Abstract
Describing the factors that shape collective behaviour is central to our understanding of animal societies. Countless studies have demonstrated an effect of group size in the emergence of collective behaviours, but comparatively few have accounted for the composition/diversity of behavioural phenotypes, which is often conflated with group size. Here, we simultaneously examine the effect of personality composition and group size on nest architecture and collective foraging aggressiveness in the social spider Stegodyphus dumicola. We created colonies of two different sizes (10 or 30 individuals) and four compositions of boldness (all bold, all shy, mixed bold and shy, or average individuals) in the field and then measured their collective behaviour. Larger colonies produced bigger capture webs, while colonies containing a higher proportion of bold individuals responded to and attacked prey more rapidly. The number of attackers during collective foraging was determined jointly by composition and size, although composition had an effect size more than twice that of colony size: our results suggest that colonies of just 10 bold spiders would attack prey with as many attackers as colonies of 110 ‘average’ spiders. Thus, personality composition is a more potent (albeit more cryptic) determinant of collective foraging in these societies.
Keywords: collective behaviour, group composition, group size, personality, temperament
1. Introduction
Understanding the factors that shape collective behaviour is vital for our understanding of animal societies because they can be key drivers of group success [1,2]. The tendency for individuals to differ consistently in their behaviour through time and across contexts (i.e. ‘animal personality’ [3–5]) is often a major determinant of collective behaviours [6,7], colony productivity [8] and survival [9,10] in a variety of taxa (fish [11,12], birds [13]). This is in part because individual traits, like personality, influence task participation [14–16], individual aptitudes for those tasks [17] (but see [18]) and the manner in which tasks are executed [19]. Yet how group personality composition interacts with other more familiar social factors like group size remains poorly resolved. Here, we explore the following question: is the presently fashionable trait of animal personality as informative or valuable as classic social traits, like group size, in predicting collective behaviour?
Countless studies have demonstrated an effect of group size in the development and emergence of collective behaviours [20–23]. For example, it has been shown that increased colony sizes can beget increased behavioural differentiation and task elitism [24], and division of labour often scales positively with colony size [25,26]. Group size can even act as a facilitator of collective behaviour in human crowds, from social facilitation [27] to social loafing [28]. Comparatively few studies, however, have accounted for the composition of behavioural phenotypes within groups of different sizes. Yet, as group size and composition could be intrinsically related social factors, simultaneously studying both attributes should provide a more complete understanding of social trade-offs, optimal group size and the execution of cooperative behaviours [20]. Most importantly, the outcomes that we have commonly attributed to group size may actually be a result of group composition, as increases in group size will frequently increase the phenotypic diversity within groups and alter their composition. Thus, classical manipulations of group size have almost universally conflated group size with group composition and/or within-group trait variation (but see [29]).
Although addressing group size and group composition independently has led to a deeper understanding of animal societies, the majority of behavioural studies are conducted under laboratory conditions devoid of ecological challenges like predation risk and abiotic stressors [7,30]. How, then, might habitats containing natural pressures influence the emergence of collective behaviour across groups of different size and personality composition? Here, we focus on collective prey capture behaviour in a highly tractable animal model, the social spider Stegodyphus dumicola. We focus on collective foraging in particular because the success of social spiders has often been attributed to their ability to cooperate to subdue larger and more profitable prey [31,32].
With the experiment herein, we examine the relative contribution of colonies' personality composition versus group size in predicting collective foraging behaviour, web architecture and anti-predator behaviour in S. dumicola. We offer three hypotheses:
Hypothesis 1: —
Colonies composed of a higher proportion of bold individuals will attack prey more rapidly, while both larger and bolder colonies will attack with a greater number of attackers.
Hypothesis 2: —
Larger and bolder colonies will build larger capture webs, though the predictive power of colony composition will be greater than that of group size.
Hypothesis 3: —
Colonies composed of a higher proportion of bold individuals will take longer to escape an aversive stimulus by evacuating the capture web into the colony retreat.
Together these hypotheses are designed to probe the (often conflated) influences of group size and personality composition in situ in a manner that is rarely achievable in other test systems.
2. Material and methods
(a). Study species and field site
Stegodyphus dumicola (Araneae: Eresidae) is an Old World social spider that lives in colonies of tens to hundreds of females that cooperate in shared web building, prey capture and alloparental care [33,34]. We collected colonies of S. dumicola in Acacia mellifera trees and along roadside fences in the southern Kalahari Desert, South Africa in January 2014. We transported colonies to our field site in Griekwastad, Northern Cape, South Africa. This site is an arid thornveld dominated by A. mellifera.
(b). Individual personality assays
Prior to personality assays, we measured the body mass and prosoma width of each spider with a digital scale and digital callipers, respectively. To determine spiders' personality, we tested their boldness by assessing their response to an aversive stimulus. Boldness is defined here as the latency to resume movement after an aversive stimulus. This metric is both highly repeatable at the individual level (repeatability = 0.63 [7]) and associated with task participation [7,14], collective behaviour [15] and social stability [35] in this and other social Stegodyphus.
