Abstract
In order to predict the fate of biodiversity in a rapidly changing world, we must first understand how species adapt to new environmental conditions. The long-term evolutionary dynamics of species' physiological tolerances to differing climatic regimes remain obscure. Here, we unite palaeontological and neontological data to analyse whether species' environmental tolerances remain stable across 3 Myr of profound climatic changes using 10 phylogenetically, ecologically and developmentally diverse mollusc species from the Atlantic and Gulf Coastal Plains, USA. We additionally investigate whether these species' upper and lower thermal tolerances are constrained across this interval. We find that these species' environmental preferences are stable across the duration of their lifetimes, even when faced with significant environmental perturbations. The results suggest that species will respond to current and future warming either by altering distributions to track suitable habitat or, if the pace of change is too rapid, by going extinct. Our findings also support methods that project species' present-day environmental requirements to future climatic landscapes to assess conservation risks.
Keywords: Atlantic coastal plain, conservation palaeobiology, fundamental niche, macroevolution, mid-Pliocene warm period, Mollusca
1. Introduction
Earth's climate is rapidly changing, altering all facets of our planet at an unprecedented rate, from the biosphere to the hydrosphere to the atmosphere. Given these changes, debate exists as to whether species can adapt their physiological tolerances, or niches, to altered environmental conditions [1–4]. Determining whether species' niches evolve or remain stable in the face of environmental change is important for implementing proper conservation measures, mitigating threats posed to biodiversity [5–7] and shedding light on macroevolutionary dynamics [8–11].
Here, we unite palaeontological and neontological data [12] to test niche stability across 3 Myr of environmental changes using 10 phylogenetically, ecologically and developmentally diverse bivalve and gastropod species from the Atlantic and Gulf Coastal Plains, USA, and surrounding region (electronic supplementary material, table S1). Species' niches were quantified using ecological niche modelling (ENM) [13] for three time periods from the Pliocene–Recent: the mid-Pliocene Warm Period (mPWP; approx. 3.264–3.025 Ma); the Eemian Last Interglacial (LIG; approx. 130–123 ka); and the present-day interval (PI). We test whether these species' niches changed across both long (Pliocene to Eemian; millions of years) and short (Eemian to present-day; thousands of years) time scales. We additionally investigate whether these species' upper and lower thermal tolerances changed across millions of years. Recent research suggests that tolerances to heat are largely conserved within terrestrial species, but that tolerances to cold are more variable [14]. This asymmetry is thought to diminish in the marine realm, where ectotherms are limited by both cold and warm conditions due to decreased aerobic capacity [15]. This study is the first to incorporate both modern and fossil data across millions of years to understand ecological and evolutionary responses of species to changes in their environment (though see [16–18] for analyses in deep time).
Theoretical [19,20] and empirical studies have both supported [21,22] and questioned [16,23,24] niche stability. The debate has even continued at the genetic level, where recent research indicates that genetic reshuffling in Drosophila species can occur in response to climate change [25,26]. Whether these genetic changes translate into evolution of actual physiological tolerances, however, remains unclear. The context in which niche evolution is considered is important with respect to whether change occurred in actual physiological tolerances (i.e. the fundamental niche; FN) or because of differences in resource utilization or underlying environmental structure (i.e. changes in the realized niche; RN). Studies may incorrectly indicate niche evolution if the environmental conditions that are available to a species are not taken into account [4,27,28].
The aforementioned studies have contributed much to our understanding of how species' environmental tolerances evolve, but questions about the relative dominance of niche evolution versus stability remain, particularly since most studies lack a temporal component that would allow for analysis of change across the entire duration of a species's lifetime, which may span millions of years [8].
The region encompassing and surrounding the Atlantic and Gulf Coastal Plains is ideal for elucidating the coevolution of species' niches and the environment. Not only has it experienced profound environmental changes associated with the closure of the Central American Seaway beginning in the Pliocene [29], but these environmental changes have been linked to patterns of extinction, species turnover and ecological change [30,31]. The mPWP is considered a climatic analogue for conditions expected at the end of this century and can contribute information on how target species may fare under future climate scenarios [32]. Results such as those presented here are vital for proper mitigation of the risks posed by current and future climate changes to the Earth's biodiversity [7,33].
2. Material and methods
In order to test for within-lineage niche stability, we used ENM, a correlative process whereby known occurrences of species are associated with environmental parameters to characterize a species's environmental requirements [13]. Models of species' abiotic niche parameters were constructed for each of three temporal intervals—the mPWP, LIG and PI—using taxon occurrence data and environmental parameters unique to each time slice. The resulting niche estimates were compared through time to statistically assess similarity using both environmental and geographical approaches [28,34,35]. In both approaches, an observed similarity metric is computed and compared with a simulated null distribution. Details of our methodology are outlined below.
(a). Taxa
We selected 10 species that occur in both the modern and fossil (from approx. 3.1 Ma to Recent) records of the Atlantic and Gulf Coastal Plains, USA, and surrounding region (table 1). Species were chosen because they have diverse phylogenetic positions, varied ecological habits and larval developmental modes, and abundant distributional data available from fossil and modern localities (electronic supplementary material, table S1). We used morphological criteria to identify target species, as each taxon is readily diagnosable. All evidence suggests that these lineages represent species that have distinct evolutionary trajectories, a supposition supported by the fact that many invertebrate species have durations greater than 3 Myr [8]. Consequently, we studied within-lineage rather than across-lineage niche evolution (although see the Discussion section for potential caveats).
Table 1.
Comparisons of multi-dimensional niches and thermal tolerance limits. Bold values indicate non-significant results. All other comparisons are statistically more similar than expected given the environmental background of the variable in question. Note that it is possible for two niches to be more similar than expected based on the environment available for one time slice, but less similar than expected based on the environment available for the other. See the electronic supplementary material, dataset S2, for graphical depictions of similarity tests.
