Abstract
Inbreeding can profoundly affect the interactions of plants with herbivores as well as with the natural enemies of the herbivores. We studied how plant inbreeding affects herbivore oviposition preference, and whether inbreeding of both plants and herbivores alters the probability of predation or parasitism of herbivore eggs. In a laboratory preference test with the specialist herbivore moth Abrostola asclepiadis and inbred and outbred Vincetoxicum hirundinaria plants, we discovered that herbivores preferred to oviposit on outbred plants. A field experiment with inbred and outbred plants that bore inbred or outbred herbivore eggs revealed that the eggs of the outbred herbivores were more likely to be lost by predation, parasitism or plant hypersensitive responses than inbred eggs. This difference did not lead to differences in the realized fecundity as the number of hatched larvae did not differ between inbred and outbred herbivores. Thus, the strength of inbreeding depression in herbivores decreases when their natural enemies are involved. Plant inbreeding did not alter the attraction of natural enemies of the eggs. We conclude that inbreeding can significantly alter the interactions of plants and herbivores at different life-history stages, and that some of these alterations are mediated by the natural enemies of the herbivores.
Keywords: Abrostola asclepiadis, experimental inbreeding, insect herbivory, natural enemies, oviposition, Vincetoxicum hirundinaria
1. Introduction
Inbreeding resulting from self-fertilization or mating between related individuals causes inbreeding depression, i.e. reduction in performance owing to inbreeding [1,2]. Inbreeding of both plants and herbivores can considerably alter interactions between them [3]. In plants, inbreeding modifies many resistance traits such as concentrations of secondary compounds [4,5], structural defences [6] and emissions of volatile compounds [7]. Consequently, inbreeding depression in plants is stronger under herbivory, and inbred plant individuals commonly experience greater herbivore damage compared with outbred individuals [3,8–10]. In herbivores, inbreeding reduces for example performance, fecundity and egg hatching rate [5,11]. The strength of inbreeding depression in the performance of herbivore larvae depends on the inbreeding of the host plant, and similarly, herbivore inbreeding can modify the strength of inbreeding depression in host plant resistance [5].
Plants and herbivorous insects interact in many ways prior to the life-history stage when the insect feeds on the plants. The traits that are central in the non-feeding life-history stages of the interaction, such as the eggs and in some species the adults, can be equally important for the antagonistic interaction as those in the feeding stage [12]. These traits can also be modified by inbreeding [13], and thereby further complicate the effects of inbreeding on plant–herbivore interactions. Plant quality and chemical cues direct the oviposition of adult insects [14–16] and they can alter plant apparency or attractiveness to the herbivore and lead to avoidance of egg deposition [17]. Theory predicts that female insects deposit their eggs on a site that maximizes the performance of the offspring [18–20], although this does not always hold true in practice [21–23]. Accordingly, inbred plants, which were of higher quality to the larvae, were favoured over outbred plants for oviposition by the adult hawk moths (Manduca sexta) [13]. Once the eggs have been laid, plants can react against the eggs directly by hypersensitive and toxic responses and indirectly by releasing volatile chemical compounds or inducing changes in leaf surface chemicals that attract natural enemies of the insect eggs [17,24,25]. Because plant inbreeding can modify emission of volatile compounds, it can also alter the interactions of plants with the natural enemies of herbivores [7,26]. So far, these studies have focused on the effects of inbreeding only in the host plant. In order to fully understand how inbreeding affects species and their interactions with one another, we should broaden the scope and investigate how simultaneous inbreeding in plants and herbivores affects interactions with the third trophic level, i.e. the natural enemies of the herbivore, and how these natural enemies alter the effects of inbreeding in plants and herbivores.
We studied whether inbreeding of the host plant Vincetoxicum hirundinaria affects the oviposition preference of the specialist herbivore Abrostola asclepiadis and whether inbreeding of either the host plant or the herbivore affects the survival of the herbivore eggs to larval stage. We know that A. asclepiadis and V. hirundinaria suffer from inbreeding depression in larval performance and resistance, respectively [5,9]. Here, we turn our focus to the effects of inbreeding on different life-history stages of the herbivore (adult, egg) and additional plant traits (apparency, indirect defences) in order to more thoroughly understand the effects of inbreeding at various stages of the plant–herbivore interaction. We specifically studied: (i) Does plant inbreeding affect oviposition preference? (ii) Does plant or herbivore inbreeding affect the rate of egg parasitism? (iii) Does plant or herbivore inbreeding affect the rate of eggs lost to predation by other insects, or to plant hypersensitive responses?
