a,b). The short-term turnover in abundance at genus and family level thus does not decline with depth within the Eocene or Plio-Pleistocene.
Figure 1.
Short-term turnover within stages (within Ypresian, Lutetian and Piacenzian–Gelasian) does not consistently vary with depth (a,b). Long-term turnover (between the Eocene and Plio-Pleistocene) markedly declines between nearshore and inner shelf at both local and regional scales (c,d), using all genera and families (small symbols) and persisting genera and families (large symbols). This decline is related to a decrease in turnover in per-lineage median species abundance (e,f) and in per-lineage species richness (g,h). Bray–Curtis dissimilarities are based on square-root transformed proportional abundances. Error bars represent 2.5th and 97.5th percentiles derived from 1000 standardizations. Dissimilarities are plotted on a logit scale.
Figure 2.
NMDS showing that Eocene and Plio-Pleistocene offshore habitats (inner shelf and outer shelf are coded light grey and white, respectively) are more similar to each other in abundance of persisting genera and families than Eocene and Plio-Pleistocene onshore habitats (peritidal and nearshore coded by dark grey shading), using Bray–Curtis dissimilarity based on square-root transformed proportional abundances. Ordinations in the upper plots show all assemblages, ordinations in the lower plots show one ordination standardized to four assemblages per habitat and per epoch and to n = 100 individuals per assemblage. Arrows visualize Eocene and Plio-Pleistocene onshore–offshore gradients: they start in centroids of shallower habitats and terminate in centroids of deeper habitats.
Table 2.
Short-term turnover within Eocene and within Plio-Pleistocene in genus and family abundances is not significantly higher onshore than offshore, using the test of HMD. (The summary statistics and the combined p-value between onshore and offshore and the 2.5 and 97.5th percentiles are based on 1000 runs standardized to four assemblages per habitat and n = 100 individuals per assemblage.)
| habitat comparison | level | mean onshore–offshore difference in dispersion | 2.5th percentile | 97.5th percentile | combined p-value | level | mean onshore–offshore difference in dispersion | 2.5th percentile | 97.5th percentile | combined p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Eocene peritidal versus nearshore | genera | 0.087 | 0.033 | 0.145 | 0.217 | families | 0.072 | 0.012 | 0.138 | 0.307 |
| Eocene peritidal versus inner shelf | genera | −0.090 | −0.138 | −0.036 | 1 | families | −0.039 | −0.100 | 0.025 | 0.941 |
| Eocene peritidal versus outer shelf | genera | −0.045 | −0.080 | −0.010 | 0.997 | families | −0.044 | −0.081 | −0.006 | 0.995 |
| Eocene nearshore versus inner shelf | genera | −0.177 | −0.242 | −0.110 | 1 | families | −0.111 | −0.193 | −0.032 | 0.999 |
| Eocene nearshore versus outer shelf | genera | −0.132 | −0.191 | −0.078 | 1 | families | −0.117 | −0.182 | −0.058 | 1 |
| Eocene inner shelf versus outer shelf | genera | 0.045 | −0.003 | 0.093 | 0.246 | families | −0.005 | −0.066 | 0.055 | 0.729 |
| Plio-Pl. peritidal versus nearshore | genera | 0.054 | −0.049 | 0.155 | 0.325 | families | 0.082 | −0.033 | 0.185 | 0.231 |
| Plio-Pl. peritidal versus inner shelf | genera | 0.026 | −0.106 | 0.173 | 0.545 | families | 0.044 | −0.093 | 0.170 | 0.425 |
| Plio-Pl. peritidal versus outer shelf | genera | 0.143 | 0.014 | 0.266 | 0.131 | families | 0.138 | 0.007 | 0.265 | 0.141 |
| Plio-Pl. nearshore versus inner shelf | genera | −0.028 | −0.148 | 0.120 | 0.784 | families | −0.038 | −0.158 | 0.079 | 0.827 |
| Plio-Pl. nearshore versus outer shelf | genera | 0.089 | −0.036 | 0.207 | 0.260 | families | 0.055 | −0.060 | 0.163 | 0.360 |
| Plio-Pl. inner shelf versus outer shelf | genera | 0.116 | −0.056 | 0.264 | 0.221 | families | 0.093 | −0.046 | 0.229 | 0.252 |
(b). Onshore–offshore gradients in temporal turnover between epochs
The turnover between the Eocene and Plio-Pleistocene in abundance of genera and families is higher onshore than in offshore. The differences between peritidal and nearshore, and between inner shelf and outer shelf, are weak, but other comparisons show significantly higher long-term turnover in onshore habitats. This demonstrates that the major decline in turnover occurs in inner shelf close to the storm-wave base (table 3 and figure 1c,d; electronic supplementary material, figures S2–S4). The decline in turnover applies to abundances of genera and families that persist from the Eocene to Plio-Pleistocene and is thus not driven solely by decline in extinction and origination but also by higher offshore conservatism in abundance. NMDS visualizes this decline in temporal turnover in genus and family abundance between the Eocene and Plio-Pleistocene, showing a marked segregation of onshore communities from Eocene and Plio-Pleistocene, whereas offshore communities from these two time intervals are close to each other (figure 2). The onshore–offshore decline in temporal turnover in abundance scales down to species-level abundance patterns, with a smaller offshore turnover: (i) in per-genus and per-family median species abundance, and (ii) in per-genus and per-family richness (figure 1e–h). Spearman's rank correlation between Eocene and Plio-Pleistocene genus and family abundances within a single habitat is significantly positive in inner shelf (r [genera] = 0.31, p < 0.011, r [families] = 0.46, p < 0.001), and becomes insignificant in peritidal (r [genera] = 0.18, p = 0.4, r [families] = 0.14, p = 0.38) and nearshore (r [genera] = 0.03, p = 0.82, r [families] = 0.16, p = 0.22), but also in outer shelf habitats (r [genera] = −0.16, p = 0.27, r [families] = −0.14, p = 0.28).