Spiders were placed in a black plastic arena (diameter = 12 cm, height = 4 cm) and given a 60 s acclimation period beneath an opaque plastic cover object. After 60 s, the cover object was removed and two rapid puffs of air were administered to the spider's anterior prosoma using an infant ear-cleaning bulb. We then measured the time until the spider moved one full body length. Trials were terminated after 600 s. Individuals with long latencies to resume movement (400–600 s) are deemed ‘shy’, individuals that resume movement rapidly are termed ‘bold’ (1–200 s) and individuals that resume movement between 200 and 400 s are considered ‘average’ (electronic supplementary material, figure S1). After their personality assays, we gave each individual spider a unique three-colour ID mark with acrylic paint atop their abdomen to permit individual identification.
(c). Collective behaviour assays
We constructed artificial colonies of S. dumicola of two different sizes (10 or 30 individuals) and four different personality compositions (all bold, all shy, 50 : 50 bold and shy, or all individuals of ‘average’ boldness) in a fully factorial design (n = 64 experimental units, n ≈ 8 of each treatment combination). These experimental colony sizes fall within the natural range of sizes found in the southern Kalahari (1 to approx. 700 spiders; C.N.K. & J.N.P. 2013, unpublished data). In these populations, all colonies sampled contain a majority of shy individuals, while the remaining individuals exhibit varying distributions ranging from average to bold (electronic supplementary material, figure S1). We allowed each colony 24 h indoors to produce a silken retreat in 240 ml plastic cups. We divided our collective foraging assays into two parts. We first tested each colony three times before they produced a capture web. Thus, each colony was operating on a web of the same volume (i.e. the size of their cup). These collective foraging assays were performed in the field under ambient temperature and natural light : dark cycles. Collective foraging assays were initiated by placing a small piece of white paper (1.5 cm2) in the centre of the web and allowing a 20 s acclimation period. We then used a battery-powered handheld vibratory device to vibrate the piece of paper to simulate a prey item caught in the silk. We recorded the latency for the first spider to emerge from the retreat, the latency for the first spider to attack the paper and the total number of attackers that participated in the prey capture event.
After the three initial assays, each colony container was fastened to a hookbush acacia (A. mellifera) branch with clothes pins in the early evening hours (20.00–21.30). This particular site contains an abundance of the widely foraging predatory ant Anoplolepis custodiens (2–8 nest entrances per m2). This ant, even in small numbers, is a top predator responsible for colony-wide death in S. dumicola in the nearby Namib Desert [36]. The following morning (05.45–06.00), we measured the capture web area produced by estimating its general shape (triangle, rectangle, etc.) and then recording the appropriate dimensions to estimate its area using a tape measure. Although relatively rare (23/64 cases), we also counted the number of individuals that had ‘dispersed’ from the plastic cup and had produced a smaller retreat on another branch. Such ancillary nests were connected to the central nest via a shared capture web. At 06.00 and 18.00 on the following days we tested the collective foraging of each colony. The foraging behaviour of each colony was measured between three and 16 times in total.
After each collective foraging event, we tested the speed at which spiders evacuated into the retreat following an aversive stimulus. The aversive stimulus was implemented by striking the branch to which the colony was attached with a blunt probe. This stimulus sends a vibration throughout the web, which caused spiders to disperse from the simulated prey item and run back into their retreat. We measured the latency for the first and the last individual to enter the retreat following this stimulus.
(d). Statistical analyses
The web size data were analysed with a general linear mixed-model ANOVA with the following predictor variables: group size, colony composition and group size × colony composition. We also included source colony ID as a random effect. We analysed the number of dispersing spiders with ANOVA with the same predictor variables and random effects as the web size data.
The latency to emerge and attack data were log-transformed to meet model assumptions. We used independent general linear mixed models to predict three response variables (latency to emerge, latency to attack, no. of attackers) with the following predictor variables: source colony ID (random), group size, colony composition, web presence (with/without capture web), group size × colony composition, group size × web presence, composition × web presence and group size × colony composition × web presence. Post hoc tests were performed using Tukey's HSD.
3. Results
(a). Web architecture and dispersal
The combined model predicting capture web size was highly significant (F16,37 = 4.84, R2 = 0.65, p = 0.007). Groups of 30 spiders produced capture webs three times larger than those created by groups of 10 individuals (F1,42.9 = 39.32, p < 0.0001) while group composition had no detectable influence on total web area (F3,40.1 = 1.16, p = 0.33). The effect of group size did not differ for different group compositions (F3,40.4 = 1.46, p = 0.23).
Larger groups also had more individuals that left the colony to create small retreats along other sections of the same capture web (F1,23 = 4.32, p = 0.05). The number of dispersers, however, was not influenced by group composition (F3,23 = 1.11, p = 0.37), and the interaction term between size and composition was also not significant (F3,23 = 0.97, p = 0.42), meaning that the number of individuals that dispersed at each colony size did not differ based on the group composition. The number of dispersers was also not influenced by the identity of the source colony from which spiders were collected (p > 0.05).