We predict that inbred plants are favoured over outbred plants by herbivore females for oviposition because they are less toxic and better-quality food for the developing larvae [5,27]. We also predict that outbred plants attract parasitoids and predators more efficiently because they are suspected to emit more volatile compounds than the inbred plants [7,13]. Lastly, we expect that parasitoids and predators favour outbred herbivore eggs because they are of higher quality than inbred eggs.
2. Material and methods
(a). Study species
Vincetoxicum hirundinaria Med. (=Cynanchum vincetoxicum (L.) Pers.) (Apocynaceae) is a perennial plant native to Europe and western Asia. It grows on calcareous substrate in rocky open habitats and along forest margins. It has a mixed mating system and in the study area, the SW archipelago of Finland, populations vary in their level of inbreeding measured as FIS [28].
The leaves of V. hirundinaria are consumed by the larvae of a specialist moth A. asclepiadis Schiff. (Noctuidae). The adult female oviposits single eggs, and egg clutches containing up to 20 eggs under the leaves of V. hirundinaria at the end of June and beginning of July [29]. The larvae hatch approximately 10 days later and complete development after five instars in approximately five to six weeks. The larvae have significant negative effects on the fitness and population growth of their host plants [30], and during local outbreaks, they defoliate the majority of plants in individual populations of V. hirundinaria (A.M. 2007, personal observation). During the past decade, we have observed strong fluctuations in population sizes of the herbivores based on estimates of damage in the plants (from close to 0–60% of leaf damage within populations). Therefore, the level of inbreeding likely varies among years and populations, being most common when population sizes are small. V. hirundinaria is considered to be toxic to mammals and generalist insect herbivores because of its high concentrations of secondary metabolite compounds [27]. In the study area, the eggs of A. aspclepiadis are commonly parasitized by egg parasitoids from the genera Telenomus sp. and Trichogramma sp. [29]. The percentage of parasitized eggs in the field ranges from 2% to 68% among populations (A.K. 2011, unpublished data). In addition to parasitoids, the eggs are predated by ants (Formicidae), spiders (Aranae) and larvae of net-winged insects (Neuroptera; A.K. personal observation).
(b). Plant and herbivore material
We obtained inbred and outbred plants by hand pollinating 10 maternal plants from one population in 2007 (Naantali, 60°27′56′′ N, 22°01′10′′ E). In each individual plant, we self-pollinated five flowers with pollen from a different flower of the same plant and cross-pollinated five flowers with pollen from another randomly chosen individual to obtain inbred and outbred plants, respectively. We allowed the seeds to germinate in the greenhouse in the following year (2008) and, once the seedlings started growing, they were transplanted into larger pots (0.9 l) with standard potting soil (Kekkilä) in 2009 (for a more detailed description of the pollination procedure, see [9]). For the experiment, we chose six inbred and six outbred seedlings that were of similar size from each of the 10 maternal families.
To obtain herbivores for the experiment, we collected eggs of A. asclepiadis from six populations in 2012. These populations were all allopatric to the plant population used here, because we wanted to avoid any potential confounding effects of local adaptation of plants and herbivores to one another. We reared the larvae hatching from the eggs individually in plastic vials until pupation. The pupae overwintered at +6°C until June of 2013, when we moved them into room temperature (+21°C) to emerge. Once the adults started to emerge at the end of June, we immediately transferred them to the experiment. We conducted the two successive experiments in Lammasluoto (60°14′03′′ N, 21°56′49′′ E), a site that harbours a large V. hirundinaria population and is allopatric to all herbivores and plants used in this study.
(c). Oviposition preference
To study the preference of the herbivores in their oviposition, we set up full-sib (n = 21) and random within-population (n = 21) herbivore pairs by crossing a male and a female from the same egg clutch (adults from the same egg clutch were considered to be siblings) or two random individuals from different egg clutches from the same population, respectively. We established these two types of pairs in order to obtain inbred and outbred eggs for the second experiment (see below). We placed the herbivores in a cylindrical mesh cage (base diameter 35 cm, height 43 cm) with one inbred and one outbred V. hirundinaria plant that originated from the same maternal family. The two plants within each cage did not differ in size (paired t-test: number of shoots, t-value = −0.61, p = 0.5439, d.f. = 41; height, t-value = −1.11, p = 0.2713, d.f. = 41; number of leaves in the tallest shoot, t-value = −1.41, p = 0.1141, d.f. = 41). The plants lay on opposite sides of the spherical cage, leaving approximately 23 cm between the plants. We released one pair (see above) of A. asclepiadis in each cage where they were allowed to mate and oviposit without restriction. We recorded the number of eggs on the plants daily and once one of the plants acquired more than 10 eggs we terminated the preference test (on average after 3–4 days) by removing that plant from the pairing cage. To obtain similar numbers of eggs on both plants, the other (non-preferred) plant was left in the pairing cage for as long as needed for 10 or more eggs to be laid on the leaves. The number of eggs was limited after acquiring 10 eggs in order to control for the number of eggs per plant and to have freshly laid eggs to offer to the predators and parasitoids in the subsequent experiment (see below). The preference test was conducted in a laboratory with ambient light and temperature during late June and early July.