Table 3.
Turnover between Eocene and Plio-Pleistocene in genus and family abundances decreases towards offshore environments, using all taxa and persisting taxa. (The test of HMD shows that although turnover is not significantly higher in two pairs of closely spaced habitats (peritidal compared with nearshore, and inner shelf compared with outer shelf), onshore turnover is significantly higher in other comparisons with offshore habitats. The mean difference in multivariate dispersion between onshore and offshore environments and the 2.5 and 97.5th percentiles are based on 1000 runs standardized to four assemblages per habitat and per epoch, and n = 100 individuals per assemblage.)
| habitat comparison | level | mean onshore–offshore difference in dispersion | 2.5th percentile | 97.5th percentile | combined p-value | level | mean onshore–offshore difference in dispersion | 2.5th percentile | 97.5th percentile | combined p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| peritidal versus nearshore | genera | 0.007 | −0.008 | 0.027 | 0.962 | families | 0.045 | 0.004 | 0.090 | 0.267 |
| peritidal versus inner shelf | genera | 0.032 | 0.008 | 0.064 | 0.006 | families | 0.198 | 0.133 | 0.268 | 0.003 |
| peritidal versus outer shelf | genera | 0.189 | 0.158 | 0.220 | 0.001 | families | 0.242 | 0.207 | 0.275 | 0.001 |
| nearshore versus inner shelf | genera | 0.025 | −0.006 | 0.061 | 0.006 | families | 0.153 | 0.079 | 0.232 | 0.004 |
| nearshore versus outer shelf | genera | 0.183 | 0.147 | 0.218 | 0.001 | families | 0.197 | 0.139 | 0.243 | 0.002 |
| inner shelf versus outer shelf | genera | 0.157 | 0.114 | 0.197 | 0.039 | families | 0.044 | −0.031 | 0.115 | 0.873 |
| peritidal versus shore face | pers. genera | 0.013 | −0.019 | 0.058 | 0.994 | pers. families | 0.047 | −0.001 | 0.098 | 0.436 |
| peritidal versus inner shelf | pers. genera | 0.077 | 0.021 | 0.143 | 0.058 | pers. families | 0.211 | 0.139 | 0.286 | 0.005 |
| peritidal versus outer shelf | pers. genera | 0.387 | 0.337 | 0.438 | 0.001 | pers. families | 0.270 | 0.216 | 0.326 | 0.002 |
| nearshore versus inner shelf | pers. genera | 0.065 | −0.006 | 0.136 | 0.013 | pers. families | 0.164 | 0.079 | 0.248 | 0.008 |
| nearshore versus outer shelf | pers. genera | 0.375 | 0.310 | 0.436 | 0.001 | pers. families | 0.224 | 0.151 | 0.290 | 0.002 |
| inner shelf versus outer shelf | pers. genera | 0.310 | 0.229 | 0.386 | 0.019 | pers. families | 0.059 | −0.031 | 0.153 | 0.724 |
A total of 82–88% of genera (42–52% of families) inhabiting peritidal and nearshore habitats in the Eocene disappear in the Plio-Pleistocene of the northeast Atlantic Province. In comparison, 72–80% of genera (33–36% of families) disappear from inner shelf and outer shelf. Although the proportions of persisting genera and families are not very high, these taxa contribute 49% and 90% of individuals, respectively, to the total abundance when both epochs are pooled. The percentages appearing in the Plio-Pleistocene are similar between onshore (67–68% of genera and 16–25% of families) and offshore (64–70% and 19–26%, respectively).