(b). Prey capture
Our combined model predicting colonies' latency to emerge was highly significant (F23,253 = 4.85, R2 = 0.37, p < 0.0001). Group composition but not group size had a significant effect on colonies' latency to emerge in response to prey (table 1). However, the effect of group composition differed in the presence versus absence of the capture web. In the absence of a capture web, colonies composed of all bold individuals were 2.2 times faster in emerging from their retreat compared with other compositions, regardless of group size (table 1 and figure 1). However, we failed to detect a relationship between latency to attack and colonies' personality composition when capture webs were present (figure 1).
Table 1.
Summary of effect tests from three general linear models predicting three aspects of colonies' collective foraging behaviour in the wild. An asterisk denotes significant values.
| source | d.f. | d.f.den | F | p-value |
|---|---|---|---|---|
| latency to emerge | ||||
| size | 1 | 260.6 | 2.56 | 0.12 |
| composition | 3 | 257.5 | 4.17 | 0.009* |
| web presence | 1 | 255.5 | 1.88 | 0.31 |
| size × composition | 3 | 259 | 0.42 | 0.85 |
| size × web presence | 1 | 254.4 | 1.76 | 0.41 |
| composition × web presence | 3 | 254.8 | 3.81 | 0.007* |
| size × composition × web presence | 3 | 254.6 | 1.05 | 0.23 |
| latency to attack | ||||
| size | 1 | 260.6 | 2.74 | 0.09 |
| composition | 3 | 257.5 | 4.56 | 0.004* |
| web presence | 1 | 255.5 | 1.55 | 0.21 |
| size × composition | 3 | 259 | 0.32 | 0.81 |
| size × web presence | 1 | 254.4 | 1.62 | 0.2 |
| composition × web presence | 3 | 254.8 | 3.94 | 0.009* |
| size × composition × web presence | 3 | 254.6 | 1.85 | 0.14 |
| number of attackers | ||||
| size | 1 | 257.5 | 26.25 | <0.0001* |
| composition | 3 | 258.2 | 17.99 | <0.0001* |
| web presence | 1 | 256.7 | 14.1 | 0.0002* |
| size × composition | 3 | 256.1 | 5.12 | 0.002* |
| size × web presence | 1 | 254.1 | 0.16 | 0.69 |
| composition × web presence | 3 | 254.9 | 0.43 | 0.73 |
| size × composition × web presence | 3 | 254.7 | 2.46 | 0.06 |
Figure 1.
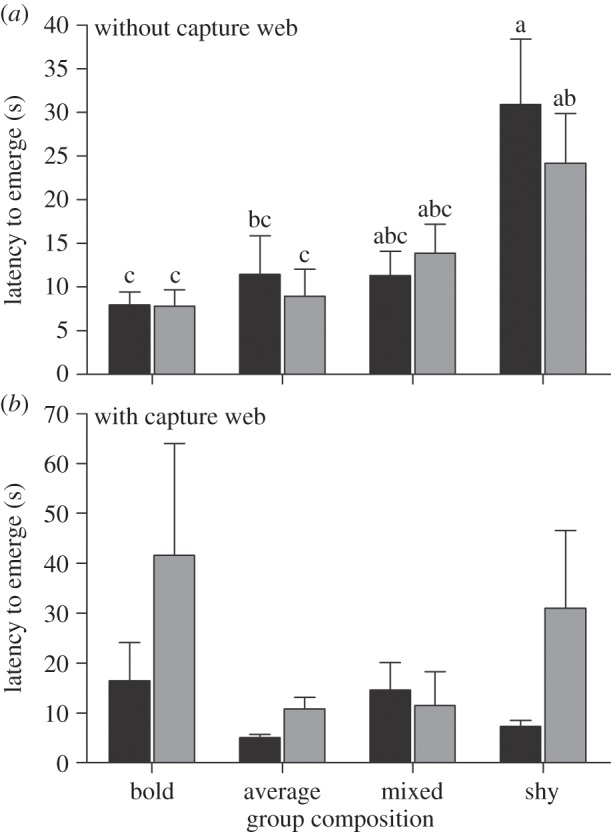
(a,b) Without a capture web, the latency for the first individual to emerge from the retreat after prey stimulus was a product of the colony's personality composition (F3,254.8 = 3.81, p = 0.007). Regardless of group size, colonies composed of all bold individuals were on average 2.2 times faster in their first emergence compared with other compositions. Values significantly different from each other are represented by different letters within each panel. Group size: black bars denote 10 and grey bars denote 30.
Our combined model predicting colonies' latency to attack was also highly significant (F23,253 = 5.43, R2 = 0.35, p = 0.004). As with latency to emerge, we detected a significant effect of group composition but not group size on colonies' latency to attack (table 1). However, the effects of group composition differed depending on whether the capture web was present versus absent. Post hoc tests revealed that, in the absence of a capture web, colonies of shy spiders were 2.6 times slower to attack the prey stimulus compared with all other colony compositions (figure 2). We failed to detect any significant effects of group size or composition when colonies were permitted to construct capture webs (figure 2).