(d). Natural enemies and plant hypersensitive responses
Using the plants and eggs obtained from the preference test (see above), we studied how inbreeding of plants and herbivores affect the interactions with the natural enemies of the herbivores and overall survival of the eggs. The leaves with eggs on them were marked with pieces of green straw, and the number of eggs on each leaf was recorded in order to keep track of the eggs that became detached. We placed the inbred and outbred plants bearing either inbred or outbred eggs on their leaves in the field where the eggs were available for naturally occurring predators and parasitoids. The average number of eggs per plant did not significantly differ between treatments (results of glmm with Poisson distribution: herbivore cross F = 2.34, p = 0.130; plant cross F = 0.67, p = 0.416; interaction F = 0.46, p = 0.501; numerator d.f. = 1, denominator d.f. = 77.87 for all). We placed the plants in blocks of four individuals (plants separated by ca 50 cm) with all the combinations of plant and herbivore inbreeding (inbred and outbred plants, inbred and outbred herbivore eggs). Altogether, there were 22 blocks that were randomly placed to areas where V. hirundinaria occurs naturally at the site. We monitored the eggs daily or every other day and recorded the number of eggs that were detached, parasitized, perforated or hatched. Here, an egg can be detached due to predation by ants or due to active hypersensitive responses of the plant (A.K. 2013, personal observation). On some leaves, we observed the formation of necrotic tissue under the egg as an indication of a hypersensitive response preceding the detachment of the egg. We classified eggs as perforated if they were empty and had visible signs of the egg surface being broken when studied under a microscope. We assumed that perforation was always caused by predation. Parasitized eggs were identified based on their black colour. Unhatched eggs attached to the plant that were not parasitized or perforated were classified as sterile.
(e). Statistical analysis
We analysed the oviposition preference of the herbivore with a repeated measures analysis (proc mixed) with the number of eggs on the inbred and on the outbred plant on the final day, i.e. the day that one or both of the plants were removed from the pairing net, as the within-response variable. We square-root-transformed the number of eggs in order to improve the normality of the data. Plant cross, herbivore cross and their interaction were included as explanatory variables. We specified the covariance structure as compound symmetry based on comparison of Akaike information criterion (AIC) values. We inspected the normality and equality of variances of the residuals by visual examination and Levene's test, respectively.
To examine whether the probability of herbivore eggs getting parasitized, detached or perforated was affected by plant or herbivore inbreeding we used the events/trials syntax for binomial data. We conducted separate analyses for the number of eggs parasitized, detached and perforated. In a fourth analysis, we used the total number of eggs lost, and thus not contributing to herbivore fitness (i.e. parasitized, detached or perforated; hereafter, lost) divided by the number of all eggs as the response variable. The ‘lost’ category was included because, for the herbivore fitness, it does not matter how the eggs are destroyed: its fitness is equally reduced by all the different activities measured. We included herbivore cross, plant cross and their interaction as explanatory variables. Total number of eggs was included as a covariate to control for any effects of egg number on parasitism and predation, as plants with more eggs may have been more attractive to the natural enemies. Plant individual was specified as a subject in the repeated statement in order to assume independence across the subjects, i.e. consider different plants as independent observations instead of each egg. We used generalized linear models (proc genmod) with logit link function. We included scale = deviance option in the model to correct for the minor overdispersion of the data when necessary.
We analysed the effects of plant and herbivore inbreeding on the proportion of sterile eggs and the number of fertile eggs. Proportion of sterile eggs was analysed using events/trials syntax for binomial data in generalized linear models (logit link function) with number of sterile eggs divided by number of all eggs as response variable and herbivore cross, plant cross and their interaction as explanatory variables. To analyse the realized fecundity of the herbivores following predation and parasitism, we constructed a generalized linear model with the number of fertile eggs as the response variable and herbivore cross, plant cross and their interaction as explanatory variables. We used Poisson distribution and log link function. Plant individual was specified as a subject in the repeated statement for both analyses. All analyses were conducted with SAS 9.4 (SAS Enterprise Guide 6.1/SAS 9.4 Cary, NC).