Ampullinidae and Batillariidae primarily contribute to high onshore turnover. They are dominant in onshore habitats during the Eocene but are absent in the Plio-Pleistocene of the northeast Atlantic and Mediterranean. Ampullinidae are presently represented by a single species occurring in the Philippines [47], and Batillariidae disappeared from the northeast Atlantic Province at the end of the Miocene [48]. In onshore habitats, Potamididae are strongly reduced, whereas Rissoidae, Pyramidellidae, Arcidae, Semelidae, Tellinidae, Cardiidae and Veneridae increase in abundance. Rissoidae radiate during the Miocene and are presently the most species-rich in the warm-temperate Mediterranean and eastern Atlantic [49]. Although some Eocene families with tropical affinities declined in abundance (Turridae, Conidae, Costellariidae, Cylichnidae) or went regionally extinct in offshore habitats (e.g. Marginellidae, which presently extend to the southernmost parts of the Mediterranean and to the Ibero-Moroccan Gulf, [50]), most families achieved similar abundance in the Eocene and Plio-Pleistocene in offshore habitats.
(c). Onshore–offshore shifts in abundance and bathymetric breadth between epochs
Although species richness increases towards inner shelf at both time intervals, the onshore–offshore gradient in bathymetric breadth changes markedly between the Eocene and Plio-Pleistocene (figure 3). The Eocene species show a significantly smaller Hurlbert's index (mean = 0.12, 95% CI = 0.11–0.13) than Plio-Pleistocene species (mean = 0.23, 95% CI = 0.21–0.24). This change is associated with a higher proportion of species occurring onshore and offshore (generalists) in the Plio-Pleistocene: 27 species (4%) belong to generalists in the Eocene, whereas 163 species (37%) belong to generalists in the Plio-Pleistocene. In contrast to the Eocene, Hurlbert's index does not decline with depth in the Plio-Pleistocene because Plio-Pleistocene offshore species show a significantly higher index than Eocene offshore species (figure 3). The differences in onshore utilization by genera and families between Plio-Pleistocene and Eocene are significantly positive: persisting genera and families are more frequent onshore in the Plio-Pleistocene than onshore in the Eocene (figure 4). These relative onshore expansions of genera and families are caused by an increase in abundance of their constituent species as well as by an increase in their species richness (figure 4).
Figure 3.
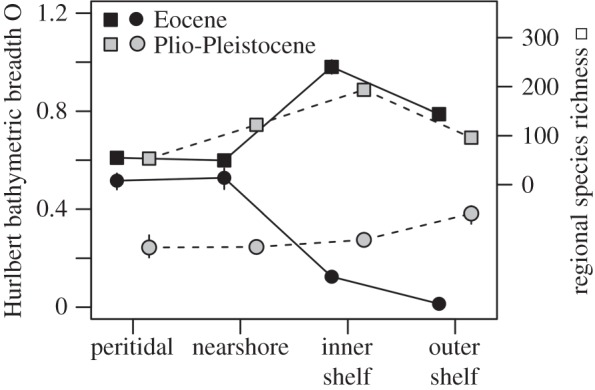
Hurlbert bathymetric breadth of Eocene species (black circles) on average declines towards offshore, whereas Hurlbert breadth of Plio-Pleistocene species (grey circles) changes weakly. Hurlbert breadth of offshore species is significantly higher in the Plio-Pleistocene than in the Eocene. Species richness (rarefied to 2477 individuals per habitat) increases towards inner shelf at both time intervals and this increase is less steep during the Plio-Pleistocene (grey squares) than during the Eocene (black squares).
Figure 4.
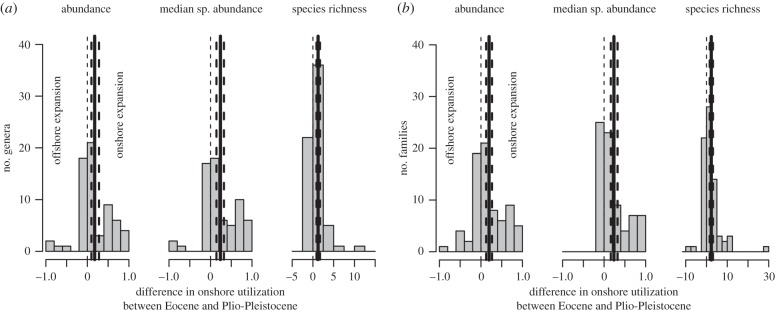
Frequency distributions of Plio-Pleistocene–Eocene differences in onshore utilization of (a) genera and (b) families that persist from Eocene to Plio-Pleistocene show that genera and families abundant in offshore habitats during Eocene expanded to onshore habitats in Plio-Pleistocene. The onshore increase in abundance also applies to an increase in per-genus and per-family median species abundance and to an increase in per-genus and per-family species richness. Thin dashed lines separate relative onshore expansion from relative offshore expansion. Thick solid and dashed lines correspond to means and bootstrapped 95% CIs. A value of zero means no difference between Eocene and Plio-Pleistocene, and positive values mean that persisting genera and families are more abundant and more species-rich onshore in the Plio-Pleistocene than in the Eocene.