Figure 2.
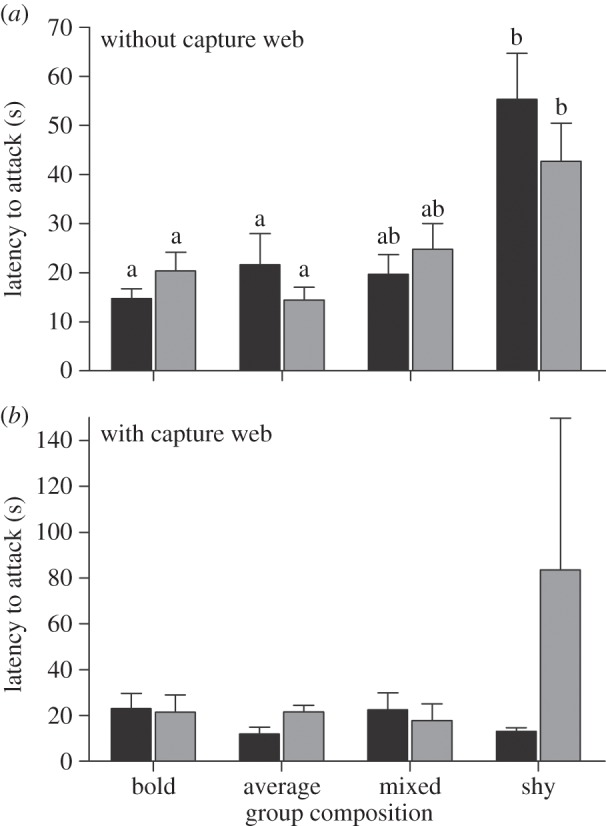
(a,b) Without a capture web, colonies composed of all shy individuals were 1.3 times faster in attacking the prey stimulus when their colony size was 30 (grey bars) as opposed to 10 (black bars) (F3,254.8 = 3.94, p = 0.009). The standard error value for 30 shy individuals exceeds the limits of the graph. Values significantly different from each other are represented by different letters within each panel.
The combined model predicting the total number of attackers was significant (F23,253 = 6.14, R2 = 0.39, p < 0.0001). This attribute was impacted by both the composition of the group and the group size (table 1). Here, however, the effects of either attribute were indistinguishable in the presence versus absence of the capture web. Groups composed of only bold individuals attacked prey with 1.8–2.6 times the number of attackers as rival colonies, while colonies of 30 individuals attacked prey with 1.26–2.1 times the number of attackers as colonies of only 10 individuals. Notably, the number of attackers participating in prey capture increased more slowly than group size, probably as a result of the behavioural composition of the colony. For comparison, the effect size of having a colony composed of only bold individuals (β = 0.39, s.e. = 0.05) was far greater than that of having a colony of 30 individuals (β = 0.15, s.e. = 0.03; figure 3).
Figure 3.
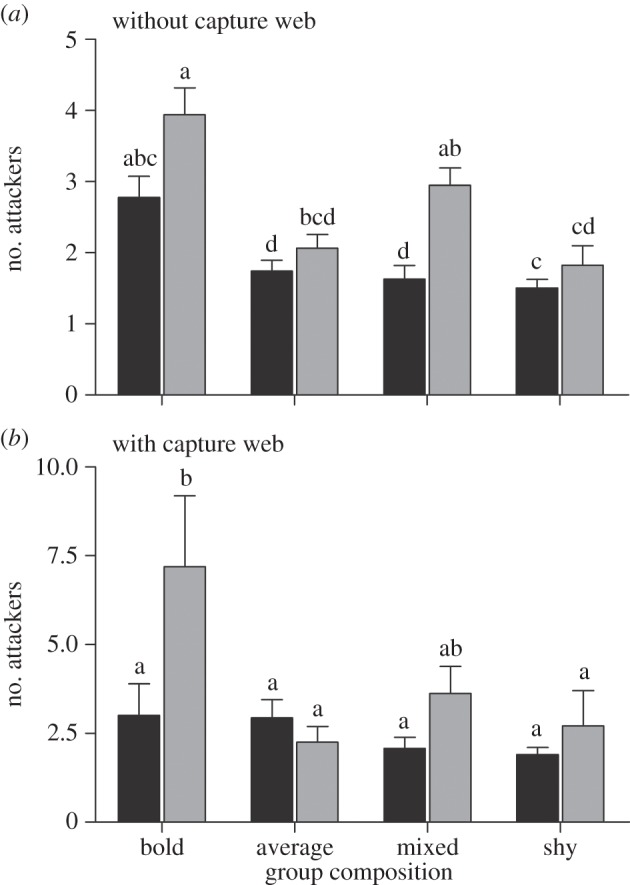
(a,b) The number of individuals that participated in the attack was influenced both by the composition of the group and the group size (F3,256.1 = 5.12, p = 0.002). For example, groups of 30 (grey bars) bold spiders always had more individuals participate in the attack compared with groups of 10 (black bars) (F1,257.5 = 26.25, p < 0.0001). Values significantly different from each other are represented by different letters within each panel.