| species/comparison |
mPWP–LIG | LIG–mPWP | PI–LIG | LIG–PI | mPWP–PI | PI–mPWP | |
|---|---|---|---|---|---|---|---|
| environmental comparison: p-values for tests using PCA on three most important variables | |||||||
| Bivalvia | Anomia simplex | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Crassostrea virginica | 0.04 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | |
| Dinocardium robustum | 0.01 | 0.00 | 0.00 | 0.02 | 0.09 | 0.00 | |
| Lucina pensylvanica | 0.25 | 0.01 | 0.00 | 0.00 | 0.03 | 0.03 | |
| Mercenaria campechiensis | 0.01 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | |
| Gastropoda | Bulla occidentalis | 0.34 | 0.54 | 0.10 | 0.29 | 0.31 | 0.20 |
| Crepidula fornicata | 0.03 | 0.00 | 0.02 | 0.00 | 0.29 | 0.58 | |
| Neverita duplicata | 0.08 | 0.00 | 0.01 | 0.44 | 0.01 | 0.00 | |
| Oliva sayana | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Terebra dislocata | 0.02 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | |
| environmental comparison: maximum surface temperature | |||||||
| Bivalvia | A. simplex | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C. virginica | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.00 | |
| D. robustum | 0.03 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | |
| L. pensylvanica | 0.00 | 0.00 | 0.00 | 0.00 | 0.81 | 0.42 | |
| M. campechiensis | 0.01 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | |
| Gastropoda | B. occidentalis | 0.00 | 0.00 | 0.02 | 0.60 | 0.96 | 0.02 |
| C. fornicata | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | |
| N. duplicata | 0.01 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | |
| O. sayana | 0.00 | 0.00 | 0.01 | 0.15 | 0.54 | 0.00 | |
| T. dislocata | 0.03 | 0.00 | 0.00 | 0.02 | 0.43 | 0.02 | |
| environmental comparison: minimum surface temperature | |||||||
| Bivalvia | A. simplex | 0.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C. virginica | 0.91 | 0.05 | 0.00 | 0.03 | 0.01 | 0.00 | |
| D. robustum | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| L. pensylvanica | 0.68 | 0.00 | 0.00 | 0.00 | 0.77 | 0.22 | |
| M. campechiensis | 0.82 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | |
| Gastropoda | B. occidentalis | 0.66 | 0.00 | 0.09 | 0.10 | 0.85 | 0.10 |
| C. fornicata | 0.17 | 0.00 | 0.01 | 0.00 | 0.44 | 0.00 | |
| N. duplicata | 0.50 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | |
| O. sayana | 0.07 | 0.00 | 0.03 | 0.00 | 0.29 | 0.00 | |
| T. dislocata | 0.18 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | |
(b). Distributional data
(i). Present day
Presence-only distributional data were derived from [36] (electronic supplementary material, table S1 and figures S1–S3). Only records with spatial uncertainty less than 15 km were retained, ensuring that they were matched correctly with corresponding environmental data of a coarser spatial resolution (i.e. 1.25° × 1.25°) [37]. We subsampled distributional data to leave one record per environmental pixel to account for sampling biases in R v. 15.2 [38], which resulted in 20–58 unique occurrences per species (electronic supplementary material, table S1). This process did not affect the resultant overall distribution of the species, but rather prevented certain localities with multiple records from being unduly weighted in the niche modelling analyses [39,40].
(ii). Fossil
We considered fossil distributional data from mPWP (approx. 3.264–3.025 Ma) and LIG (approx. 130–123 ka) strata of the Atlantic and Gulf Coastal Plains, USA, and surrounding region. To ensure that distributional data were derived from geologic units of similar ages to our periods of interest, we generated a stratigraphic database for all Pliocene–Recent geologic units of the Atlantic Coastal Plain (electronic supplementary material, dataset S1). Correlations and unit ages were determined by extensive literature survey and use of various stratigraphic databases, resulting in 10 viable formations for the Pliocene and 16 for the LIG. The formations from which occurrence data were derived are documented in the electronic supplementary material, dataset S1.
Distributional records were obtained from on-site investigations of collections to ensure proper species identification, including the Florida Museum of Natural History, Paleontological Research Institution, Virginia Museum of Natural History, Academy of Natural Sciences of Drexel University and Yale Peabody Museum. As with present-day distributional data, we subsampled fossil distributional data to leave one record per environmental pixel, resulting in six to 16 unique occurrences per species (electronic supplementary material, table S1). At least six spatially explicit distributional records were used for model calibration for any given species/time period; studies have shown this number to be statistically robust for extant species [41,42].
(c). Environmental data
Environmental data were derived from the coupled atmosphere–ocean HadCM3 global climate model (GCM) [43,44] for three time slices: mPWP (approx. 3.264–3.025 Ma), LIG (approx. 130–123 ka) and PI (considering the pre-industrial interval from approx. 1850 to 1890). Ideally, we would use an ensemble-modelling approach that considered multiple GCMs [45]; however, model output for the LIG was available to us only from HadCM3, and consisted of variations of temperature and salinity parameters. This GCM has been successfully used in a variety of Quaternary and pre-Quaternary modelling studies [46–48]. Boundary conditions for the LIG were from [47,49]. Here, atmospheric gas concentrations were derived from ice core records [50–52], and orbital parameters were from Berger & Loutre [53]. The mPWP GCM used the alternate PRISM3D PlioMIP dataset [54], and the pre-industrial experiment was equivalent to the study of Braconnot et al. [55]. All GCM experiments were run for 500 model years, and environmental parameters were averaged from the final 30 years of each experiment at 1.25° × 1.25° resolution (approx. 140 × 140 km at the equator). Where ocean data were unavailable (i.e. sites presenting macrofossil data, but where the GCM indicated land), we used an inverse-distance weighted algorithm to extrapolate model data.
Modelled monthly salinity and temperature outputs were converted to maximum, minimum and average yearly coverages for both surface and bottom conditions using ArcGIS. From these 12 coverages, we eliminated variables that significantly covaried (assessed using the ‘cor’ function in R). Ultimately, two bottom variables (yearly average salinity and temperature) and four surface variables (maximum and minimum salinity, and maximum and minimum temperature) were retained. These six variables were preserved because they did not significantly covary and are deemed biologically important for marine ectotherms [56–58].
(d). Modelling algorithm
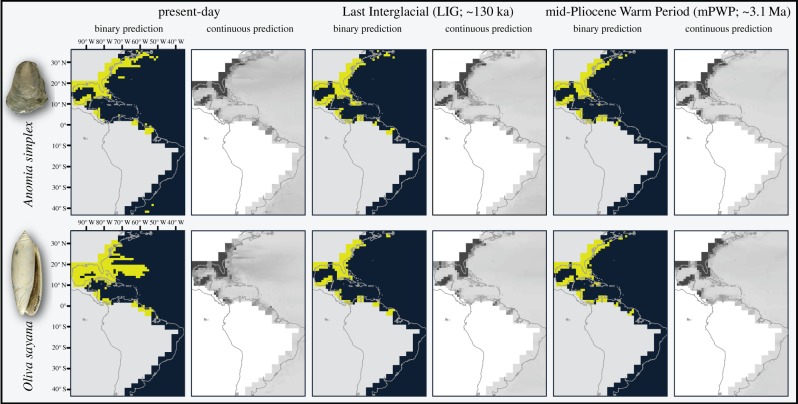
To approximate niche parameters for these species, we generated ENMs using MaxEnt v. 3.3.3 [59] (figure 1; electronic supplementary material, figures S4 and S5). MaxEnt finds suitable environmental combinations for species under a null expectation that suitability is proportional to availability. Thus, MaxEnt minimized the relative entropy of observed environments relative to those in the background [60]. We enabled only quadratic features to simulate realistic bell-shaped response curves that are known from physiological experiments of plants and animals [61–63]. We calibrated models within a region bounded by the Americas and 34° W longitude, and 48° N and 44° S latitude (figure 1). We sought the union of the area sampled by researchers and most likely to be accessible to the species across spatial and temporal dimensions [13,64,65]. We used all spatially explicit data points for each species/time slice, running 100 bootstrap replicates with a 10% random test percentage. The mean value of the suitability grids was used to threshold to binary predictions [66,67].