4. Discussion
(a). Onshore–offshore gradients at short time-scales
The moderately high between-sequence rank correlations in the Eocene and the lack of relation with time imply that the short-term turnover is coupled with some degree of recurrence in abundance patterns at individual depths, as observed in many other time series with benthic assemblages in the fossil record [51,52]. The smaller dissimilarity at regional scales at each depth implies that this recurrence is related to processes operating at larger spatial scales that can reduce extinction risk and slow down temporal change in metacommunity composition. Such processes include those that allow broad geographical range size via high dispersal or high effective habitat area [53–56]. Geographical range size is a key predictor of extinction [57], and its role in reducing extinction risk is generally much stronger than the role of eurytopy [58]. The onshore–offshore decline in the steepness of latitudinal temperature gradients may contribute to the lack of onshore–offshore gradients in geographical range size (and thus in extinction rate at short time-scales) because the latitudinal range of shallow habitats (0–50 m) along the eastern ocean margins [59] remains on average within a 5°C range for approximately 1500 km, whereas the latitudinal range of deeper habitats (100–200 m) remains on average within a 5°C range for approximately 2500–3000 km (electronic supplementary material, figure S7), and spatial variation in seawater temperature drives the large-scale distribution of ectotherms [60,61]. Therefore, marine ectotherms inhabiting onshore, temporally variable habitats can achieve broad geographical ranges because they are eurytopic [30], whereas offshore, temperature-specialized species can attain broad geographical ranges because they face milder latitudinal climatic gradients [62].
(b). Onshore extinction at long time-scales
The emergence of an onshore–offshore decline in temporal turnover over longer time-scales, together with the lack of relationship between the short-term turnover and temporal separation between sequences over Myr scales, implies that the onshore increase in turnover between the Eocene and the Plio-Pleistocene is not a simple extrapolation of small, incremental changes that can be observed from sequence to sequence. Rather, the decline in turnover depends on climatic or oceanographic perturbations that occurred between the Eocene and Plio-Pleistocene and affected onshore lineages more strongly than offshore lineages, as revealed: (i) by a smaller offshore proportion of extinct genera and families, (ii) by a smaller variability in offshore abundance of persisting genera and families, and (iii) by a smaller variability in offshore abundance of their constituent species. Therefore, this decline is not merely a function of declining extinction rates but also a function of declining variability in population abundances, leading to a higher ecological conservatism of offshore lineages. Climatic changes may contribute to higher long-term onshore volatility because: (i) Cenozoic environments show larger temperature fluctuations in the superficial waters than in the deep-shelf not only at short but also at long time-scales [24], and (ii) the Cenozoic record shows a higher long-term persistence of milder latitudinal gradients in mean temperature at 200 m than at the surface during the Cenozoic [63]. Multiple climatic reversals between Ypresian–Lutetian and Plio-Pleistocene, including Lutetian cooling, Mid-Eocene climatic optimum, glaciation at the Eocene/Oligocene boundary and mid-Miocene climatic optimum [64], could thus contribute to the higher extinction and population variability in the onshore habitats. Temporal fluctuations in the location and extent of oxygen minimum zones can generate instability that is larger offshore than onshore [65], but the Eocene and Plio-Pleistocene assemblages of the northeast Atlantic and Mediterranean do not occur in dysoxic habitats. The Pliocene molluscan communities in the Mediterranean were assembled primarily from the tropical eastern Atlantic province along western Africa (that was formed after the closure of the Indo-Pacific seaway during the Early-Middle Miocene), but offshore and onshore lineages should be not affected differently by such oceanographic changes. Regardless of the actual climatic or oceanographic causes, we suggest that onshore lineages face abrupt and high-amplitude regional-scale perturbations over longer durations that are not experienced over shorter durations [66]. Present-day offshore and bathyal habitats apparently support deep-water lineages since the Mesozoic, also suggesting that offshore lineages can be less affected by such perturbations and thus can conserve their niches more strongly than onshore lineages [67,68].