3. Results
The herbivores preferred to oviposit on the outbred over the inbred plants (table 1 and figure 1). At the end of the experiment, the outbred plants had on average 20.38 ± 2.48 eggs, whereas the inbred plants had 13.07 ± 2.24 eggs (mean ± s.e.). Plant or herbivore inbreeding did not affect the probability of eggs getting parasitized or perforated (table 1 and figure 2). However, on the outbred plants, the outbred eggs were more likely to be detached than the inbred eggs (proportion of detached outbred eggs = 0.440, inbred eggs = 0.235; Tukey's test p = 0.0178; table 1 and figure 2). When we analysed perforation, parasitism and detachment of the eggs together, the outbred eggs were more likely to be lost (proportion of lost outbred eggs = 0.506, inbred eggs = 0.367; table 1 and figure 2). The proportion of sterile eggs was higher in the inbred eggs (proportion of sterile outbred eggs = 0.375, inbred eggs = 0.567; table 1 and figure 2). Altogether, the realized fecundity of the herbivores was very low: only 10% of the eggs laid produced a viable larva. The average number of hatched eggs per moth pair was not statistically significantly higher in outbred (3.33 ± 1.31 eggs) compared with inbred herbivore eggs (1.37 ± 1.01), although there was a tendency to that direction (table 1 and figure 2). Plant cross did not affect herbivore fecundity.
Table 1.
The effect of plant and herbivore inbreeding on herbivore A. asclepiadis oviposition preference and herbivore egg survival on its host plant V. hirundinaria. ‘Lost’ refers to parasitized, detached and perforated eggs combined. Italicized values denote p-values of <0.05. d.f. = numerator, denominator d.f. for F-values for oviposition.
| oviposition |
parasitized |
detached |
perforated |
lost |
sterile |
fecundity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| source | F | p | x2 | p | x2 | p | x2 | p | x2 | p | x2 | p | x2 | p |
| herbivore cross | 2.03 | 0.162 | 0.04 | 0.845 | 2.30 | 0.130 | 2.87 | 0.090 | 4.83 | 0.028 | 7.70 | 0.006 | 2.91 | 0.088 |
| plant cross | 4.55 | 0.039 | 0.07 | 0.786 | 0.03 | 0.859 | 0.07 | 0.796 | 0.03 | 0.866 | 0.30 | 0.587 | 1.72 | 0.190 |
| herbivore cross × plant cross | 0.27 | 0.607 | 0.05 | 0.826 | 5.10 | 0.024 | 0.82 | 0.364 | 2.48 | 0.115 | 0.33 | 0.564 | 1.76 | 0.185 |
| no. of eggs | 0.10 | 0.754 | 1.17 | 0.279 | 2.71 | 0.100 | 0.01 | 0.923 | ||||||
| d.f. | 1, 40 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
Figure 1.
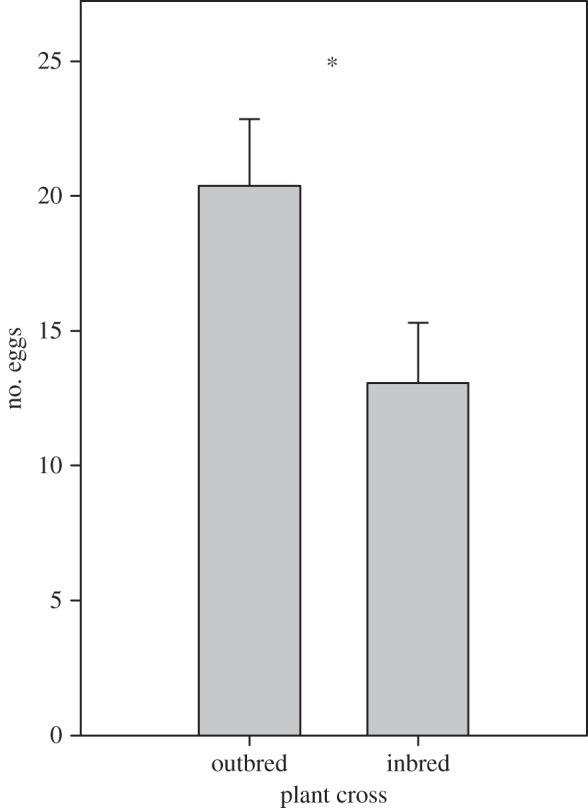
Oviposition preference of the herbivore A. asclepiadis in an experiment with inbred and outbred V. hirundinaria host plants. The average (+s.e.) number of eggs laid per plant in the two breeding treatments. Statistically significant difference in herbivore preference is indicated by asterisk *p < 0.05.
Figure 2.