Figure 1.
Representative ecological niche models. Model results for the present, LIG and mPWP for two species: Anomia simplex and Oliva sayana. Binary and continuous predictions are presented, with binary predictions thresholded using the mean suitability value from the continuous output. For the binary predictions, yellow indicates suitable and dark blue indicates unsuitable, whereas for the continuous predictions, darker greys indicate higher suitability. All analyses were conducted within the geographical extent shown. Note that the modelled shorelines do not match the continental shorelines because of the nature of our GCM data and the need to capture the higher sea levels characteristic of the mPWP. See the electronic supplementary material, figures S4 and S5, for remaining species analysed.
To correct for potential biases in fossil distributional data, we implemented what is called a ‘bias file’ within MaxEnt for past modelling [68]. The bias file describes the probability that an area was sampled; thus, regions with rock outcrop (i.e. areas where species may actually be sampled) were weighted twice as heavily as regions without rock outcrop. MaxEnt will then factor out this bias during the modelling process (see [68] for details). This method essentially accounts for incomplete knowledge of species' distributions (sensu [69]).
Although characterizing the entirety of a species's FN is often difficult without mechanistic studies [14], we study close approximations here, given that recent biophysical approaches have determined that FNs can be represented by limited sets of parameters such as temperature [70,71]. This is particularly true for marine ectotherms, which have been shown to closely match range limits within their thermal tolerances [15]. That being said, our estimates may reflect some quantity between the RN and the FN, as our niche parameters are ultimately derived from the areas occupied by a species [13,14,27].
(e). Model verification
Two model validation methods were used, depending on the prevalence of distributional records (electronic supplementary material, table S2). For species/time slices with less than 25 points, we assessed statistical significance using a jack-knife procedure under a least training presence threshold [42]. This method, however, may produce over-optimistic estimates of predictive power for sample sizes of more than 25, and these species/time slices were tested using partial receiver operating characteristic analyses [72].
(f). Niche comparisons
Characterizations of species' niches were compared through time using two statistical approaches: a kernel smoothing script [28] and ENMTools [35]. Both frameworks use randomization tests to compare observed similarity to that expected under a null hypothesis. The null is rejected if models are more or less similar to what is expected by chance, based on the environment within the geographical regions of interest. Similarity is quantified using Schoener's D [73], with values ranging from 0 to 1, or from more to less similar, respectively.
For each of the 10 species, we compared observed niches across the three different time periods (mPWP, LIG and PI). Comparisons were made in two directions [28,35] (for example, comparing the mPWP to the LIG, and the LIG to the mPWP), as it is possible for two niches to be more similar than expected based on the environment available for one time slice, but less similar than expected based on the environment available for the other. If the observed value fell outside the null distribution to the high end, niches were more similar than expected by chance, whereas if the observed value fell outside the null distribution to the lower end, niches were more different than expected by chance. Observed values that fell within the null distribution did not allow for discrimination of similarity or differences based on the environment available to the entities in question. We performed similarity tests using (i) a principal component analysis (PCA) applied to all six environmental variables, (ii) a PCA applied to the three most important environmental variables, (iii) raw average bottom temperature and maximum surface temperature in two-dimensional environmental space, (iv) maximum surface temperature only; (v) minimum surface temperature only and (vi) ENMTools on projections of ecological niche models. The first five sets of tests compared niches in environmental space, with the first three multi-dimensional in nature, whereas the sixth compared niches in geographical space. Each of these types of test resulted in 60 comparisons (i.e. 10 species × three time slices × two directions), for a total of 360 tests. Details of the comparisons are provided below.
(i). Environmental comparisons
We calculated metrics of niche overlap in gridded environmental space using the methodology of Broennimann et al. [28]. Here, ordination techniques [74] allow for direct comparison of species–environment relationships in environmental space [27]. Observed densities for each region are corrected in light of the availability of environmental space using kernel density functions (table 1; electronic supplementary material, table S3 and dataset S2). Niche overlap is measured along gradients of a multivariate analysis, and statistical significance is assessed using the framework described above.
We tested for similarity using a PCA (i) applied to all six environmental parameters, and (ii) when niche dimensionality was reduced to three variables, including surface coverages for maximum salinity, maximum temperature and minimum temperature. These variables were retained because they explained the most variance in the dataset [58,75,76]. Analyses performed with this reduced set of variables are potentially more informative, as over-parametrization can constrict niche estimates and lead to approximations closer to the RN [13]. PCA analyses were calibrated on environments of both time slices (setting project equal to false). We used both the PCA-occ and PCA-env functions; the former calibrates the PCA based only on the distributional data, whereas the latter uses data from the entire environmental space of the two study systems. The results were equivalent, and thus we present only those from PCA-env. A bin size of 100 was used to characterize the environment, running 1000 replicates for similarity tests. As prevalence of distributional data varies through time, we generated input data from ENMs outside of the framework of Broennimann et al. [28], subsampling one point per pixel in binary predictions such that comparisons were unbiased with regard to the quantity of input data. Doing so ensures that we capture all of the environments that a species finds suitable, rather than the portion that happened to be occupied most frequently.
We also tested similarity in raw variables (table 1; electronic supplementary material, table S3 and dataset S2). We used the script of Broennimann et al. [28] to analyse maximum and minimum surface temperature individually, and we modified the script to compare raw variables in two dimensions, while still accounting for differences in availability of environments in a given time period. We were interested in testing for evolution in overall temperature parameters, and thus we assessed similarity using average bottom temperature and maximum surface temperature.
(ii). Geographical projections
In addition to the comparisons made entirely in environmental space, we used ENMTools [35] to compare the geographical projections of niches. Null distributions consisted of 100 random models generated within MaxEnt, with model parameters drawn from and constrained by the study system. To ensure accurate response curves when projecting, we disabled clamping and enabled extrapolation within MaxEnt [77].