Finally, the identity of the source colony from which individual spiders were obtained had no significant effect on any of the collective traits assessed here (all 95% CIs overlapped zero).
(c). Web evacuation
No independent variables (i.e. composition, size, etc.) had a significant influence on the latency for the first individual (all p ≥ 0.12) or the last individual (all p ≥ 0.18) to evacuate the capture web following an aversive stimulus.
4. Discussion
Determining the traits that underlie collective behaviour is important, partly because it provides clues to how evolution can hone the collective and/or emergent traits of groups. Given its intuitive appeal and ease of manipulation, group size has been manipulated in an extraordinary number of systems, and it seems to be important in determining a variety of collective traits. However, groups of different sizes are inherently different in respect of other, more cryptic traits. For example, larger groups might also contain more informed individuals [37] or have an increased likelihood that keystone individuals will be present in the mélange [38]. Here, we demonstrate that the relative importance of personality composition versus group size varies depending on the trait under consideration. In some cases, the effects of personality composition effectively dwarf those attributable to group size.
(a). Web architecture and dispersal
For web architecture, the only significant predictor was group size. Here, the estimated effect of group size was three times that of personality composition, where groups of 30 individuals produced webs three times larger than colonies of only 10 individuals. Thus, group size has a roughly additive effect (i.e. linear scaling) on capture web size. For this collective trait, the influence of group size is far greater than that of personality composition. This corroborates previous evidence in this social system which suggested that individual personality does not influence the propensity for individuals to produce capture web silk, though smaller individuals are more likely to participate in web building [7]. In other social spiders, larger capture webs increase prey capture rate but decrease per capita food intake [31], so a linear scaling between group size and capture web size does not confer per capita foraging benefits.
Similarly, the number of individuals that dispersed to create new retreats along the capture web was influenced only by group size, where larger groups contained more dispersers. Colonies of S. dumicola in the field are often polydomous (i.e. containing multiple retreats), where smaller colonies will ‘bud’ off from the natal colony and share a single large capture web [36]. Although it is unknown if these budding individuals are also more likely to engage in long-distance dispersal to find new clusters of colonies [39], evidence from mitochondrial DNA suggests that colonies in close proximity are formed from single matri-lineage propagules, and thus local population dynamics are strongly influenced by individual behavioural decisions [40]. The propensity for individuals to leave the colony, in turn, seems to be influenced by group size (as in other social groups [41]), but an individual's decision to bud off from the natal colony may be influenced by individual-level traits (i.e. hunger state, personality).
(b). Prey capture
The effects of personality composition were larger than group size for collective foraging behaviour. Admittedly, it is not terribly surprising that both group size and personality composition impact some aspects of collective foraging behaviour. Dozens of studies from a diversity of systems have demonstrated these sorts of associations. The surprising findings from our data are the sizable differences in effect size. Personality composition was the only trait significantly associated with colonies' latency to emerge or latency to attack under any condition. Without a capture web, colonies of bold spiders attacked prey three to eight times more rapidly than other compositions. As for the number of attackers, the effect size of personality composition was more than twice that of group size. To place this in relative terms, colonies of 10 bold spiders are predicted to attack prey with as many attackers as colonies of 110 spiders with only average boldness, assuming linear relationships (figure 4). Given that the foraging success of social spiders is often linked to the number of attackers [31,42,43], our results suggest that foraging success may hinge more heavily on the types of individuals in the colony rather than the mere number of members.
Figure 4.
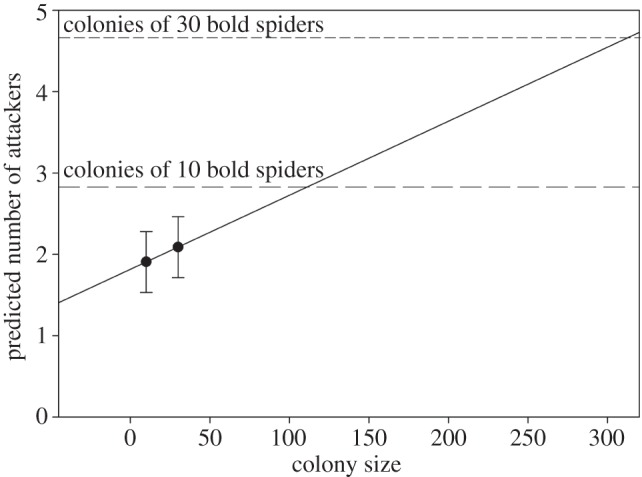
A speculative depiction of the predicted relationship between colony size and participation in collective prey capture for shy/average colonies (solid line). If this trend line were extrapolated linearly, then colonies of 10 bold spiders (long dashed line) would attack colonies with as many attackers as colonies of 110 shy/average spiders. Furthermore, colonies of 30 bold spiders (short dashed line) should attack prey with as many attackers as colonies of nearly 300 shy/average spiders.
