Abstract
Free-ranging common tenrecs, Tenrec ecaudatus, from sub-tropical Madagascar, displayed long-term (nine months) hibernation which lacked any evidence of periodic interbout arousals (IBAs). IBAs are the dominant feature of the mammalian hibernation phenotype and are thought to periodically restore long-term ischaemia damage and/or metabolic imbalances (depletions and accumulations). However, the lack of IBAs in tenrecs suggests no such pathology at hibernation Tbs > 22°C. The long period of tropical hibernation that we report might explain how the ancestral placental mammal survived the global devastation that drove the dinosaurs and many other vertebrates to extinction at the Cretaceous–Palaeogene boundary following a meteorite impact. The genetics and biochemistry of IBAs are of immense interest to biomedical researchers and space exploration scientists, in the latter case, those envisioning a hibernating state in astronauts for deep space travel. Unravelling the physiological thresholds and temperature dependence of IBAs will provide new impetus to these research quests.
Keywords: mammals, hibernation, Cretaceous–Palaeogene boundary, Madagascar, Tenrec ecaudatus
1. Introduction
At the Cretaceous–Palaeogene (K-Pg) boundary about 65.5 million years ago (Ma) the ancestor of the placental mammals (Eutheria) survived the meteorite impact at Chicxulub, Mexico [1], which killed the non-avian dinosaurs and many other animals and plants [2–4]. A small (6–245 g), insectivorous ancestor inherited a tropical Earth, along with a few egg-laying mammals (Montremata), some marsupials (Metatheria) and a few lineages (e.g. Multituberculata) which went extinct during the Cenozoic [2]. Ecological release from the vice grip which the dinosaurs held over Mesozoic mammals drove remarkably rapid evolutionary processes within several hundred thousand years of the extinction event [2]. Four new placental lineages (Xenarthra, Afrotheria, Laurasiatheria and Euarchontoglires) [2] appeared very suddenly, harbouring forms that displayed spectacular new morphological and physiological characteristics [5–8]. The long ca. 160 Myr stint of the nocturnal, small, insectivorous mammal was over, and gave way to the age of the mammals, the Cenozoic. Yet, no placental mammal would exist today had this ancestor not survived the global short- and long-term effects of the Chicxulub impact [3,4] and an Indian Ocean meteorite impact 40 000 years later [9]. How did this placental ancestor, as well as the monotremes and the marsupials, survive when so many other vertebrate groups did not?
There is growing debate about whether the ballistic impact ejecta that re-entered the Earth's atmosphere following the Chicxulub impact could have generated an initial infrared heat pulse sufficient to ignite wildfires globally [10–15]. If global wildfires did occur, they would undoubtedly have been responsible for the extermination of all mammals that could not seek safe refuge from the short- and long-term effects of the fires. Indeed, it is the differential survival of vertebrates following the impact which currently provides one avenue of support for the wildfires notion [3,11]. No large-sized, non-marine reptile, bird or mammal, that is, a vertebrate too large to seek refuge underground, in tree cavities or underwater, survived the K-Pg boundary extinction event [3]. The predominant hypothesis is that small mammals survived both the short- and long-term effects of the impact by hibernating in a safe refuge, typically, underground [3,16].
It has been estimated that the long-term effects of the impact lasted for a year or more [3,4]. However, in extant tropical placental mammals there is no known hibernation phenotype that would allow such long periods of hibernation. Long-term hibernation (more than six months) is typically associated with mammals which inhabit temperate, high-latitude and/or high-altitude, highly seasonal cold environments [17]. For example, year-long hibernation has been reported in captive mountain pygmy possums (a marsupial) [18]. Cold environments appeared in the Late Cenozoic only, and certainly did not exist at the K-Pg boundary [19,20]. Indeed, until relatively recent reports of hibernation in Malagasy primates [21,22] and tenrecs [23,24], and in the echidna (a monotreme) from semi-tropical Queensland, Australia, [25,26], it was thought that hibernation was a physiological capacity limited to high latitude, temperate mammals only [17].
In this study, we report a plesiomorphic (ancestral) capacity for long-term hibernation that exists in an extant, phylogenetically basal, tropical placental mammal, the common tenrec, Tenrec ecaudatus. The Tenrecidae belong to the superorder Afrotheria, which includes elephants, dugongs and manatees, hyraxes, golden moles, elephant-shrews (sengis) and the aardvark [27]. The tenrecs colonized Madagascar from Africa ca. 47 Ma and underwent spectacular adaptive radiation on the island thereafter [28]. We measured the core body temperature (Tb) of free-ranging animals in the dry deciduous forests of western Madagascar. We argue that the capacity for long-term tropical hibernation in basal placental mammals was the key attribute which enabled them to survive the K-Pg extinction event along with presumably heterothermic marsupial [29] and monotreme [25,26,30] ancestors.