3. Results
Model verification exercises suggest that models of species' niches are statistically significant for each time slice (p < 0.05; see the electronic supplementary material, table S2). The niche model depictions are shown in figure 1 and the electronic supplementary material, figures S4 and S5.
Together, the suite of niche comparisons (360 in total) indicates these species' environmental preferences are stable across millions of years. In 359 of 360 cases, we found no evidence of niche dissimilarity across all comparisons. Indeed, of the 10 ecologically diverse species studied, nine show the opposite pattern: statistically similar niches for the majority of the comparisons. Probabilistically, this result would be obtained less than 1% of the time, assuming equal likelihood for evolution versus stability of niche attributes. We obtain evidence of niche similarity for tests on both PCAs and raw variables. Moreover, minimum and maximum temperature tolerances are generally conserved through time.
(a). Environmental comparisons
Comparisons on multi-dimensional niches indicate overwhelming signals of niche stability across the time slices. Of these 180 comparisons, 149 indicate statistically similar niches through time, and no comparison found evidence of niche dissimilarity.
Comparisons considering all six environmental variables indicate niches are statistically similar for most species and time slices (46 of 60 comparisons; electronic supplementary material, table S3). When niche dimensionality was reduced to the most important variables, nine species show statistically similar niches for all comparisons, with the exception of one or two inconclusive tests for Crepidula fornicata, Dinocardium robustum, Lucina pensylvanica and Neverita duplicata (49 of 60 comparisons; figure 2 and table 1). Bulla occidentalis is the only species with non-significant tests across multiple time slices. This species does not have any readily identifiable traits—such as larval strategy or feeding ecology—that would predispose it to occupying new environments relative to the other species that we studied. Niches also show stability when raw variables are considered. Seven of the 10 species have statistically similar niches across all time comparisons (42 of 60 comparisons; electronic supplementary material, table S3). Two other species, Oliva sayana and Crassostrea virginica, have statistically similar niches with the exception of one and two inconclusive tests, respectively. Quantifying niche similarity for B. occidentalis proved more difficult, as three of six niche comparisons are non-significant (but not statistically different).
Figure 2.
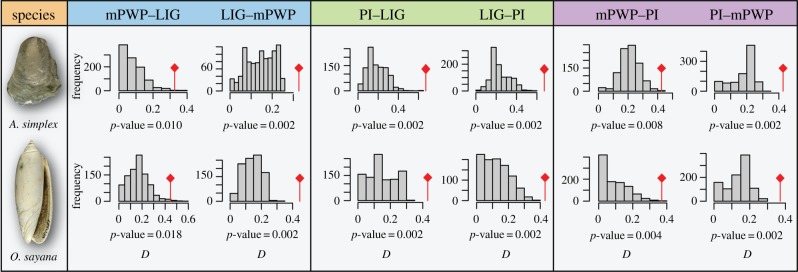
Representative results from niche comparison analyses. Comparisons for Anomia simplex and Oliva sayana using a PCA on the three most important environmental variables: maximum and minimum surface temperature, and maximum surface salinity. Comparisons are shown for the LIG (approx. 130 ka), mPWP (approx. 3.1 Ma) and PI. The histograms show the null distribution of similarity values (D) drawn from the study area, with the observed similarity value in red. All comparisons indicate that niches are statistically more similar than expected given the environmental backgrounds. For other comparisons, see table 1 and the electronic supplementary material, table S3 and dataset S2.
Species seem to conserve their upper thermal tolerance limits, but results are less conclusive for minimum temperature tolerances (table 1; electronic supplementary material, dataset S2). Across the suite of species, the majority of comparisons are statistically similar with regard to maximum surface temperature, although six species have one or two comparisons that are inconclusive (B. occidentalis, D. robustum, L. pensylvanica, N. duplicata, O. sayana and Terebra dislocata). Comparisons also indicate statistical similarity with regard to minimum temperature tolerances. However, the structure of this variable changes through time, making it difficult to quantify similarities or differences. For example, all mPWP–LIG comparisons are inconclusive with the exception of N. duplicata, as are at least half of the comparisons for B. occidentalis and L. pensylvanica.
(b). Geographical comparisons
Results from comparisons of the geographical projections of niches mirror those from the environmental comparisons. Niches are statistically similar for seven of the 10 species across all comparisons (42 of 60 comparisons; electronic supplementary material, table S3 and dataset S2). Crassostrea virginica and L. pensylvanica have one comparison that is inconclusive (LIG–mPWP and PI–mPWP, respectively), while the niche of B. occidentalis is significantly dissimilar for the LIG–mPWP comparison and non-significant for the PI–mPWP comparison.
4. Discussion
Our analyses find no support for niche evolution. Instead, we observe statistically significant niche stability across 3 Myr of considerable environmental changes, from extreme warmth during the mPWP to glacial cycles during the Pleistocene [29]. This is true for all 10 of the species analysed. Importantly, niche stability will not be recovered within analyses for reasons other than similarity, whereas niche differences can be obtained as a function of changing parameters of the RN [14]. Therefore, the lack of any net change suggests that species were either shifting their niche preferences in response to oscillating climatic conditions at scales too rapid to be detected by our analyses, or their preferences remained stable across this temporal interval. In either case, overall niche stability has profound implications for understanding conservation priorities and for elucidating macroevolutionary dynamics.
(a). Implications for survival of taxa during times of change
These results aid our understanding of how species may respond to climate change on both long and short time scales. As climate continues to change, species that are unable to adapt to new conditions face two futures: extinction or shifting distributions to follow suitable areas. Already, both responses have been documented or predicted as a result of current climate change. Marine and terrestrial species are forecast to experience climate-driven extinctions into the twenty-second century [78,79]. Indeed, the niche stability we have documented may doom many marine species to extinction over the next 100+ years, particularly if they live at their thermal tolerance limits and are unable to alter their upper thresholds [58]. The target species considered here are predicted to experience severe distributional reductions by the end of this century when variables other than temperature and salinity are considered, but wholesale extinction is unlikely [36]. This prediction is supported by their survival in the Pliocene, albeit in geographically reduced areas, when conditions were purportedly similar to those expected at the end of this century [32]. These small areas of suitability—or refugia—are thought to have played an important role in species' survival during past episodes of climate change [80].