(c). Onshore expansion at long time-scales
Plio-Pleistocene genera and families that were frequent offshore during the Eocene expanded onshore during the Plio-Pleistocene and also maintained their presence offshore. An increase in the proportion of bathymetric generalists and the flattening of the onshore–offshore gradient in bathymetric breadth imply that this long-term onshore expansion observed in our study can be modulated by climatic changes that led to the net shift from tropical Eocene to warm-temperate Plio-Pleistocene conditions. Poleward latitudinal shifts are typically associated with the emergence of colder deep-shelf waters and with declining steepness of thermal bathymetric gradients at higher latitudes. Such changes in temperature and in the structure of the thermal bathymetric gradient produce a latitudinal increase in the bathymetric range size of marine ectotherms and their shallow-water emergence at higher latitudes [69,70].
Supplementary Material
Acknowledgements
We thank P. Novack-Gottshall and T. D. Olszewski for critical reviews, M. Stachowitsch for comments and S. Danise for help with sample processing. This work benefited from visits to Paris and Vienna museum collections under the Synthesys program.
Data accessibility
Source data are available at dx.doi.org/10.5061/dryad.943j7.
Funding statement
This work was supported by the Austrian Science Fund (FWF) projects P19013-Bio, the Slovak Research and Development Agency (APVV 0644–10), the Slovak Scientific Grant Agency (VEGA 0068–10) and the NSF (DEB-0919451).
References
- 1.Pandolfi JM. 1996. Limited membership in Pleistocene reef coral assemblages from the Huon Peninsula, Papua New Guinea: constancy during global change. Paleobiology 22, 152–176. [Google Scholar]
- 2.Ivany LC, Brett CE, Wall HLB, Wall PD, Handley JC. 2009. Relative taxonomic and ecologic stability in Devonian marine faunas of New York State: a test of coordinated stasis. Paleobiology 35, 499–524. ( 10.1666/0094-8373-35.4.499) [DOI] [Google Scholar]
- 3.Holland SM, Zaffos A. 2011. Niche conservatism along an onshore–offshore gradient. Paleobiology 37, 270–286. ( 10.1666/10032.1) [DOI] [Google Scholar]
- 4.Buzas M, Hayek LC, Culver SJ, Hayward BW, Osterman LE. 2014. Ecological and evolutionary consequences of benthic community stasis in the very deep sea (<1500m). Paleobiology 40, 102–112. ( 10.1666/13010) [DOI] [Google Scholar]
- 5.Zuschin M, Harzhauser M, Hengst B, Mandic O, Roetzel R. 2014. Long-term ecosystem stability in a Lower Miocene estuarine succession. Geology 42, 1–4. ( 10.1130/G34761.1) [DOI] [Google Scholar]
- 6.Roy K, Jablonski D, Valentine JW. 1995. Thermally anomalous assemblages revisited: patterns in the extraprovincial latitudinal range shifts of Pleistocene marine mollusks. Geology 23, 1071–1074. () [DOI] [Google Scholar]
- 7.Jackson ST, Overpeck JT. 2000. Responses of plant populations and communities to environmental changes of the Late Quaternary. Paleobiology 26(Suppl. 4), 194–220. ( 10.1666/0094-8373(2000)26[194:ROPPAC]2.0.CO;2) [DOI] [Google Scholar]
- 8.Yasuhara M, Hunt G, Cronin TM, Okahashi H. 2009. Temporal latitudinal-gradient dynamics and tropical instability of deep-sea species diversity. Proc. Natl Acad. Sci. USA 106, 21 717–21 720. ( 10.1073/pnas.0910935106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomašových A, Kidwell SM. 2010. The effects of temporal resolution on species turnover and on testing metacommunity models. Am. Nat. 175, 587–606. ( 10.1086/651661) [DOI] [PubMed] [Google Scholar]
- 10.Roy K. 2001. Analyzing temporal trends in regional diversity: a biogeographic perspective. Paleobiology 24, 631–645. () [DOI] [Google Scholar]
- 11.Roy K, Goldberg EE. 2007. Origination, extinction, and dispersal: integrative models for understanding present-day diversity gradients. Am. Nat. 170, S71–S85. ( 10.1086/519403) [DOI] [PubMed] [Google Scholar]
- 12.Holland SM, Patzkowsky M. 2007. Gradient ecology of a biotic invasion: biofacies of the type Cincinnatian series (Upper Ordovician), Cincinnati, Ohio region, USA. Palaios 22, 392–407. ( 10.2110/palo.2006.p06-066r) [DOI] [Google Scholar]