2. Material and methods
(a). Study animals
The common tenrec, T. ecaudatus is the largest species (1–2 kg) within Tenrecidae (Afrotheria) on Madagascar and is also one of the largest extant insectivores [31]. It has a very cosmopolitan distribution inhabiting virtually every habitat type on Madagascar, including arable land, pastureland, plantations, rural gardens and urban areas [31,32]. Tenrec ecaudatus is known to hibernate during the austral winter [26,33,34], but supporting data are scarce. Local hunters from Amparafara southeast of Antananarivo have reported anecdotal evidence that males hibernate earlier than females and juveniles [35,36]. Males apparently start hibernating between February and April, whereas females commence as late as May. Both sexes were reported to arouse in October and November which is the mating season [31].
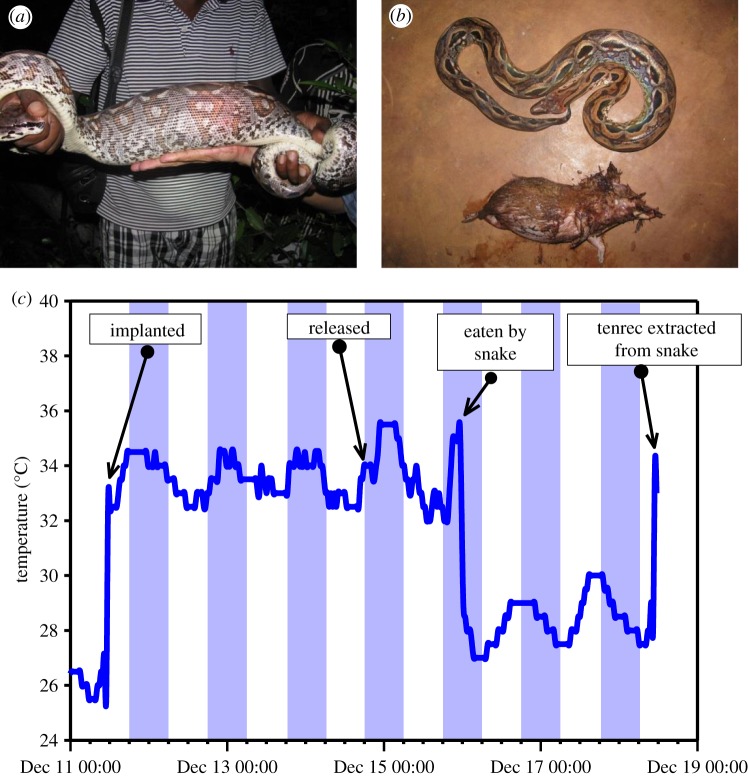
Over two seasons (2010 and 2011) at Ankarafantsika (see ‘Study site’), 22 tenrecs were captured; seven adult males (781–992 g), five sub-adult males (316–377 g), three adult females (661–762 g), two sub-adult females (184 and 413 g) and five sub-adults of undetermined sex (312–467 g). All animals were caught by hand, by walking the established trails in the area at night with local guides. Individuals were located, captured and transported back to the research camp where they were housed in plastic containers lined with paper towels and provided with live insects and tinned sardines. Fifteen animals were surgically implanted with a combination of radio transmitter and body temperature data logger (see ‘Procedures’), and released back into the wild within several days of capture. Animals that were not implanted were either small males (184–374 g) or were individuals in poor condition heavily infected with internal parasites. The fate of the implanted and released tenrecs varied; three were killed by feral dogs, two by unknown natural predators, three by Malagasy boas (figure 1), three disappeared from the study area either through poaching or natural movement, one hibernated for eight months in the same site, emerged and then left the study area, and three were excavated from their hibernacula after nine months of austral winter hibernation (see ‘Results’). These data confirm the high rates of mortality at Ankarafantsika by feral dogs, snakes, natural predators and poachers [35], as reported also for greater hedgehog tenrecs, Setifer setosus [24].
Figure 1.
An illustration of predation of common tenrecs by snakes, one cause of high mortality at the Ankarafantsika site. (a) A captured Malagasy ground boa (Acrantophis madagascariensis) 2 days after swallowing an adult male tenrec. (b) The tenrec that was extracted from the live boa. (c) The body temperatures of the tenrec and the dead tenrec within the snake, captured by an iButton transmitter–logger package that was implanted into the tenrec. (Online version in colour.)
(b). Study site
The study was conducted over two rainy seasons, from November 2009 to November 2011 in the Jardin Botanique A research area adjacent to the Ampijoroa Forestry Station in Ankarafantiska National Park (16°19′ S, 46°48′ E), Madagascar. The study site has been described by Levesque et al. [37]. Detailed ambient temperature (Ta) data were recorded at various locations throughout the study site using DS1922L Thermochron iButtons (Dallas Semiconductor, Dallas, TX, USA) programmed to record once every 30 min at a resolution of 0.0625°C. DS1922L iButtons are inexpensive data loggers that measure and record temperature at user-defined rates which are being used increasingly in physiological and ecological research [38]. iButtons are encased in a stainless steel cap and communicate with a host computing device through a serial 1-Wire protocol. Ambient temperatures were measured by placing iButtons in black bodies (plastic cylinders spray-painted matt black) in Stevenson screens 1 m from the ground in shaded areas. Soil temperatures were recorded by burying iButtons at 0, 250 and 500 mm at various sites in the forest depending upon where animals were suspected of hibernating.