If species are able to keep pace with the changing environment, distributional shifts, rather than extinctions, are expected [33]. Under this scenario, dispersal ability becomes an important parameter predicting species' responses to climate change [81]. Present-day elevational, latitudinal and bathymetric shifts [82] have already been observed in response to current warming patterns, and indeed the fossil record provides abundant evidence for habitat tracking during rapid Pleistocene climate cycles [83], often creating non-analogous community assemblages [84]. The rate at which climate changes also dictates whether species can track preferred environments, and future rates are anticipated to exceed those experienced during the geologic intervals analysed within this study [58,85,86]. In a rapidly changing world, species will probably be forced to move to suitable areas or face extinction. That is, it seems unlikely that they will alter their abiotic preferences on extremely short time scales if they are unable to do so on longer time scales, as we demonstrated here.
Methodologically, niche stability provides support for ENM and species distribution modelling (SDM) analyses that attempt to predict how species will respond to altered climatic conditions [13]. In particular, our results may somewhat alleviate concerns over inaccurate forecasts due to changing niches [1,3]. Problems still remain, however, in that ENM and SDM methods typically do not account for dispersal limitations or altered biotic interactions [87] (though see [85]); nor do they consider that species can alter their behaviour or microhabitat preferences to buffer against environmental changes [2,88].
(b). Macroevolutionary implications of stable niches
We show that large-scale parameters of species' niches, in this case temperature and salinity, do not change for a phylogenetically and ecologically diverse set of marine molluscs. Although species may modify their behaviour or resource utilization, the FN places constraints on species' interactions with the environment, which potentially governs speciation and extinction processes over long time scales [10,89]. Some researchers have suggested that niche stability may promote allopatric speciation [90,91]. That is, environmental perturbations may separate two populations, with those populations prevented from merging back together because of constraints imposed by the FN, which will then eventually lead to diversification.
Niche stability also provides a potential mechanism for the morphological stasis observed within species over millions of years [8]. More specifically, niche stability requires species to track preferred habitats as the environment changes, thereby continuously joining and separating populations on scales of less than 10 000 years or so. In this framework, any localized phenotypic adaptation is unlikely to be fixed across an entire species, such that no overall net changes are observed for the species as a whole [8].
(c). Potential caveats
Although our analyses are quantitatively robust, our study is not without limitations. First, our models may approximate the existing or RN, rather than the FN [92], because FNs are difficult to characterize without detailed physiological studies [13,14]. With that said, niche estimates were calculated from environmental preferences that were averaged over a period of time, which may broaden estimates such that real physiological limits are captured [58]. The recovered pattern of niche stability would be even more robust if we studied RNs, as change is expected to occur over time in RN parameters owing to differences in resource utilization or underlying environmental structure [4,13,27]. Second, estimates of present-day and past niches may not be equivalent, and thus may be incomparable. This, however, is of less concern here because we documented niche stability rather than niche evolution. Third, we acknowledge that recognition of ‘species'—especially in the fossil record—is sometimes contentious, and while these species are diagnosably distinct throughout their duration, they may not constitute single evolutionary lineages. Our results, however, would be more robust if we studied aggregated collections of closely related lineages, as we would expect more change in niche parameters at speciation. We support conservatism of niches across speciation events if the entities in question represent closely related species complexes. Fourth, we analysed data from warm time periods, as distributional data do not exist for glacial periods (e.g. the last glacial maximum; approx. 21 ka). Therefore, our analyses may have missed rapid (but reversible) niche evolution that occurred in response to these colder conditions. Although possible, the scenario is unlikely because of the rate at which niche evolution would have had to occur and because of the paucity of evidence for niche adaptation both in the fossil record [83] and in experimental studies [14]. Moreover, environmental conditions at the mPWP, LIG and PI differ to a significant degree, such that we are still able to discern whether species adapted to new conditions or tracked stable climate envelopes. Finally, and related to this issue, because palaeoclimate models were only available for certain key temporal intervals, we could not capture the entire temporal history of these species in the context of an ENM framework. We did, however, examine changes across both long (mPWP to LIG) and short (LIG to PI) time scales.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Alycia Stigall, Geerat Vermeij, Gary Carvalho, Norman MacLeod and an anonymous reviewer for improving the quality of this contribution. We thank the following individuals for museum collection help: Lauck Ward and Alton Dooley (Virginia Museum of Natural History), Judith Nagel-Myers, Greg Dietl, David Campbell and Warren Allmon (Paleontological Research Institution), Paul Callomon (The Academy of Natural Sciences of Drexel University), Jessica Utrup (Yale Peabody Museum), and Alex Kittle and Sean Roberts (Florida Museum of Natural History). We also thank numerous geologists for discussions on stratigraphy, including: Lauck Ward (Virginia Museum of Natural History), Christopher Williams and Guy Means (Florida Geological Survey), Paul Huddlestun (Georgia Geological Survey), Helaine Markewich (US Geological Survey), Kathleen Farrell (North Carolina Geological Survey), John Wehmiller (University of Delaware), Ervin Otvos (University of Southern Mississippi) and Lyell Campbell (University of South Carolina Upstate). We benefited from discussion of ENM with Narayani Barve, Andres Lira, A. Townsend Peterson, Jorge Soberón and the KU ENM Working Group (University of Kansas). Olivier Brönnimann is thanked for assistance with his niche overlap script. A.H. and S.J.H. thank Paul Valdes and Joy Singarayer for making available LIG model boundary conditions.
Data accessibility
The stratigraphic database (dataset S1) and output from niche comparison tests (dataset S2) are available in the electronic supplementary material. Climate and distributional data are available on Dryad, doi:10.5061/dryad.qd08b.
Funding statement
An NSF GK-12 Fellowship, KU Madison and Lila Self Graduate Fellowship, KU Biodiversity Institute Panorama Grant and John W. Wells Grant-in-Aids of Research provided funding for this work to E.E.S. B.S.L. was supported by NSF grant no. EF-1206757. A.H. and S.J.H. acknowledge that the research leading to these results received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no. 278636.