These results are exciting because countless studies on collective behaviour have overlooked personality composition, partly because it is a cryptic aspect of trait variation and can be difficult to manipulate in the field. However, our results suggest that future studies should attempt to account for this variation. Moreover, classic studies that considered group size alone may have inadvertently attributed effects to group size that are actually produced by differences in group composition or within-group behavioural variation. This might also explain why different studies often find conflicting effects of group size (reviewed in [22]): by manipulating group size, studies are simultaneously and unknowingly shifting groups' personality compositions.
Collective foraging behaviours differed drastically based on whether or not colonies had produced a capture web. In fact, when colonies were allowed to produce a capture web in an Acacia tree, the effect of both group size and composition were lost on two of the three collective foraging behaviours measured. Importantly, even before we allowed colonies to produce a capture web, they were still exposed to many environmental cues (e.g. wind, olfactory cues, light/dark cycles). However, they admittedly lacked any potential interactions with live prey or predators in these initial assays. By contrast, when colonies were permitted to construct a capture web, we noted live prey in nearly every web, and we often observed individuals and groups of spiders interacting with predatory ants. Although mostly speculative, we propose that antagonistic species interactions may decouple the influence of both group size and group composition on some collective behaviours, but not others, as has been observed in some other systems [44].
Contrary to the seemingly linear relationship between group size and some collective behaviours (e.g. web production), the strong effect of group composition on collective behaviour may be subtle and nonlinear. For instance, in some extreme examples, colonies' collective behaviour can be driven by the traits of one or a few highly influential group members [38,43]. Under such circumstances, the effects of group size on collective behaviour are small or undetectable [15]. Data from laboratory studies suggest that the presence of bold Stegodyphus is particularly important in determining colonies' behaviour because bold spiders somehow instigate or catalyse more aggressive foraging in their fellow (often shy) colony mates [43].
(c). Web evacuation
Neither colony composition nor group size affected the latency for individuals to evacuate the capture web following a vibratory aversive stimulus. This is surprising because one would intuitively predict that web evacuation, which is a measure of colonies' collective boldness/fear, would be intimately associated with the boldness of the colony constituents. Thus, it appears that the relationships between the collective behaviour of colonies and the personalities of their constituents are not always easy to predict and may easily vary depending on the ecological context (e.g. foraging versus anti-predator behaviour) under consideration and/or how we measure them.
5. Conclusion
The results of separate studies on personality composition or group size have often attributed similar findings to either mechanism independently (e.g. invasion biology [45,46]), while others have profitably considered the eco-evolutionary consequences of both colony composition and size simultaneously [10,29]. In our system, the relative influence of group size and personality composition depended on the collective trait being considered. In some cases, the differences in effect size were considerable and counterintuitive. As such, we urge that future studies on common models of collective behaviour (e.g. fish schools, bird flocks, ant colonies, etc.) should manipulate both personality composition and group size simultaneously to elucidate their interplay and relative contributions on a variety of collective behaviours. In S. dumicola, naturally occurring colonies vary considerably in their size–composition relationship, where larger groups generally contain a larger proportion of bold individuals (F1,15 = 16.3, p = 0.001, r2 = 0.54; electronic supplementary material, figure S2). Furthermore, colony composition is probably not driven by within-group relatedness, as variable colony compositions arise among colonies that share similar levels of within-group relatedness [40]. We further wonder whether or how group size and group composition naturally covary in diverse systems; for instance, along environmental gradients. We argue that these are vital next steps towards a comprehensive understanding of the behavioural and evolutionary ecology of collective behaviours.
Supplementary Material
Supplementary Material
Acknowledgements
We thank C. Tate Hollbrook, Sasha Dall, Simona Kralj-Fišer and one anonymous reviewer for significantly improving the quality of this manuscript. We are extremely thankful for the kindness and helpfulness provided by Koekais Guest Farm, and to Colin M. Wright and Lauren Keiser for field assistance.
Ethics statement
We thank the South Africa Department of Tourism, Environment and Conservation for providing permits for field research (FAUNA 1060/2012).
Data accessibility
The data associated with this manuscript have been deposited at Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.895b2/1).
Funding statement
Funding for this research was provided by the University of Pittsburgh and the National Science Foundation (IOS 1352705).