(c). Procedures
The animals were implanted with a combination of radio transmitter and DS1922L Thermochron iButtons. Two iButtons were encapsulated in surgical wax (Paramat Extra-Merck KGaA, Darmstadt, Germany) together with a modified 2-stage collar transmitter (Merlin Systems Inc., Boise, ID, USA). The modification involved the removal of the collar and the replacement of the whip aerial with a coiled aerial. The resulting transmitter–logger packages had a total mass of around 13.0 g (mean 13.0 g, range 11.7–13.5 g). The iButtons were programmed to record body temperature at 30 or 36 min intervals with an accuracy of 0.5°C. One iButton was programmed with a delayed measurement onset that coincided with the memory limit of the other iButton. All iButtons were calibrated against a mercury thermometer prior to implantation as well as post-recovery to the nearest 0.1°C. No drift was observed.
During anaesthesia, oxygen and vapourized anaesthetic (isoflurane) were delivered to the animal through a mask at a rate of 700 ml min−1. Anaesthesia was induced at 1–2% isoflurane and maintained at 0.5%. The transmitter–logger package was inserted via an incision in the peritoneal cavity which was then sutured using 3/0 catgut and sealed with Vetbond tissue adhesive (3M, London, Ontario, Canada). An intramuscular injection of antibiotics (1 ml/10 g of Duplocillin) was administered to prevent post-operative infection. The animals were kept for 1 day for post-surgery observations and were released at the site of capture. The locations of the animals were recorded frequently, usually daily, using either a R-1000 Telemetry Receiver (Communications Specialists, Orange, CA, USA) or a IC-R10 Communications Receiver (ICOM, Tokyo, Japan), connected to an RA-23K ‘H’ antenna (Telonics, Mesa, AZ, USA) or a standard 150-MHz Yagi antenna (manufactured by Cliff Dearden, Pietermaritzburg, KZN, South Africa) and a 150-MHz power booster (Merlin Systems Inc).
3. Results
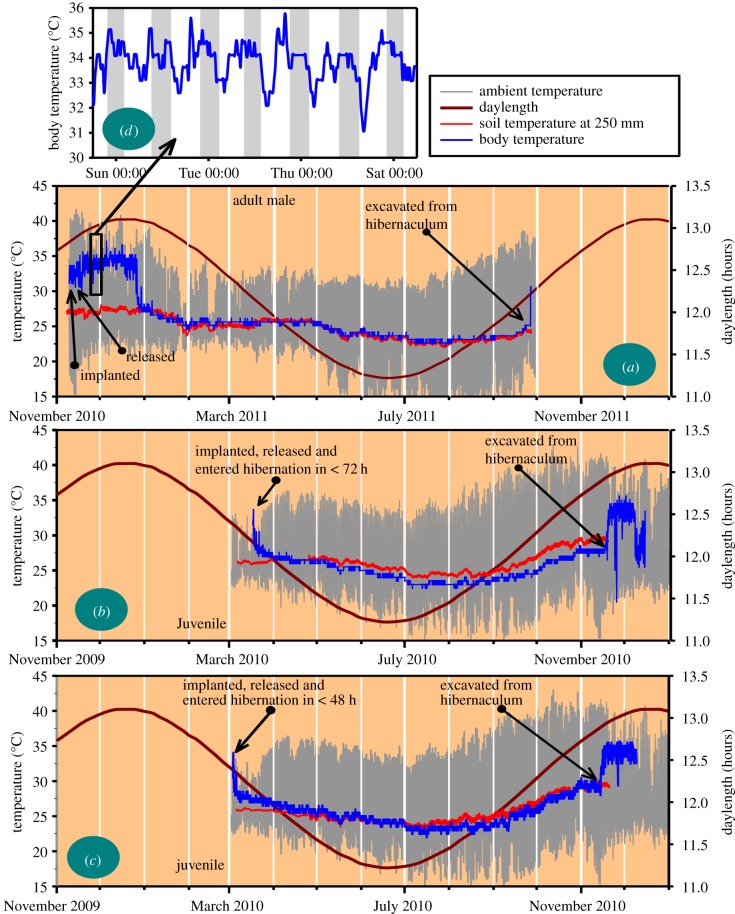
Continuous measures of Tb spanning up to nine months were obtained from three animals, two sub-adults of undetermined sex (377 and 413 g) in 2010, and one adult male (781 g) in 2011. After implantation and release, these three tenrecs avoided predation by burrowing into the sandy soil at the site at Ankarafantsika National Park. They remained in the same position until they were excavated after the transmitter signals began to weaken. We decided to excavate the animals to avoid losing the animals (and the body temperature (Tb) data) through failed signal transmissions, and to avoid a repetition of the fate of one animal that was located daily for eight months in the same location, but which then aroused from hibernation and left the study area.