References
- 1.Pearman PB, Guisan A, Broennimann O, Randin CF. 2008. Niche dynamics in space and time. Trends Ecol. Evol. 23, 149–158. ( 10.1016/j.tree.2007.11.005) [DOI] [PubMed] [Google Scholar]
- 2.Lavergne S, Mouquet N, Thuiller W, Ronce O. 2010. Biodiversity and climate change: integrating evolutionary and ecological responses of species and communities. Annu. Rev. Ecol. Evol. Syst. 41, 321–350. ( 10.1146/annurev-ecolsys-102209-144628) [DOI] [Google Scholar]
- 3.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 4.Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C. 2014. Unifying niche shift studies: insights from biological invasions. Trends Ecol. Evol. 29, 260–269. ( 10.1016/j.tree.2014.02.009) [DOI] [PubMed] [Google Scholar]
- 5.Pereira HM, et al. 2010. Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501. ( 10.1126/science.1196624) [DOI] [PubMed] [Google Scholar]
- 6.Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. 2011. Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–58. ( 10.1126/science.1200303) [DOI] [PubMed] [Google Scholar]
- 7.Moritz C, Agudo R. 2013. The future of species under climate change: resilience or decline? Science 341, 504–508. ( 10.1126/science.1237190) [DOI] [PubMed] [Google Scholar]
- 8.Eldredge N, et al. 2005. The dynamics of evolutionary stasis. Paleobiology 31, 133–145. ( 10.1666/0094-8373(2005)031[0133:TDOES]2.0.CO;2) [DOI] [Google Scholar]
- 9.Romdal T, Araújo MB, Rahbek C. 2013. Life on a tropical planet: niche conservatism explains the global diversity gradient. Glob. Ecol. Biogeogr. 22, 344–350. ( 10.1111/j.1466-8238.2012.00786.x) [DOI] [Google Scholar]
- 10.Jablonski D, Belanger CL, Berke SK, Huang S, Krug AZ, Roy K, Tomasovych A, Valentine JW. 2013. Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient. Proc. Natl Acad. Sci. USA 110, 10 487–10 494. ( 10.1073/pnas.1308997110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeij GJ. 1991. When biotas meet: understanding biotic interchange. Science 253, 1099–1104. ( 10.1126/science.253.5024.1099) [DOI] [PubMed] [Google Scholar]
- 12.Fritz SA, Schnitzler J, Eronen JT, Hof C, Böhning-Gaese K, Graham CH. 2013. Diversity in time and space: wanted dead and alive. Trends Ecol. Evol. 28, 509–516. ( 10.1016/j.tree.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 13.Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, Araújo MB. 2011. Ecological niches and geographic distributions. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Araújo MB, Ferri-Yanez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 15.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stigall AL. 2012. Using ecological niche modelling to evaluate niche stability in deep time. J. Biogeogr. 39, 772–781. ( 10.1111/j.1365-2699.2011.02651.x) [DOI] [Google Scholar]
- 17.Stigall AL. In press. When and how do species achieve niche stability over long time scales? Ecography ( 10.1111/ecog.00719) [DOI] [Google Scholar]
- 18.Malizia RW, Stigall AL. 2011. Niche stability in Late Ordovician articulated brachiopod species before, during, and after the Richmondian invasion. Palaeogeogr. Palaeoclimatol. Palaeoecol. 311, 154–170. ( 10.1016/j.palaeo.2011.08.017) [DOI] [Google Scholar]
- 19.Holt RD. 1996. Adaptive evolution in source–sink environments: direct and indirect effects of density-dependence on niche evolution. Oikos 75, 182–192. ( 10.2307/3546242) [DOI] [Google Scholar]
- 20.Kawecki TJ. 1995. Demography of source–sink populations and the evolution of ecological niches. Evol. Ecol. 9, 38–44. ( 10.1007/BF01237695) [DOI] [Google Scholar]
- 21.Martínez-Meyer E, Peterson AT. 2006. Conservatism of ecological niche characteristics in North American plant species over the Pleistocene-to-Recent transition. J. Biogeogr. 33, 1779–1789. ( 10.1111/j.1365-2699.2006.01482_33_10.x) [DOI] [Google Scholar]
- 22.Strubble D, Broennimann O, Chiron F, Matthysen E. 2013. Niche conservatism in non-native birds in Europe: niche unfilling rather than niche expansion. Glob. Ecol. Biogeogr. 22, 962–970. ( 10.1111/geb.12050) [DOI] [Google Scholar]
- 23.Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A. 2007. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 10, 701–709. ( 10.1111/j.1461-0248.2007.01060.x) [DOI] [PubMed] [Google Scholar]
- 24.Rödder D, Lötters S. 2009. Niche shift versus niche conservatism? Climatic characteristics of the native and invasive ranges of the Mediterranean house gecko (Hemidactylus turcicus). Glob. Ecol. Biogeogr. 18, 674–687. ( 10.1111/j.1466-8238.2009.00477.x) [DOI] [Google Scholar]
- 25.Umina PA, Weeks AR, Kearney MR, McKechnie SW, Hoffmann AA. 2005. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 308, 691–693. ( 10.1126/science.1109523) [DOI] [PubMed] [Google Scholar]
- 26.Balanyá J, Oller JM, Huey RB, Gilchrist GW, Serra L. 2006. Global genetic change tracks global warming in Drosophila subobscura. Science 313, 1773–1775. ( 10.1126/science.1131002) [DOI] [PubMed] [Google Scholar]
- 27.Araújo MB, Peterson AT. 2012. Uses and misuses of bioclimatic envelope modeling. Ecology 93, 1527–1539. ( 10.1890/11-1930.1) [DOI] [PubMed] [Google Scholar]
- 28.Broennimann O, et al. 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 21, 481–497. ( 10.1111/j.1466-8238.2011.00698.x) [DOI] [Google Scholar]
- 29.Cronin TM. 1988. Evolution of marine climates of the US Atlantic coast during the past four million years. Phil. Trans. R. Soc. Lond. B 318, 661–678. ( 10.1098/rstb.1988.0029) [DOI] [Google Scholar]
- 30.Todd JA, Jackson JBC, Johnson KG, Fortunato HM, Heitz A, Alvarez M, Jung P. 2002. The ecology of extinction: molluscan feeding and faunal turnover in the Caribbean Neogene. Proc. R. Soc. Lond. B 269, 571–577. ( 10.1098/rspb.2001.1923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeij GJ. 2009. One-way traffic in the western Atlantic: causes and consequences of Miocene to early Pleistocene molluscan invasions in Florida and the Caribbean. Paleobiology 31, 624–642. ( 10.1666/04066.1) [DOI] [Google Scholar]
- 32.Robinson MM, Dowsett HJ. 2008. Pliocene role in assessing future climate impacts. EOS Trans. Am. Geophys. Union 89, 501–502. ( 10.1029/2008EO490001) [DOI] [Google Scholar]
- 33.Warren R, et al. 2013. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Change 3, 678–682. ( 10.1038/nclimate1887) [DOI] [Google Scholar]
- 34.Warren DL, Glor RE, Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883. ( 10.1111/j.1558-5646.2008.00482.x) [DOI] [PubMed] [Google Scholar]
- 35.Warren DL, Glor RE, Turelli M. 2010. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33, 607–611. ( 10.1111/j.1600-0587.2009.06041.x) [DOI] [Google Scholar]
- 36.Saupe EE, Hendricks JR, Townsend AT, Lieberman BS. 2014. Climate change and marine molluscs of the western North Atlantic: future prospects and perils. J. Biogeogr. 41, 1352–1366. ( 10.1111/jbi.12289) [DOI] [Google Scholar]
- 37.Graham CH, Elith J, Hijmans RJ, Guisan A, Peterson AT, Loiselle BA, Group TNPSDW. 2008. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 45, 239–247. ( 10.1111/j.1365-2664.2007.01408.x) [DOI] [Google Scholar]
- 38.R Core Team. 2012.