References
- 1.Ingram KK, Pilko A, Heer J, Gordon DM. 2013. Colony life history and lifetime reproductive success of red harvester ant colonies. J. Anim. Ecol. 82, 540–550. ( 10.1111/1365-2656.12036) [DOI] [PubMed] [Google Scholar]
- 2.Gordon DM. 2013. The rewards of restraint in the collective regulation of foraging by harvester ant colonies. Nature 498, 91–93. ( 10.1038/nature12137) [DOI] [PubMed] [Google Scholar]
- 3.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 4.Kralj-Fišer S, Schuett W. 2014. Studying personality variation in invertebrates: why bother? Anim. Behav. 91, 41–52. ( 10.1016/j.anbehav.2014.02.016) [DOI] [Google Scholar]
- 5.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 6.Brown C, Irving E. 2014. Individual personality traits influence group exploration in a feral guppy population. Behav. Ecol. 25, 95–101. ( 10.1093/beheco/art090) [DOI] [Google Scholar]
- 7.Keiser CN, Jones DK, Modlmeier AP, Pruitt JN. 2014. Exploring the effects of individual traits and within-colony variation on task differentiation and collective behavior in a desert social spider. Behav. Ecol. Sociobiol. 68, 839–850. ( 10.1007/s00265-014-1696-9) [DOI] [Google Scholar]
- 8.Modlmeier AP, Liebmann JE, Foitzik S. 2012. Diverse societies are more productive: a lesson from ants. Proc. R. Soc. B 279, 2142–2150. ( 10.1098/rspb.2011.2376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pruitt JN. 2012. Behavioural traits of colony founders affect the life history of their colonies. Ecol. Lett. 15, 1026–1032. ( 10.1111/j.1461-0248.2012.01825.x) [DOI] [PubMed] [Google Scholar]
- 10.Pruitt JN. 2013. A real-time eco-evolutionary dead-end strategy is mediated by the traits of lineage progenitors and interactions with colony invaders. Ecol. Lett. 7, 879–886. ( 10.1111/ele.12123) [DOI] [PubMed] [Google Scholar]
- 11.Harcourt JL, Ang TZ, Sweetman G, Johnstone RA, Manica A. 2009. Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252. ( 10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]
- 12.Dyer JR, Croft DP, Morrell LJ, Krause J. 2009. Shoal composition determines foraging success in the guppy. Behav. Ecol. 20, 165–171. ( 10.1093/beheco/arn129) [DOI] [Google Scholar]
- 13.Kurvers RHJM, Prins HHT, van Wieren SE, van Oers K, Nolet BA, Ydenberg RC. 2010. The effect of personality on social foraging: shy barnacle geese scrounge more. Proc. R. Soc. B 277, 601–608. ( 10.1098/rspb.2009.1474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinsted L, Pruitt JN, Settepani V, Bilde T. 2013. Individual personalities shape task differentiation in a social spider. Proc. R. Soc. B 280, 20131407 ( 10.1098/rspb.2013.1407) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Pruitt JN, Grinsted L, Settepani V. 2013. Linking levels of personality: personalities of the ‘average’ and ‘most extreme’ group members predict colony-level personality. Anim. Behav. 86, 391–399. ( 10.1016/j.anbehav.2013.05.030) [DOI] [Google Scholar]
- 16.Settepani V, Grinsted L, Granfeldt J, Jensen JL, Bilde T. 2013. Task specialization in two social spiders, Stegodyphus sarasinorum (Eresidae) and Anelosimus eximius (Theridiidae). J. Evol. Biol. 26, 51–62. ( 10.1111/jeb.12024) [DOI] [PubMed] [Google Scholar]
- 17.Wright CM, Holbrook CT, Pruitt JN. 2014. Animal personality aligns task specialization and task proficiency in a spider society. Proc. Natl Acad. Sci. USA 111, 9533–9537. ( 10.1073/pnas.1400850111) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Dornhaus A. 2008. Specialization does not predict individual efficiency in an ant. PLoS Biol. 6, e285 ( 10.1371/journal.pbio.0060285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang AT, Sih A. 2013. Multilevel selection and effects of keystone hyperaggressive males on mating success and behavior in stream water striders. Behav. Ecol. 24, 1166–1176. ( 10.1093/beheco/art044) [DOI] [Google Scholar]
- 20.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Clark CW, Mangel M. 1986. The evolutionary advantages of group foraging. Theor. Popul. Biol. 30, 45–75. ( 10.1016/0040-5809(86)90024-9) [DOI] [Google Scholar]
- 22.Dornhaus A, Powell S, Bengston S. 2012. Group size and its effects on collective organization. Annu. Rev. Entomol. 57, 123–141. ( 10.1146/annurev-ento-120710-100604) [DOI] [PubMed] [Google Scholar]
- 23.Leticia A, Paul T. 1998. Colony size and individual fitness in the social spider Anelosimus eximius. Am. Nat. 152, 403–418. ( 10.1086/286178) [DOI] [PubMed] [Google Scholar]