The animals were located at a depth between 250 and 500 mm in sandy soil. No discernable hibernaculum cavity or tunnel could be found during excavation. All three tenrecs were found to be torpid, and aroused fully within 1 h (figure 2).
Figure 2.
Hibernation bouts of eight to nine months in three common tenrecs (Tenrec ecaudatus) measured in the dry deciduous forest of western Madagascar. Note the complete lack of IBAs. The measures of soil temperature in (a) were obtained less than 3 m from the hibernaculum, whereas those in (b,c) were measured about 200 m from the hibernaculum, accounting for the lack of precise fit of the soil temperatures to the corresponding Tbs in (b,c). The torpor bouts in (b,c) in the period following excavation were induced in the laboratory when the animals were exposed to an ambient temperature of 18°C. The data in (d) illustrate the daily rhythms of Tb in a normothermic adult male tenrec prior to entry into hibernation. (Online version in colour.)
The unique and most salient characteristic of the hibernation bouts which all three animals displayed was that they did not arouse once in a continuous period of hibernation lasting for eight to nine months. The Tb during hibernation never decreased below 22°C (figures 2 and 3). The hibernation Tb tracked soil temperature which also did not decrease below 22°C throughout the austral winter (figures 2 and 3). Unfortunately, we do not know how long the hibernation bouts might have lasted had we not excavated the animals.
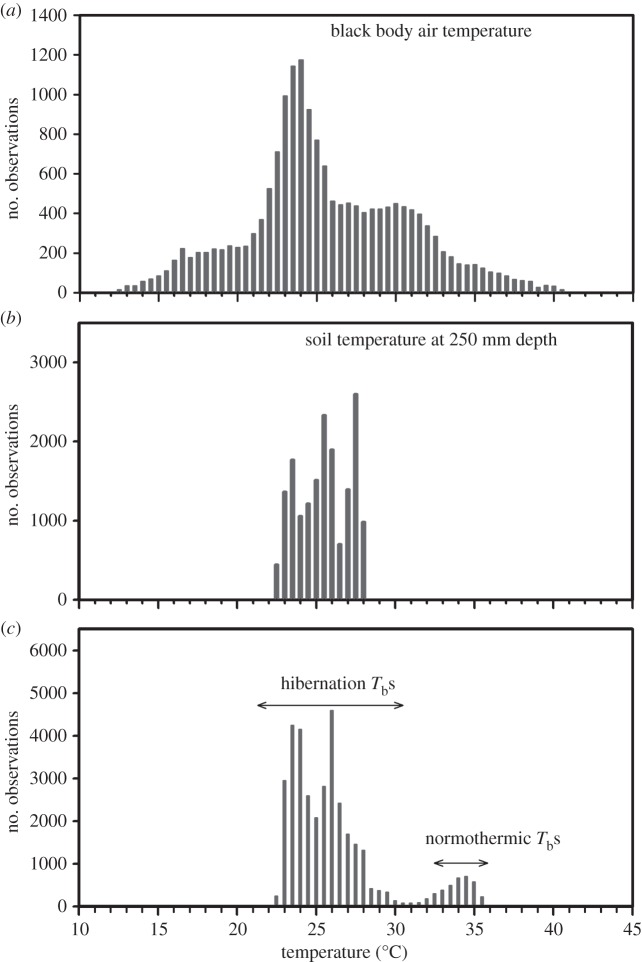
Figure 3.
Frequency distributions of black body air temperatures (a), soil temperatures at a depth of 250 mm (b) and body temperatures (c) of an adult male tenrecs (data from figure 2a). Note that, throughout the austral winter, neither the soil nor the body temperature decreased below 22°C. (Online version in colour.)
4. Discussion
The lack of interbout arousals (IBAs) in tenrecs is particularly noteworthy because all temperate hibernators arouse periodically to normal Tbs for 12–24 h and then re-enter hibernation [39,40]. The mammalian hibernation phenotype sustains cardiovascular, biochemical and neuronal functions at low hibernation Tbs that are not expressed in non-hibernators [41–43]. For example, whereas the heart continues to function at a dramatically reduced rate during hibernation, in non-hibernators the onset of arrhythmia occurs at 32°C, atrial fibrillation at 30°C and cardiac arrest at 15°C [41]. These hypothermic influences do not occur in hibernators. In the most extreme case, Arctic ground squirrels (Urocitellus parryii) maintain Tb at −2°C to −3°C during three-week hibernation bouts [44].