- 39.Royle JA, Chandler RB, Yackulic C, Nichols JD. 2012. Likelihood analysis of species occurrence probability from presence-only data for modelling species distributions. Methods Ecol. Evol. 3, 545–554. ( 10.1111/j.2041-210X.2011.00182.x) [DOI] [Google Scholar]
- 40.Yackulic CB, Chandler R, Zipkin EF, Royle JA, Nichols JD, Campbell Grant EH, Veran S. 2013. Presence-only modelling using MAXENT: when can we trust the inferences? Methods Ecol. Evol. 4, 236–243. ( 10.1111/2041-210x.12004) [DOI] [Google Scholar]
- 41.Hernandez PA, Graham CH, Master LL, Albert DL. 2006. The effect of sample size and species characteristics on performance of different distribution modeling methods. Ecography 29, 773–785. ( 10.1111/j.0906-7590.2006.04700.x) [DOI] [Google Scholar]
- 42.Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT. 2007. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 34, 102–117. ( 10.1111/j.1365-2699.2006.01594.x) [DOI] [Google Scholar]
- 43.Gordon C, Cooper C, Senior CA, Banks H, Gregory JM, Johns TC, Mitchell JFB, Wood RA. 2000. The simulation of SST, sea ice extents and ocean heat transports in a version of the Hadley Centre coupled model without flux adjustments. Clim. Dyn. 16, 147–168. ( 10.1007/s003820050010) [DOI] [Google Scholar]
- 44.Pope VD, Gallani ML, Rowntree PR, Stratton RA. 2000. The impact of new physical parameterizations in the Hadley Centre Climate model: HadAM3. Clim. Dyn. 16, 123–146. ( 10.1007/s003820050009) [DOI] [Google Scholar]
- 45.Fordham DA, Wigley TML, Watts MJ, Brook BW. 2012. Strengthening forecasts of climate change impacts with multi-ensemble averaged projections using MAGICC/SCENGEN 5.3. Ecography 45, 4–8. ( 10.1111/j.1600-0587.2011.07398.x) [DOI] [Google Scholar]
- 46.Eriksson A, Betti L, Friend AD, Lycett SJ, Singarayer JS, von Cramon-Taubadel N, Valdes PJ, Balloux F, Manica A. 2012. Late Pleistocene climate change and the global expansion of anatomically modern humans. Proc. Natl Acad. Sci. USA 109, 16 089–16 094. ( 10.1073/pnas.1209494109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singarayer JS, Valdes PJ. 2010. High-latitude climate sensitivity to ice-sheet forcing over the last 120 kyr. Quat. Sci. Rev. 29, 43–55. ( 10.1016/j.quascirev.2009.10.011) [DOI] [Google Scholar]
- 48.Haywood AM, Ridgwell A, Lunt DJ, Hill DJ, Pound MJ, Dowsett HJ, Dolan AM, Francis JE, Williams M. 2011. Are there pre-Quaternary geological analogues for a future greenhouse warming? Phil. Trans. R. Soc. A 369, 933–956. ( 10.1098/rsta.2010.0317) [DOI] [PubMed] [Google Scholar]
- 49.Singarayer JS, Valdes PJ, Friedlingstein P, Nelson S, Beerling DJ. 2011. Late Holocene methane rise caused by orbitally controlled increase in tropical sources. Nature 470, 82–85. ( 10.1038/nature09739) [DOI] [PubMed] [Google Scholar]
- 50.Petit JR, et al. 1999. Climate and atmospheric history of the past 420,000 years from the Vostok Ice Core, Antarctica. Nature 399, 429–436. ( 10.1038/20859) [DOI] [Google Scholar]
- 51.Loulergue L, et al. 2008. Orbital and millennial-scale features of atmospheric CH4 over the past 800,000 years. Nature 453, 383–386. ( 10.1038/nature06950) [DOI] [PubMed] [Google Scholar]
- 52.Spahni R, et al. 2005. Atmospheric methane and nitrous oxide of the Late Pleistocene from Antarctic ice cores. Science 310, 1317–1321. ( 10.1126/science.1120132) [DOI] [PubMed] [Google Scholar]
- 53.Berger A, Loutre MF. 1991. Insolation values for the climate of the last 10 million years. Quat. Sci. Rev. 10, 297–317. ( 10.1016/0277-3791(91)90033-Q) [DOI] [Google Scholar]
- 54.Haywood AM, Dowsett HJ, Robinson MM, Stoll DK, Dolan AM, Lunt DJ, Otto-Bliesner B, Chandler MA. 2011. Pliocene Model Intercomparison Project (PlioMIP): experimental design and boundary conditions (Experiment 2). Geosci. Model Dev. 4, 571–577. ( 10.5194/gmd-4-571-2011) [DOI] [Google Scholar]
- 55.Braconnot P, et al. 2007. Results of PMIP2 coupled simulations of the Mid-Holocene and Last Glacial Maximum. Part 1: experiments and large-scale features. Clim. Past 3, 261–277. ( 10.5194/cp-3-261-2007) [DOI] [Google Scholar]
- 56.Tomašových A, Kidwell SM. 2009. Preservation of spatial and environmental gradients by death assemblages. Paleobiology 35, 119–145. ( 10.1666/07081.1) [DOI] [Google Scholar]
- 57.Buckley LB, Hurlbert AH, Jetz W. 2012. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob. Ecol. Biogeogr. 9, 873–885. ( 10.1111/j.1466-8238.2011.00737.x) [DOI] [Google Scholar]
- 58.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690. [Google Scholar]
- 59.Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. ( 10.1016/j.ecolmodel.2005.03.026) [DOI] [Google Scholar]
- 60.Elith J, Phillips SJ, Hastie T, Dudik M, Chee YE, Yates CJ. 2011. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57. ( 10.1111/j.1472-4642.2010.00725.x) [DOI] [Google Scholar]
- 61.Austin MP, Nicholls AO, Doherty MD, Meyers JA. 1994. Determining species response functions to an environmental gradient by means of a beta-function. J. Veg. Sci. 5, 215–228. ( 10.2307/3236154) [DOI] [Google Scholar]
- 62.Angilletta M. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 63.Hooper HL, Connon R, Callaghan A, Fryer G, Yarwood-Buchanan S, Biggs J, Maund SJ, Hutchinson TH, Sibly RM. 2008. The ecological niche of Daphnia magna characterized using population growth rate. Ecology 89, 1015–1022. ( 10.1890/07-0559.1) [DOI] [PubMed] [Google Scholar]
- 64.Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, Leathwick J, Ferrier S. 2009. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol. Appl. 19, 181–197. ( 10.1890/07-2153.1) [DOI] [PubMed] [Google Scholar]