- 13.Miller AI, Foote M. 2009. Epicontinental seas versus open-ocean settings: the kinetics of mass extinction and origination. Science 326, 1106–1109. ( 10.1126/science.1180061) [DOI] [PubMed] [Google Scholar]
- 14.Sepkoski JJ., Jr 1987. Environmental trends in extinction during the Phanerozoic. Science 235, 64–66. ( 10.1126/science.11539724) [DOI] [PubMed] [Google Scholar]
- 15.Sepkoski JJ., Jr 1991. A model on onshore-offshore change in faunal diversity. Paleobiology 17, 58–77. [DOI] [PubMed] [Google Scholar]
- 16.Kiessling W, Aberhan M. 2007. Environmental determinants of marine benthic biodiversity dynamics through Triassic-Jurassic times. Paleobiology 33, 414–434. ( 10.1666/06069.1) [DOI] [Google Scholar]
- 17.Bottjer DJ, Jablonski D. 1988. Paleoenvironmental patterns in the evolution of post-Paleozoic benthic marine invertebrates. Palaios 3, 540–560. ( 10.2307/3514444) [DOI] [Google Scholar]
- 18.Jablonski D, Lidgard S, Taylor PS. 1997. Comparative ecology of bryozoan radiations: origin of novelties in cyclostomes and cheilostomes. Palaios 12, 505–523. ( 10.2307/3515408) [DOI] [Google Scholar]
- 19.Kiessling W, Simpson C, Foote M. 2010. Reefs as cradles of evolution and sources of biodiversity in the Phanerozoic. Science 327, 196–198. ( 10.1126/science.1182241) [DOI] [PubMed] [Google Scholar]
- 20.Jablonski D, Belanger CL, Berke SK, Huang S, Krug AZ, Roy K, Tomasovych A, Valentine JW. 2013. Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient. Proc. Natl Acad. Sci. USA 110, 10 487–10 494. ( 10.1073/pnas.1308997110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith KF, Gaines SD. 2003. Rapoport's bathymetric rule and the latitudinal species diversity gradient for northeast Pacific fishes and northwest Atlantic gastropods: evidence against a causal link. J. Biogeogr. 30, 1153–1159. ( 10.1046/j.1365-2699.2003.00898.x) [DOI] [Google Scholar]
- 22.Jablonski D. 2005. Evolutionary innovations in the fossil record: the intersection of ecology, development, and macroevolution. J. Exp. Zool. 304B, 504–519. ( 10.1002/jez.b.21075) [DOI] [PubMed] [Google Scholar]
- 23.Stanley SM, Wetmore KL, Kennett JP. 1988. Macroevolutionary differences between the two major clades of Neogene planktonic Foraminifera. Paleobiology 14, 235–249. [Google Scholar]
- 24.Nikolaev SD, Oskina NS, Blyum NS, Bubenshchikova NV. 1998. Neogene-Quaternary variations of the ‘Pole-Equator’ temperature gradient of the surface oceanic waters in the north Atlantic and north Pacific. Glob. Planet. Change 18, 85–111. ( 10.1016/S0921-8181(98)00009-5) [DOI] [Google Scholar]
- 25.Stevens GC. 1996. Extending Rapoport's rule to Pacific marine fishes. J. Biogeogr. 23, 149–154. ( 10.1046/j.1365-2699.1996.00977.x) [DOI] [Google Scholar]
- 26.Dynesius M, Jansson R. 2000. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA 97, 9115–9120. ( 10.1073/pnas.97.16.9115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt RD, Barfield M, Gomulkiewicz R. 2004. Temporal variation can facilitate niche evolution in harsh sink environments. Am. Nat. 164, 187–200. ( 10.1086/422343) [DOI] [PubMed] [Google Scholar]
- 28.Rangel TFLVB, Diniz-Filho AF. 2005. An evolutionary tolerance model explanining spatial patterns in species richness under environmental gradients and geometric constraints. Ecography 25, 253–263. ( 10.1111/j.0906-7590.2005.04038.x) [DOI] [Google Scholar]
- 29.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 30.Jackson JBC. 1974. Biogeographic consequences of eurytopy and stenotopy among marine bivalves and their evolutionary significance. Am. Nat. 108, 541–560. ( 10.1086/282933) [DOI] [Google Scholar]
- 31.Jablonski D, Valentine JW. 1981. Onshore-offshore gradients in Recent eastern Pacific shelf faunas and their paleobiogeographic significance. In Evolution today (eds Scudder GCE, Reveal JL.), pp. 441–453. Pittsburg, PA: Carnegie-Mellon University Hunt Institute of Botany Documents. [Google Scholar]
- 32.Allmon WD. 2001. Nutrients, temperature, disturbance, and evolution: a model for the late Cenozoic marine record of the western Atlantic. Palaeogeogr. Palaeoclim. Palaeoecol. 166, 9–26. ( 10.1016/S0031-0182(00)00199-1) [DOI] [Google Scholar]