There is much debate surrounding the fitness benefits of IBAs in temperate hibernators given the very high energetic costs that are involved in arousals [40,41,45]. IBAs may be associated with the restoration of neuronal function and metabolic imbalances (depletion and accumulation of metabolites) as well as gluconeogenesis [41,45–47]. It is becoming increasingly apparent that hibernation is a plesiomorphic trait in mammals [7,25,48] (although see [49]) so IBAs could be a derived trait in the temperate hibernation phenotype which evolved following the onset of global cooling ca. 50 Ma [7]. However, lesser hedgehog tenrecs (Echinops telfairi) display IBAs when held at a constantly low temperature in the laboratory [50,51], and early laboratory data suggest that T. ecaudataus can self-arouse from hibernation at Tbs below 20°C [52]. IBAs cannot be discerned, though, from data from free-ranging E. telfairi or S. setosus during the austral winter [23,53]. The lack of IBAs that we report here may therefore represent a temperature-dependent response and may not necessarily constitute a characteristic of the plesiomorphic hibernation phenotype.
The lack of IBAs in free-ranging T. ecaudatus may point to a critical threshold Tb only below which accumulated metabolic imbalances are sufficient to necessitate periodic restorative euthermy. Data from free-ranging Cheirogaleus dwarf lemurs from Madagascar illustrate and support this threshold concept well [54]. During hibernation, dwarf lemurs lacked IBAs when Tb passively tracked large daily fluctuations in hibernaculum temperatures (daily Tb minima 15.5–19.3°C, maxima 28.5–31.2°C). However, in well-insulated hibernacula, IBAs did occur because daily minima were 18.4–20.3°C but maxima only 23.1–25.0°C, i.e. never exceeding 30°C. These data suggest that, in poorly insulated hibernacula, daily increases in Tb to approximately 30°C through passive heating may be sufficient to restore accumulated metabolic imbalances that occured when daily Tb decreased below 20°C, whereas the Tb maxima in well-insulated hibernacula were too low for restorative metabolic homeostasis.
The identification of putative Tb thresholds for metabolic imbalance, as well as the minimum Tb threshold for restorative metabolic homeostasis, has important implications for biomedical research and future space travel. It should provide new impetus in understanding the limits of induced metabolic suppression and hypothermia for prolonged medical treatments, such as trauma, strokes, asphyxiated neonates, or for hibernation induction in astronauts.
It has been predicted that manned space flights to Mars will occur within 20–30 years and that the astronauts will travel in a hibernating state for the six to nine months that it will take to get there [55–57]. This prediction assumes, of course, that ischaemic damage, for example to the heart, will be avoided at Tbs that currently compromise function. Our tenrec data suggest that periodic arousals from hibernation will not be necessary if the Tb of hibernating astronauts is maintained above a certain threshold Tb which, in the case of tenrecs, was approximately 22°C. Reductions of Tb below this threshold, though, are likely to induce metabolic imbalances and ischaemic damage. Pinpointing the lowest safe limits of a human hibernating Tb is important because it will have profound logistical and cost implications for deep space travel.
Hibernation lowers the risk of extinction [58], increases winter survival rates [59], and facilitates reproductive and life-history flexibility [24,60]. Indeed, T. ecaudatus display some of the most unique mammalian life-history traits, such as the largest litter sizes (as many as 30), of all mammals [61]. Our study also revealed an exceptionally high mortality rate during the non-hibernating season at the site from predation [24]. Thus, selective factors involved in long-term tropical hibernation seem to be different from those which drove derived seasonal hibernation in temperate hibernators. Our hibernation data show some affinities to the ‘protoendothermy’ first noted in echidnas [25,26], suggesting retention of plesiomorphic characteristics of hibernation on Madagascar through phylogenetic inertia.
In tropical hibernators, there is arguably no fitness benefit to remaining active above ground and exposed to predators when not directly involved in reproduction [25]. Thus, we support the argument that, in addition to the seasonal reduction in food availability and the prohibitive costs of normothermy which make hibernation essential in temperate hibernators, the minimization of predation risk and hence the increase in longevity also confers huge fitness benefits to tropical hibernators. Predation-avoidance hibernation may be an ancient plesiomorphic characteristic in mammals and is a legacy, perhaps, of the 163 Myr of ecological suppression by the dinosaurs. It enabled the ancestral placentals, as well as the marsupials and monotremes also known to hibernate [30,39], to endure the short- and long-term devastations of the K-Pg asteroid impact, a capacity which is possibly the sole explanation for the existence of mammals today. Although the lack of IBAs would undoubtedly have conserved energy during sustained hibernation, they probably represent a temperature-dependent physiological response which was not necessarily a critical component of the plesiomorphic hibernation phenotype.
Supplementary Material
Acknowledgements
We are grateful to Dr Akira Mori, Kyoto University, for extracting the tenrec from the snake without harm to the snake. We also thank the following individuals and institutions for their assistance and logistical support: the local guides (Tosy, Alpha and Ndrema), Dr Cindy Canale, Dr Belinda Rose (MRCVS), Mark Downey, the Malagasy National Parks, the Départment de Biologie Animale of the University of Antananarivo, the Malagasy Institute for the Conservation of Tropical Environments and Cliff Dearden. We are grateful to two anonymous reviewers for their encouraging, positive suggestions.