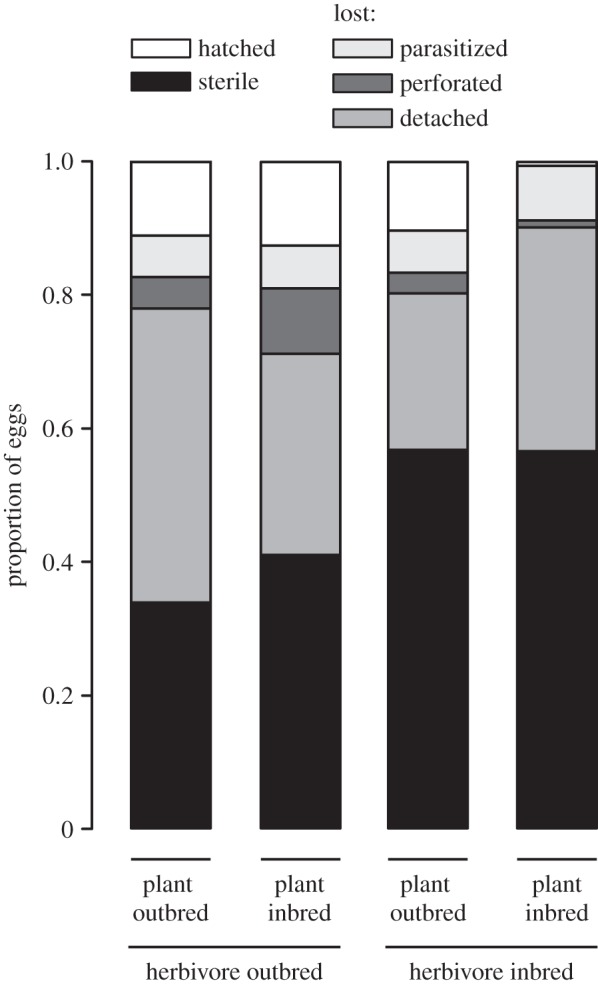
Proportions of the herbivore A. asclepiadis eggs that hatched, became parasitized, detached or perforated, or were sterile on inbred and outbred V. hirundinaria host plants. Lost refers to parasitized, detached and perforated eggs combined.
4. Discussion
We discovered that the herbivores deposited more eggs on outbred compared with inbred plants. For a specialist moth, a vigorous outbred host plant may appear to be a more suitable egg deposition site compared with an inbred plant [31]. However, the preference was unexpected given that A. asclepiadis larvae reach higher biomasses on inbred plants [5,9]. Other species of Lepidoptera have previously been found to oviposit on plants on which the performance of their offspring is not maximized [21–23]. We know the chemical signals used by herbivores for host recognition upon contact with the host at oviposition [12,15,17] are likely to be stronger in the outbred V. hirundinaria plants based on their higher concentrations of some foliar phenolic compounds compared with the inbred plants [5]. Therefore, the outbred plants are chemically more apparent to the female herbivores relative to the inbred plants, which, in turn, may result in the observed discrepancy between preference and performance of the herbivore. Contrary to our results, Kariyat et al. [13] discovered that adult hawk moth (M. sexta) females prefer to oviposit on inbred plants, which are also of higher quality to the larvae. Then again, similar to our results, higher apparency of outbred plants relative to inbred plants in terms of plant size or vigour resulted in higher rates of infestation by weevils (Trichobaris soror) in Datura stramonium [32] and insect-borne pathogens (Erwinia tracheiphila) in wild gourd (Cucurbita pepo ssp. texana) [33]. Finally, herbivore oviposition preference can also have implications for the performance of the host plant and the evolution of self-fertilization. Inbreeding depression in plants is commonly stronger when herbivores are present [3], and likewise, V. hirundinaria also exhibits inbreeding depression in resistance [5,9]. If the higher egg deposition on outbred than inbred plants also leads to greater damage and reduced fitness on the outbred plants, herbivore preference in oviposition can potentially modify the negative effects of inbreeding in plants. However, more work on this topic is required before definitive conclusions can be made.
Outbred herbivore eggs were lost more frequently than inbred eggs. In addition, detachment of the outbred eggs on outbred plants (owing to predation or plant hypersensitive responses) was more likely than that of the inbred eggs. The eggs from the outbred herbivore pairings may have been more viable than the inbred eggs [11] and, therefore, of higher quality to the enemies and more susceptible to be lost to predation and parasitism, although parasitism and predation alone were not significant. It seems that the higher probability of destruction of outbred herbivore eggs by predation, parasitism and plant hypersensitive responses evens out the inbreeding depression in herbivore fecundity, as the number of fertile eggs (i.e. eggs that produced a larva) did not differ statistically significantly at the end of the experiment between inbred and outbred herbivore crosses. It is likely that without the natural enemies and plant hypersensitive responses, inbreeding depression in egg hatching rate would have been stronger judging by the relatively higher proportion of sterile inbred eggs. However, we have to note that there were no control eggs that were protected from predation altogether. It is also possible that a higher proportion of the inbred eggs were sterile from the beginning of the experiment, which may mean that if the embryo was dead early on in the development, these eggs were not suitable for the natural enemies and perhaps did not elicit hypersensitive responses in the plant. In fact, if we consider only hatched and lost eggs (excluding sterile eggs), the percentages of eggs lost are very similar in the two treatments, with 85% and 81% for the inbred and outbred eggs, respectively. On the other hand, sterility of the eggs could have resulted later in the egg development from unsuccessful parasitoid attack or predation.