- 33.Harley CDG, Smith KF, Moore VL. 2003. Environmental variability and biogeography: the relationship between bathymetric distribution and geographical range size in marine algae and gastropods. Glob. Ecol. Biogeogr. 12, 499–506. ( 10.1046/j.1466-822X.2003.00062.x) [DOI] [Google Scholar]
- 34.Escarguel G, Brayard A, Bucher H. 2008. Evolutionary rates do not drive latitudinal diversity gradients. J. Zool. Syst. Evol. Res. 46, 82–86. ( 10.1111/j.1439-0469.2007.00443.x) [DOI] [Google Scholar]
- 35.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell G. 2010. Fluctuating selection: the perpetual renewal of adaptation in variable environments. Phil. Trans. R. Soc. B 365, 87–97. ( 10.1098/rstb.2009.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foote M, Raup D. 1996. Fossil preservation and the stratigraphic ranges of taxa. Paleobiology 22, 121–140. [DOI] [PubMed] [Google Scholar]
- 38.Raffi S, Stanley SM, Marasti R. 1985. Biogeographic patterns and Plio-Pleistocene extinction of Bivalvia in the Mediterranean and southern North Sea. Paleobiology 11, 368–388. [Google Scholar]
- 39.Dominici S, Kowalke T. 2007. Depositional dynamics and the record of ecosystem stability: Early Eocene faunal gradients in the Pyrenean Foreland, Spain. Palaios 22, 268–284. ( 10.2110/palo.2005.p05-022r) [DOI] [Google Scholar]
- 40.Huyghe D, Merle D, Lartaud F, Cheype F, Emmanuel L. 2012. Middle Lutetian climate in the Paris Basin: implications for a marine hotspot of paleobiodiversity. Facies 58, 587–604. ( 10.1007/s10347-012-0307-3) [DOI] [Google Scholar]
- 41.Draut AE, Raymo ME, McManus JF, Oppo DW. 2003. Climate stability during the Pliocene warm period. Paleoceanography 18, 1078 ( 10.1029/2003PA000889) [DOI] [Google Scholar]
- 42.Blois JL, Williams JW, Fitzpatrick MC, Jackson ST, Ferrier S. 2013. Space can substitute for time in predicting climate-change effects on biodiversity. Proc. Natl Acad. Sci. USA 110, 9374–9379. ( 10.1073/pnas.1220228110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellin C, Bradshaw CJA, Fordham DA, Caley MJ. 2014. Strong but opposing β diversity—stability relationships in coral reef fish communities. Proc. R. Soc. B 281, 20131993 ( 10.1098/rspb.2013.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson MJ, Ellingsen KE, McArdle BH. 2006. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 9, 683–693. ( 10.1111/j.1461-0248.2006.00926.x) [DOI] [PubMed] [Google Scholar]
- 45.Vovk V. 2012. Combining p-values via averaging. See http://arxiv.org/abs/1212.4966.
- 46.Hurlbert SH. 1978. The measurement of niche overlap and some relatives. Ecology 59, 67–77. ( 10.2307/1936632) [DOI] [Google Scholar]
- 47.Caze B, Merle D, Le Meur M, Pacaud J-M, Ledon D, Saint Martin J-P. 2011. Taxonomic implications of the residual colour patterns of ampullinid gastropods and their contribution to the discrimination from naticids. Acta Palaeontol. Pol. 56, 329–347. ( 10.4202/app.2009.0084) [DOI] [Google Scholar]
- 48.Ozawa T, Kohler F, Reid DG, Glaubrecht M. 2009. Tethyan relicts on continental coastlines of the northwestern Pacific Ocean and Australasia: molecular phylogeny and fossil record of batillariid gastropods (Caenogastropoda, Cerithioidea). Zool. Scr. 38, 503–525. ( 10.1111/j.1463-6409.2009.00390.x) [DOI] [Google Scholar]
- 49.Kowalke T, Harzhauser M. 2004. Early ontogeny and palaeoecology of the Mid-Miocene rissoid gastropods of the Central Paratethys. Acta Palaeontol. Pol. 49, 111–134. [Google Scholar]
- 50.Silva CMD, Landau B, La Perna R. 2011. Biogeography of Iberian Atlantic Neogene marginelliform gastropods (Marginellidae, Cystiscidae): global change and transatlantic colonization. J. Paleontol. 85, 1052–1066. ( 10.1666/11-104.1) [DOI] [Google Scholar]
- 51.Olszewski TD, Patzkowsky ME. 2001. Measuring recurrence of marine biotic gradients: a case study from the Pennsylvanian-Permian Midcontinent. Palaios 16, 440–460. () [DOI] [Google Scholar]