Ethics statement
All procedures involving the use of animals were approved by Madagascar National Parks (Permit 218/09/MEF/SG/DGF/DCB.SAP/SLRSE and 158/10/MEF/SG/DGF/DCB.SAP/SCBSE) and comply with all national Malagasy laws.
Data accessibility
All data reported in this study can be accessed from the electronic supplementary file.
Funding statement
This research was financed by a UKZN incentive grant, an NRF competitive grant, and an NRF incentive grant to B.G.L., and a National Science and Engineering Research Council (Canada) postgraduate scholarship to D.L.L. We greatly appreciated an equipment donation for the acquisition of GPS devices from Idea Wild.
References
- 1.Hildebrand AR, Penfield GT, Kring DA, Pilkington M, Camargo A, Jacobsen SB, Boynton WV. 1991. Chicxulub Crater: a possible Cretaceous Tertiary boundary impact crater on the Yucatan Peninsula, Mexico. Geology 19, 867–871. ( 10.1130/0091-7613) [DOI] [Google Scholar]
- 2.O'Leary MA, et al. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339, 662–667. ( 10.1126/science.1229237) [DOI] [PubMed] [Google Scholar]
- 3.Robertson DS, McKenna MC, Toon OB, Hope S, Lillegraven JA. 2004. Survival in the first hours of the Cenozoic. Geol. Soc. Am. Bull. 116, 760–768. ( 10.1130/b25402.1) [DOI] [Google Scholar]
- 4.Schulte P, et al. 2010. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 327, 1214–1218. ( 10.1126/science.1177265) [DOI] [PubMed] [Google Scholar]
- 5.Lovegrove BG, Mowoe MO. 2014. The evolution of micro-cursoriality in mammals. J. Exp. Biol. 217, 1316–1325. ( 10.1242/jeb.095737) [DOI] [PubMed] [Google Scholar]
- 6.Lovegrove BG, Mowoe MO. 2013. The evolution of mammal body sizes: responses to Cenozoic climate change in North American mammals. J. Evol. Biol. 26, 1317–1329. ( 10.1111/jeb.12138) [DOI] [PubMed] [Google Scholar]
- 7.Lovegrove BG. 2012. The evolution of endothermy in Cenozoic mammals: a plesiomorphic–apomorphic continuum. Biol. Rev. 87, 128–162. ( 10.1111/j.1469-185X.2011.00188.x) [DOI] [PubMed] [Google Scholar]
- 8.Lovegrove BG. 2012. The evolution of mammalian body temperature: the Cenozoic supraendothermic pulses. J. Comp. Physiol. B 182, 579–589. ( 10.1007/s00360-011-0642-7) [DOI] [PubMed] [Google Scholar]
- 9.Lerbekmo JF. 2014. The Chicxulub-Shiva extraterrestrial one-two killer punches to Earth 65 million years ago. Mar. Pet. Geol. 49, 203–207. ( 10.1016/j.marpetgeo.2013.05.014) [DOI] [Google Scholar]
- 10.Morgan J, Artemieva N, Goldin T. 2013. Revisiting wildfires at the K-Pg boundary. J. Geophys. Res. Biogeosci. 118, 1508–1520. ( 10.1002/2013jg002428) [DOI] [Google Scholar]
- 11.Robertson DS, Lewis WM, Sheehan PM, Toon OB. 2013. K-Pg extinction: reevaluation of the heat-fire hypothesis. J. Geophys. Res. Biogeosci. 118, 329–336. ( 10.1002/jgrg.20018) [DOI] [Google Scholar]
- 12.Adair RK. 2010. Wildfires and animal extinctions at the Cretaceous/Tertiary boundary. Am. J. Phys. 78, 567–573. ( 10.1119/1.3192770) [DOI] [Google Scholar]
- 13.Goldin TJ, Melosh HJ. 2009. Self-shielding of thermal radiation by Chicxulub impact ejecta: firestorm or fizzle? Geology 37, 1135–1138. ( 10.1130/g30433a.1) [DOI] [Google Scholar]
- 14.Harvey MC, Brassell SC, Belcher CM, Montanari A. 2008. Combustion of fossil organic matter at the Cretaceous-Paleogene (K-P) boundary. Geology 36, 355–358. ( 10.1130/g24646a.1). [DOI] [Google Scholar]
- 15.Kring DA, Durda DD. 2002. Trajectories and distribution of material ejected from the Chicxulub impact crater: implications for postimpact wildfires. J. Geophys. Res. Planets 107, 22 ( 10.1029/2001je001532) [DOI] [Google Scholar]
- 16.Kikuchi R, Vanneste M. 2010. A theoretical exercise in the modeling of ground-level ozone resulting from the K-T asteroid impact: its possible link with the extinction selectivity of terrestrial vertebrates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 288, 14–23. ( 10.1016/j.palaeo.2010.01.027) [DOI] [Google Scholar]
- 17.Geiser F. 2013. Hibernation. Curr. Biol. 23, R188–R193. ( 10.1016/j.cub.2013.01.062) [DOI] [PubMed] [Google Scholar]
- 18.Geiser F. 2007. Yearlong hibernation in a marsupial mammal. Naturwissenschaften 94, 941–944. ( 10.1007/s00114-007-0274-7) [DOI] [PubMed] [Google Scholar]