- 65.VanDerWal J, Shoo LP, Graham C, Williams SE. 2009. Selecting pseudo-absence data for presence-only distribution modeling: how far should you stray from what you know? Ecol. Modell. 220, 589–594. ( 10.1016/j.ecolmodel.2008.11.010) [DOI] [Google Scholar]
- 66.Liu C, Berry PM, Dawson TP, Pearson RG. 2005. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28, 385–393. ( 10.1111/j.0906-7590.2005.03957.x) [DOI] [Google Scholar]
- 67.Freeman EA, Moisen GG. 2008. A comparison of the performance of threshold criteria for binary classification in terms of predicted prevalence and kappa. Ecol. Modell. 217, 48–58. ( 10.1016/j.ecolmodel.2008.05.015) [DOI] [Google Scholar]
- 68.Dudík M, Schapire RE, Phillips SJ. 2005. Correcting sample selection bias in maximum entropy density estimation. In Advances in neural information process systems, pp. 323–330. Cambridge, MA: MIT Press. [Google Scholar]
- 69.Svenning J-C, Fløjgaard C, Marske KA, Nógues-Bravo D, Normand S. 2011. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 30, 2930–2947. ( 10.1016/j.quascirev.2011.06.012) [DOI] [Google Scholar]
- 70.Kearney MR, Simpson SJ, Raubenheimer D, Kooijman SALM. 2013. Balancing heat, water and nutrients under environmental change: a thermodynamic framework. Funct. Ecol. 27, 950–966. ( 10.1111/1365-2435.12020) [DOI] [Google Scholar]
- 71.Kearney MR, Wintle BA, Porter WP. 2010. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 3, 203–213. ( 10.1111/j.1755-263X.2010.00097.x) [DOI] [Google Scholar]
- 72.Peterson AT, Papeş M, Soberón J. 2008. Rethinking receiver operating characteristic analysis. Ecol. Modell. 213, 63–72. ( 10.1016/j.ecolmodel.2007.11.008) [DOI] [Google Scholar]
- 73.Schoener TW. 1968. Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology 49, 704–726. ( 10.2307/1935534) [DOI] [Google Scholar]
- 74.Hof C, Rahbek C, Araújo MB. 2010. Phylogenetic signals in the climatic niches of the world's amphibians. Ecography 33, 242–250. [Google Scholar]
- 75.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296–1297. ( 10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 76.Tunnell JW, Andrews J, Barrera NC, Moretzsohn F. 2010. Encyclopedia of Texas seashells: identification, ecology, distribution and history. College Station, TX: Texas A&M University Press. [Google Scholar]
- 77.Owens HL, et al. 2013. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Modell. 263, 10–18. ( 10.1016/j.ecolmodel.2013.04.011) [DOI] [Google Scholar]
- 78.Bijma J, Pörtner H-O, Yesson C, Rogers AD. 2013. Climate change and the oceans: what does the future hold? Mar. Pollut. Bull. 74, 495–505. ( 10.1016/j.marpolbul.2013.07.022) [DOI] [PubMed] [Google Scholar]
- 79.Zu Ermgassen PSE, et al. 2012. Historical ecology with real numbers: past and present extent and biomass of an imperilled estuarine habitat. Proc. R. Soc. B 279, 3393–3400. ( 10.1098/rspb.2012.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Willis KJ, MacDonald GM. 2011. Long-term ecological records and their relevance to climate change predictions for a warmer world. Annu. Rev. Ecol. Evol. Syst. 42, 267–287. ( 10.1146/annurev-ecolsys-102209-144704) [DOI] [Google Scholar]
- 81.Trakhtenbrot A, Nathan R, Perry G, Richardson DM. 2005. The importance of long-distance dispersal in biodiversity conservation. Divers. Distrib. 11, 173–181. ( 10.1111/j.1366-9516.2005.00156.x) [DOI] [Google Scholar]
- 82.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 83.Hof C, Levinsky I, Araújo MB, Rahbek C. 2011. Rethinking species’ ability to cope with rapid climate change. Glob. Change Biol. 17, 2987–2990. ( 10.1111/j.1365-2486.2011.02418.x) [DOI] [Google Scholar]
- 84.Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482. ( 10.1890/070037) [DOI] [Google Scholar]
- 85.Fordham DA, et al. 2013. Population dynamics can be more important than physiological limits for determining range shifts under climate change. Glob. Change Biol. 19, 3224–3237. ( 10.1111/gcb.12289) [DOI] [PubMed] [Google Scholar]
- 86.IPCC. 2013. Climate change 2013: the physical science basis. Geneva, Switzerland: Intergovernmental Panel on Climate Change Secretariat. [Google Scholar]
- 87.Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. 1998. Making mistakes when predicting shifts in species range in response to global warming. Science 391, 783–786. [DOI] [PubMed] [Google Scholar]
- 88.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Dam JA, Aziz HA, Álvarez Sierra MÁ, Hilgen FJ, van den Hoek Ostende LW, Lourens LJ, Mein P, van der Meulen AJ, Palaez-Campomanes P. 2006. Long-period astronomical forcing of mammal turnover. Nature 443, 687–691. ( 10.1038/nature05163) [DOI] [PubMed] [Google Scholar]
- 90.Peterson AT, Soberón J, Sanchez-Cordero V. 1999. Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267. ( 10.1126/science.285.5431.1265) [DOI] [PubMed] [Google Scholar]
- 91.Kozak KH, Wiens JJ. 2006. Does niche conservatism promote speciation? A case study in North American salamanders. Evolution 60, 2604–2621. ( 10.1111/j.0014-3820.2006.tb01893.x) [DOI] [PubMed] [Google Scholar]
- 92.Soberón J, Nakamura M. 2009. Niches and distributional areas: concepts, methods, and assumptions. Proc. Natl Acad. Sci. USA 106, 19 644–19 650. ( 10.1073/pnas.0901637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The stratigraphic database (dataset S1) and output from niche comparison tests (dataset S2) are available in the electronic supplementary material. Climate and distributional data are available on Dryad, doi:10.5061/dryad.qd08b.