- 52.Pandolfi JM, Jackson JBC. 2006. Ecological persistence interrupted in Caribbean coral reefs. Ecol. Lett. 9, 818–826. ( 10.1111/j.1461-0248.2006.00933.x) [DOI] [PubMed] [Google Scholar]
- 53.Loreau M, Mouquet N, Gonzalez A. 2003. Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl Acad. Sci. USA 100, 12 765–12 770. ( 10.1073/pnas.2235465100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkov I, Banavar JR, Hubbell SP, Maritan A. 2007. Patterns of relative species abundance in rainforests and coral reefs. Nature 450, 45–49. ( 10.1038/nature06197) [DOI] [PubMed] [Google Scholar]
- 55.Olszewski TD. 2012. Persistence of high diversity in non-equilibrium ecological communities: implications for modern and fossil ecosystems. Proc. R. Soc. B 279, 230–236. ( 10.1098/rspb.2011.0936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foote M. 2014. Environmental controls on geographic range size in marine animal genera. Paleobiology 40, 440–458. ( 10.1666/13056) [DOI] [Google Scholar]
- 57.Payne JL, Finnegan S. 2007. The effect of geographic range on extinction risk during background and mass extinction. Proc. Natl Acad. Sci. USA 104, 10 506–10 511. ( 10.1073/pnas.0701257104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harnik PG, Simpson C, Payne JL. 2012. Long-term differences in extinction risk among the seven forms of rarity. Proc. R. Soc. B 279, 4969–4976. ( 10.1098/rspb.2012.1902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Locarnini RA, Mishonov AV, Antonov JI, Boyer TP, Garcia HE, Baranova OK, Zweng MM, Johnson DR. 2010. World ocean atlas 2009, volume 1: temperature. In NOAA Atlas NESDIS 68 (ed. Levitus S.), pp. 1–184. Washington, DC: US Government Printing Office. [Google Scholar]
- 60.Belanger CL, Jablonski D, Roy K, Berke SK, Krug AZ, Valentine JW. 2012. Global environmental predictors of benthic marine biogeographic structure. Proc. Natl Acad. Sci. USA 109, 14 046–14 051. ( 10.1073/pnas.1212381109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckley LB, Hurlbert AH, Jetz W. 2012. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob. Ecol. Biogeogr. 21, 873–885. ( 10.1111/j.1466-8238.2011.00737.x) [DOI] [Google Scholar]
- 62.Jocque M, Field R, Brendonck L, De Meester L. 2010. Climatic control of dispersal-ecological specialization trade-offs: a metacommunity process at the heart of the latitudinal diversity gradient? Glob. Ecol. Biogeogr. 19, 244–252. ( 10.1111/j.1466-8238.2009.00510.x) [DOI] [Google Scholar]
- 63.Zachos JC, Stott LD, Lohmann KC. 1994. Evolution of early Cenozoic marine temperatures. Paleoceanography 9, 353–387. ( 10.1029/93PA03266) [DOI] [Google Scholar]
- 64.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2003. Trends, rhythms, and aberrations in global Climate 65 Ma to present. Science 292, 686–693. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 65.Jacobs DK, Lindberg DR. 1998. Oxygen and evolutionary patterns in the sea: onshore/offshore trends and recent recruitment of deep-sea faunas. Proc. Natl Acad. Sci. USA 95, 9396–9401. ( 10.1073/pnas.95.16.9396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liow LH, Skaug HJ, Ergon T, Schweder T. 2010. Global occurrence trajectories of microfossils: environmental variability and the rise and fall of individual species. Paleobiology 36, 224–252. ( 10.1666/08080.1) [DOI] [Google Scholar]
- 67.Lindner A, Cairns SD, Cunningham CW. 2008. From offshore to onshore: multiple origins of shallow-water corals from deep-sea ancestors. PLoS ONE 3, e2429 ( 10.1371/journal.pone.0002429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thuy B, Gale AS, Kroh A, Kucera M, Numberger-Thuy LD, Reich M, Stohr S. 2013. Ancient origin of the modern deep-sea fauna. PLoS ONE 7, e46913 ( 10.1371/journal.pone.0046913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carney RS. 2005. Zonation of deep biota on continental margins. Oceanogr. Mar. Biol. 43, 211–278. ( 10.1201/9781420037449.ch6) [DOI] [Google Scholar]
- 70.Menzies RJ, George RY, Rowe GT. 1973. Abyssal environment and ecology of the world oceans. New York, NY: John Wiley & Sons. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are available at dx.doi.org/10.5061/dryad.943j7.