Contrary to our expectations, outbred plants were not significantly more effective in attracting natural enemies of the herbivores than inbred plants. In contrast to our results, damaged outbred horsenettle plants (Solanum carolinense) were more effective than inbred plants in attracting predatory hymenopterans, the natural enemies of the herbivores [26]. This difference was accounted for by the stronger induction of volatiles in the outbred plants relative to inbred plants [26]. It may be that the egg deposition as such does not alter the emission of volatile compounds or other cues used by predators and parasitoids in V. hirundinaria. Even though changes in volatile emissions and parasitoid attraction following egg deposition are reported from several different plant species [34–38], they are not universal [17,39]. Alternatively, egg parasitoids can also use cues left behind on plant leaves by the adult herbivore, such as scales, faeces and residues of sex pheromones [40–43]. If host-derived cues are the main causes of attraction for the parasitoids and predators in this system, effects of inbreeding on indirect defences against the eggs would be negligible.
5. Conclusion
Our results confirm that herbivore and plant inbreeding can affect plant–herbivore interactions already at the oviposition and egg stage before the larval feeding begins. Unexpectedly, we observed mainly positive effects of inbreeding, as outbred plants were preferred for oviposition by herbivores and outbred herbivore eggs were lost more often than inbred eggs. Thus, we can conclude that the negative effects of inbreeding expressed in one specific plant trait or at a specific herbivore life-history stage (biomass of herbivore larvae, plant resistance [5]) do not precisely predict the effects on other traits (survival of herbivore eggs, plant apparency to herbivores; this study). In addition, our results suggest that natural enemies coupled with plant hypersensitive responses can alter the strength of inbreeding depression in the herbivores at the egg stage. Taken together, these results highlight the importance of studying inbreeding depression in different life-history stages and under natural conditions in order to thoroughly understand its effects on plant–herbivore interactions.
Acknowledgements
We thank Liisa Laukkanen, Niek Scheepens, Anneli Asplund, Alba Martin Fernandez, Salla Mikkola, Moriz Halbmeier, Alicia Leroux, Laura Stöcklin, Hanna Kalliolevo, Naaja Perander and Tuija Koivisto for assistance with conducting the experiment; Veli Vikberg for assistance with identifying the parasitoids; Archipelago Research institute in Seili and Ruissalo Botanical Garden for providing the facilities.
Data accessibility
The full datasets are available at Dryad depository (doi:10.5061/dryad.0qg71).
Funding statement
This study was financially supported by the Academy of Finland (grant 138308 to R.L.).
References
- 1.Charlesworth B, Charlesworth D. 1999. The genetic basis of inbreeding depression. Genet. Res. 74, 329–340. ( 10.1017/S0016672399004152) [DOI] [PubMed] [Google Scholar]
- 2.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. ( 10.1016/s0169-5347(02)02489-8) [DOI] [Google Scholar]
- 3.Carr DE, Eubanks MD. 2014. Interactions between insect herbivores and plant mating systems. Annu. Rev. Entomol. 59, 185–203. ( 10.1146/annurev-ento-011613-162049) [DOI] [PubMed] [Google Scholar]
- 4.Campbell SA, Thaler JS, Kessler A. 2013. Plant chemistry underlies herbivore-mediated inbreeding depression in nature. Ecol. Lett. 16, 252–260. ( 10.1111/ele.12036) [DOI] [PubMed] [Google Scholar]
- 5.Kalske A, Mutikainen P, Muola A, Scheepens JF, Laukkanen L, Salminen J-P, Leimu R. 2014. Simultaneous inbreeding modifies inbreeding depression in a plant–herbivore interaction. Ecol. Lett. 17, 229–238. ( 10.1111/ele.12223) [DOI] [PubMed] [Google Scholar]
- 6.Kariyat RR, Balogh CM, Moraski RP, De Moraes CM, Mescher MC, Stephenson AG. 2013. Constitutive and herbivore-induced structural defenses are compromised by inbreeding in Solanum carolinense (Solanaceae). Am. J. Bot. 100, 1014–1021. ( 10.3732/ajb.1200612) [DOI] [PubMed] [Google Scholar]