- 19.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 20.Janis CM. 1993. Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annu. Rev. Ecol. Syst. 24, 467–500. ( 10.1146/annurev.es.24.110193.002343) [DOI] [Google Scholar]
- 21.Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. 2005. Hibernation in the tropics: lessons from a primate. J. Comp. Physiol. B 175, 147–155. ( 10.1007/s00360-004-0470-0) [DOI] [PubMed] [Google Scholar]
- 22.Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. 2004. Hibernation in a tropical primate. Nature 429, 825–826. ( 10.1038/429825a) [DOI] [PubMed] [Google Scholar]
- 23.Lovegrove BG, Canale C, Levesque D, Fluch G, Řeháková-Petrů M, Ruf T. 2014. Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiol. Biochem. Zool. 87, 30–45. ( 10.1086/673313) [DOI] [PubMed] [Google Scholar]
- 24.Levesque DL, Lovasoa OMA, Rakotoharimalala SN, Lovegrove BG. 2013. High mortality and annual fecundity in a free-ranging basal placental mammal, Setifer setosus (Tenrecidae: Afrosoricida). J. Zool. Lond. 291, 205–212. ( 10.1111/jzo.12063) [DOI] [Google Scholar]
- 25.Grigg G, Beard L. 2000. Hibernation by echidnas in mild climates: hints about the evolution of endothermy? In Life in the cold (eds Heldmaier G, Klingenspor M.), pp. 5–19. Berlin, Germany: Springer. [Google Scholar]
- 26.Grigg GC, Beard LA, Augee ML. 2004. The evolution of endothermy and its diversity in mammals and birds. Physiol. Biochem. Zool. 77, 982–997. ( 10.1086/425188) [DOI] [PubMed] [Google Scholar]
- 27.Springer MS, Cleven GC, Madsen O, de Jong WW, Waddell VG, Amrine HM, Stanhope MJ. 1997. Endemic African mammals shake the phylogenetic tree. Nature 388, 61–64. ( 10.1038/40386) [DOI] [PubMed] [Google Scholar]
- 28.Poux C, Madsen O, Glos J, de Jong WW, Vences M. 2008. Molecular phylogeny and divergence times of Malagasy tenrecs: influence of data partitioning and taxon sampling on dating analyses. BMC Evol. Biol. 8, 102, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geiser F. 1994. Hibernation and daily torpor in marsupials: a review. Aust. J. Zool. 42, 1–16. ( 10.1071/ZO9940001) [DOI] [Google Scholar]
- 30.Nicol SC, Morrow G, Andersen NA. 2008. Hibernation in monotremes: a review. In Hypometabolism in animals: hibernation, torpor and cryobiology (eds Lovegrove BG, McKechnie AE.), p. 424 Pietermaritzburg, South Africa: University of KwaZulu-Natal. [Google Scholar]
- 31.Garbutt N. 2007. Mammals of Madagascar. London, UK: A & C Black. [Google Scholar]
- 32.Eisenberg JF, Gould E. 1969. The tenrecs: a study in mammalian behavior and evolution Smithson. Contrib. Zool. 27, 1–156. [Google Scholar]
- 33.Nicoll ME. 1986. Diel variation in body temperature in Tenrec ecaudatus during seasonal hypothermia. J. Mamm. 67, 759–762. ( 10.2307/1381143) [DOI] [Google Scholar]
- 34.Nicoll ME. 1985. Respones to Seychelles tropical forest seasons by a litter-foraging mammalina insectivore, Tenrec ecaudatus, native to Madagascar. J. Anim. Ecol. 54, 71–88. ( 10.2307/4621) [DOI] [Google Scholar]
- 35.Randrianjafy V. 2003. Contribution à l’étude de la biologie de conservation de la communauté micromammalienne d'Ankarafantsika. PhD thesis, University of Antananarivo, Antananarivo, Madagascar. [Google Scholar]
- 36.Andriatsarafara FR. 1981. Quelques observation sur l'ontogenie et le comportement de Tenrec ecaudatus (Schreber 1777) en captivité: comparaisons avec les données connus chez d'autres insectivores. Diplôme d’Études Approfondies de Sciences, Université d'Antananarivo, Antananarivo. [Google Scholar]
- 37.Levesque DL, Rakotondravony D, Lovegrove BG. 2012. Home range and shelter site selection in the greater hedgehog tenrec in the dry deciduous forest of Western Madagascar. J. Zool. 287, 161–168. ( 10.1111/j.1469-7998.2012.00899.x) [DOI] [Google Scholar]
- 38.Lovegrove BG. 2009. Modification and miniaturization of Thermochron iButtons for surgical implantation into small animals. J. Comp. Physiol. B 179, 451–458. ( 10.1007/s00360-008-0329-x) [DOI] [PubMed] [Google Scholar]
- 39.Geiser F, Ruf T. 1995. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol. Zool. 68, 935–966. [Google Scholar]