- 7.Delphia CM, Rohr JR, Stephenson AG, De Moraes CM, Mescher MC. 2009. Effects of genetic variation and inbreeding on volatile production in a field population of horsenettle. Int. J. Plant Sci. 170, 12–20. ( 10.1086/593039) [DOI] [Google Scholar]
- 8.Carr DE, Eubanks MD. 2002. Inbreeding alters resistance to insect herbivory and host plant quality in Mimulus guttatus (Scrophulariaceae). Evolution 56, 22–30. ( 10.1111/j.0014-3820.2002.tb00846.x) [DOI] [PubMed] [Google Scholar]
- 9.Muola A, Mutikainen P, Laukkanen L, Lilley M, Leimu R. 2011. The role of inbreeding and outbreeding in herbivore resistance and tolerance in Vincetoxicum hirundinaria. Ann. Bot. 108, 547–555. ( 10.1093/aob/mcr174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivey CT, Carr DE, Eubanks MD. 2004. Effects of inbreeding in Mimulus guttatus on tolerance to herbivory in natural environments. Ecology 85, 567–574. ( 10.1890/02-0730) [DOI] [Google Scholar]
- 11.Saccheri IJ, Brakefield PM, Nichols RA. 1996. Severe inbreeding depression and rapid fitness rebound in the butterfly Bicyclus anynana (Satyridae). Evolution 50, 2000–2013. ( 10.2307/2410758) [DOI] [PubMed] [Google Scholar]
- 12.Knolhoff LM, Heckel DG. 2014. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 59, 263–278. ( 10.1146/annurev-ento-011613-161945) [DOI] [PubMed] [Google Scholar]
- 13.Kariyat RR, Mauck KE, Balogh CM, Stephenson AG, Mescher MC, De Moraes CM. 2013. Inbreeding in horsenettle (Solanum carolinense) alters night-time volatile emissions that guide oviposition by Manduca sexta moths. Proc. R. Soc. B 280, 20130020 ( 10.1098/rspb.2013.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraenkel GS. 1959. The raison d’être of secondary plant substances. Science 129, 1466–1470. ( 10.1126/science.129.3361.1466) [DOI] [PubMed] [Google Scholar]
- 15.Simmonds MSJ. 2001. Importance of flavonoids in insect–plant interactions: feeding and oviposition. Phytochemistry 56, 245–252. ( 10.1016/s0031-9422(00)00453-2) [DOI] [PubMed] [Google Scholar]
- 16.Langan AM, Wheater CP, Dunleavy PL. 2001. Does the small white butterfly (Pieris rapae L.) aggregate eggs on plants with greater gas exchange activity? J. Insect Behav. 14, 459–468. ( 10.1023/a:1011167905126) [DOI] [Google Scholar]
- 17.Hilker M, Meiners T. 2011. Plants and insect eggs: how do they affect each other? Phytochemistry 72, 1612–1623. ( 10.1016/j.phytochem.2011.02.018) [DOI] [PubMed] [Google Scholar]
- 18.Gripenberg S, Mayhew PJ, Parnell M, Roslin T. 2010. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 13, 383–393. ( 10.1111/j.1461-0248.2009.01433.x) [DOI] [PubMed] [Google Scholar]
- 19.Thompson JN, Pellmyr O. 1991. Evolution of oviposition behavior and host preference in Lepidoptera. Annu. Rev. Entomol. 36, 65–89. ( 10.1146/annurev.ento.36.1.65) [DOI] [Google Scholar]
- 20.Jaenike J. 1978. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 14, 350–356. ( 10.1016/0040-5809(78)90012-6) [DOI] [PubMed] [Google Scholar]
- 21.Rausher MD. 1979. Larval habitat suitability and oviposition preference in three related butterflies. Ecology 60, 503–511. ( 10.2307/1936070) [DOI] [Google Scholar]
- 22.Gripenberg S, Morriën E, Cudmore A, Salminen J-P, Roslin T. 2007. Resource selection by female moths in a heterogeneous environment: what is a poor girl to do? J. Anim. Ecol. 76, 854–865. ( 10.1111/j.1365-2656.2007.01261.x) [DOI] [PubMed] [Google Scholar]
- 23.Underwood DLA. 1994. Intraspecific variability in host-plant quality and ovipositional preferences in Eucheira socialis (Lepidoptera: Pieridae). Ecol. Entomol. 19, 245–256. ( 10.1111/j.1365-2311.1994.tb00416.x) [DOI] [Google Scholar]
- 24.Blenn B, Bandoly M, Küffner A, Otte T, Geiselhardt S, Fatouros NE, Hilker M. 2012. Insect egg deposition induces indirect defense and epicuticular wax changes in Arabidopsis thaliana. J. Chem. Ecol. 38, 882–892. ( 10.1007/s10886-012-0132-8) [DOI] [PubMed] [Google Scholar]
- 25.Hilker M, Meiners T. 2006. Early herbivore alert: insect eggs induce plant defense. J. Chem. Ecol. 32, 1379–1397. ( 10.1007/s10886-006-9057-4) [DOI] [PubMed] [Google Scholar]
- 26.Kariyat RR, Mauck KE, De Moraes CM, Stephenson AG, Mescher MC. 2012. Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol. Lett. 15, 301–309. ( 10.1111/j.1461-0248.2011.01738.x) [DOI] [PubMed] [Google Scholar]
- 27.Muola A, Mutikainen P, Lilley M, Laukkanen L, Salminen J-P, Leimu R. 2010. Associations of plant fitness, leaf chemistry, and damage suggest selection mosaic in plant–herbivore interactions. Ecology 91, 2650–2659. ( 10.1890/09-0589.1) [DOI] [PubMed] [Google Scholar]
- 28.Leimu R, Mutikainen P. 2005. Population history, mating system, and fitness variation in a perennial herb with a fragmented distribution. Conserv. Biol. 19, 349–356. ( 10.1111/j.1523-1739.2005.00480.x) [DOI] [Google Scholar]
- 29.Förare J. 1995. The biology of the noctuid moth Abrostola asclepiadis Schiff. (Lepidoptera, Noctuidae) in Sweden. Ent. Tidskr. 116, 179–186. [Google Scholar]
- 30.Leimu R, Lehtilä K. 2006. Effects of two types of herbivores on the population dynamics of a perennial herb. Basic Appl. Ecol. 7, 224–235. ( 10.1016/j.baae.2005.09.002) [DOI] [Google Scholar]
- 31.Hull-Sanders HM, Eubanks MD. 2005. Plant defense theory provides insights into interactions involving inbred plants and insect herbivores. Ecology 86, 897–904. ( 10.1890/04-0935) [DOI] [Google Scholar]
- 32.Bello-Bedoy R, Cruz LL, Núñez-Farfán J. 2011. Inbreeding alters a plant–predispersal seed predator interaction. Evol. Ecol. 25, 815–829. ( 10.1007/s10682-010-9448-4) [DOI] [Google Scholar]
- 33.Ferrari MJ, Du D, Winsor JA, Stephenson AG. 2007. Inbreeding depression of plant quality reduces incidence of an insect-borne pathogen in a wild gourd. Int. J. Plant Sci. 168, 603–610. ( 10.1086/513487) [DOI] [Google Scholar]
- 34.Meiners T, Hilker M. 2000. Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol. 26, 221–232. ( 10.1023/A:1005453830961) [DOI] [Google Scholar]
- 35.Colazza S, Fucarino A, Peri E, Salerno G, Conti E, Bin F. 2004. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 207, 47–53. ( 10.1242/jeb.00732) [DOI] [PubMed] [Google Scholar]
- 36.Bruce TJA, Midega CAO, Birkett MA, Pickett JA, Khan ZR. 2010. Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol. Lett. 6, 314–317. ( 10.1098/rsbl.2009.0953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME. 2012. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7, e43607 ( 10.1371/journal.pone.0043607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reymond P. 2013. Perception, signaling and molecular basis of oviposition-mediated plant responses. Planta 238, 247–258. ( 10.1007/s00425-013-1908-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fatouros NE, Bukovinszkine'Kiss G, Kalkers LA, Soler Gamborena R, Dicke M, Hilker M. 2005. Oviposition-induced plant cues: do they arrest Trichogramma wasps during host location? Entomol. Exp. Appl. 115, 207–215. ( 10.1111/j.1570-7458.2005.00245.x) [DOI] [Google Scholar]
- 40.Lewis WJ, Jones RL, Sparks AN. 1972. A host-seeking stimulant for the egg parasite Trichogramma evanescens: its source and a demonstration of its laboratory and field activity. Ann. Entomol. Soc. Am. 65, 1087–1089. [Google Scholar]
- 41.Noldus LPJJ, Potting RPJ, Barendregt HE. 1991. Moth sex pheromone adsorption to leaf surface: bridge in time for chemical spies. Physiol. Entomol. 16, 329–344. ( 10.1111/j.1365-3032.1991.tb00571.x) [DOI] [Google Scholar]
- 42.Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M. 2008. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 19, 677–689. ( 10.1093/beheco/arn011) [DOI] [Google Scholar]
- 43.Meiners T, Hilker M. 1997. Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae). Oecologia 112, 87–93. ( 10.1007/s004420050287) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full datasets are available at Dryad depository (doi:10.5061/dryad.0qg71).