- 40.Geiser F, Kenagy GJ. 1988. Torpor duration in relation to temperature and metabolism in hibernating ground squirrels. Physiol. Zool. 61, 442–449. [Google Scholar]
- 41.Carey HV, Andrews MT, Martin SL. 2003. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153–1181. ( 10.1152/physrev.00008.2003) [DOI] [PubMed] [Google Scholar]
- 42.Hochachka PW. 1986. Defense strategies against hypoxia and hypothermia. Science 231, 234–241. ( 10.1126/science.2417316) [DOI] [PubMed] [Google Scholar]
- 43.Storey KB, Storey JM. 1990. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q. Rev. Biol. 65, 145–174. ( 10.1086/416717) [DOI] [PubMed] [Google Scholar]
- 44.Barnes B. 1989. Freeze avoidance in a mammal: body temperatures below 0°C in an Arctic hibernator. Science 244, 1593–1595. ( 10.1126/science.2740905) [DOI] [PubMed] [Google Scholar]
- 45.Jinka TR, Rasley BT, Drew KL. 2012. Inhibition of NMDA-type glutamate receptors induces arousal from torpor in hibernating arctic ground squirrels (Urocitellus parryii). J. Neurochem. 122, 934–940. ( 10.1111/j.1471-4159.2012.07832.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epperson LE, Karimpour-Fard A, Hunter LE, Martin SL. 2011. Metabolic cycles in a circannual hibernator. Physiol. Genomics 43, 799–807. ( 10.1152/physiolgenomics.00028.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hampton M, Melvin RG, Andrews MT. 2013. Transcriptomic analysis of brown adipose tissue across the physiological extremes of natural hibernation. PLoS ONE 8, 12 ( 10.1371/journal.pone.0085157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovegrove BG. 2012. A single origin of heterothermy in mammals. In Living in a seasonal world: thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 3–11. Berlin, Germany: Springer. [Google Scholar]
- 49.Geiser F. 2008. Ontogeny and phylogeny of endothermy and torpor in mammals and birds. Comp. Biochem. Physiol. A 150, 176–180. ( 10.1016/j.cbpa.2007.02.041) [DOI] [PubMed] [Google Scholar]
- 50.Wein J. 2010. Effects of ambient temperature on tropical hibernation in the lesser hedgehog tenrec, Echinops telfairi. Hamburg, Germany: University of Hamburg. [Google Scholar]
- 51.Oelkrug R, et al. 2013. Brown fat in a protoendothermic mammal fuels eutherian evolution. Nature 4, Article number 2140 ( 10.1038/ncomms3140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kayser C. 1961. The physiology of natural hibernation. New York, NY: Pergamon Press. [Google Scholar]
- 53.Lovegrove BG, Génin F. 2008. Torpor and hibernation in a basal placental mammal, the lesser hedgehog tenrec Echinops telfairi. J. Comp. Physiol. B 178, 691–698. ( 10.1007/s00360-008-0257-9) [DOI] [PubMed] [Google Scholar]
- 54.Dausmann KH, Glos J, Heldmaier G. 2009. Energetics of tropical hibernation. J. Comp. Physiol. B 179, 345–357. ( 10.1007/s00360-008-0318-0) [DOI] [PubMed] [Google Scholar]
- 55.Singer D. 2006. Human hibernation for space flight: utopistic vision or realistic possibility? J. Br. Interplanet Soc. 59, 139. [Google Scholar]
- 56.Ayre M, Zancanaro C, Malatesta M. 2004. Morpheus—hypometabolic stasis in humans for long term space flight. J. Br. Interplanet Soc. 57, 325–339. [Google Scholar]
- 57.Rossini L, Seidl T, Izzo D, Summerer L. 2006. Beyond astronaut's capabilities: a critical review. In 58th International Astronautical Congress, Hyderabad, paper IAC-07-A5.2.04. American Institute of Aeronautics and Astronautics. [Google Scholar]
- 58.Geiser F, Turbill C. 2009. Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96, 1235–1240. ( 10.1007/s00114-009-0583-0) [DOI] [PubMed] [Google Scholar]
- 59.Turbill C, Bieber C, Ruf T. 2011. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B 278, 3355–3363. ( 10.1098/rspb.2011.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bieber C, Juskaitis R, Turbill C, Ruf T. 2012. High survival during hibernation affects onset and timing of reproduction. Oecology 169, 155–166. ( 10.1007/s00442-011-2194-7) [DOI] [PubMed] [Google Scholar]
- 61.Eisenberg JF. 1981. The mammalian radiations: an analysis of trends in evolution, adaptation, and behavior. London, UK: Athlone Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this study can be accessed from the electronic supplementary file.