- 24.Gautrais J, Theraulaz G, Deneubourg J-L, Anderson C. 2002. Emergent polyethism as a consequence of increased colony size in insect societies. J. Theor. Biol. 215, 363–373. ( 10.1006/jtbi.2001.2506) [DOI] [PubMed] [Google Scholar]
- 25.Jeanson R, Fewell JH, Gorelick R, Bertram SM. 2007. Emergence of increased division of labor as a function of group size. Behav. Ecol. Sociobiol. 62, 289–298. ( 10.1007/s00265-007-0464-5) [DOI] [Google Scholar]
- 26.Holbrook CT, Barden PM, Fewell JH. 2011. Division of labor increases with colony size in the harvester ant Pogonomyrmex californicus. Behav. Ecol. 22, 960–966. ( 10.1093/beheco/arr075) [DOI] [Google Scholar]
- 27.Milgram S, Bickman L, Berkowitz L. 1969. Note on the drawing power of crowds of different size. J. Pers. Soc. Psychol. 13, 79 ( 10.1037/h0028070) [DOI] [Google Scholar]
- 28.Karau SJ, Williams KD. 1993. Social loafing: a meta-analytic review and theoretical integration. J. Pers. Soc. Psychol. 65, 681–706. ( 10.1037/0022-3514.65.4.681) [DOI] [Google Scholar]
- 29.Jeanson R, Fewell JH. 2008. Influence of the social context on division of labor in ant foundress associations. Behav. Ecol. 19, 567–574. ( 10.1093/beheco/arn018) [DOI] [Google Scholar]
- 30.Purcell J, Avilés L. 2007. Smaller colonies and more solitary living mark higher elevation populations of a social spider. J. Anim. Ecol. 76, 590–597. ( 10.1111/j.1365-2656.2007.01228.x) [DOI] [PubMed] [Google Scholar]
- 31.Yip EC, Powers KS, Avilés L. 2008. Cooperative capture of large prey solves scaling challenge faced by spider societies. Proc. Natl Acad. Sci. USA 105, 11 818–11 822. ( 10.1073/pnas.0710603105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harwood G, Avilés L. 2013. Differences in group size and the extent of individual participation in group hunting may contribute to differential prey-size use among social spiders. Biol. Lett. 9, 20130621 ( 10.1098/rsbl.2013.0621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avilés L. 1997. 23• Causes and consequences of cooperation and permanent-sociality in spiders. In The evolution of social behavior in insects and arachnids (eds Choe JC, Crespi BJ.), pp. 476–498. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Lubin Y, Bilde T. 2007. The evolution of sociality in spiders. Adv. Study Behav. 37, 83–145. ( 10.1636/Hi11-51.1) [DOI] [Google Scholar]
- 35.Laskowski KL, Pruitt JN. 2014. Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proc. R. Soc. B 281, 20133166 ( 10.1098/rspb.2013.3166) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Henschel JR. 1998. Predation on social and solitary individuals of the spider Stegodyphus dumicola (Araneae, Eresidae). J. Arachnol. 26, 61–69. [Google Scholar]
- 37.Sumpter DJ, Krause J, James R, Couzin ID, Ward AJ. 2008. Consensus decision making by fish. Curr. Biol. 18, 1773–1777. ( 10.1016/j.cub.2008.09.064) [DOI] [PubMed] [Google Scholar]
- 38.Modlmeier AP, Keiser CN, Watters JV, Sih A, Pruitt JN. 2014. The keystone individual concept: an ecological and evolutionary overview. Anim. Behav. 89, 53–62. ( 10.1016/j.anbehav.2013.12.020) [DOI] [Google Scholar]
- 39.Schneider JM, Roos J, Lubin Y, Henschel JR. 2001. Dispersal of Stegodyphus dumicola (Araneae, Eresidae): they do balloon after all! J. Arachnol. 29, 114–116. ( 10.2307/3706128) [DOI] [Google Scholar]
- 40.Johannesen J, Hennig A, Dommermuth B, Schneider JM. 2002. Mitochondrial DNA distributions indicate colony propagation by single matri-lineages in the social spider Stegodyphus dumicola (Eresidae). Biol. J. Linnean Soc. 76, 591–600. ( 10.1046/j.1095-8312.2002.00082.x) [DOI] [Google Scholar]
- 41.Smith ML, Ostwald MM, Loftus JC, Seeley TD. 2014. A critical number of workers in a honeybee colony triggers investment in reproduction. Naturwissenschaften 101, 783–790. ( 10.1007/s00114-014-1215-x) [DOI] [PubMed] [Google Scholar]
- 42.Nentwig W. 1985. Social spiders catch larger prey: a study of Anelosimus eximius (Araneae: Theridiidae). Behav. Ecol. Sociobiol. 17, 79–85. ( 10.1007/BF00299433) [DOI] [Google Scholar]
- 43.Pruitt JN, Keiser CN. 2014. The personality types of key catalytic individuals shape colonies’ collective behaviour and success. Anim. Behav. 93, 87–95. ( 10.1016/j.anbehav.2014.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cote J, Fogarty S, Tymen B, Sih A, Brodin T. 2013. Personality-dependent dispersal cancelled under predation risk. Proc. R. Soc. B 280, 20132349 ( 10.1098/rspb.2013.2349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fogarty S, Cote J, Sih A. 2011. Social personality polymorphism and the spread of invasive species: a model. Am. Nat. 177, 273–287. ( 10.1086/658174) [DOI] [PubMed] [Google Scholar]
- 46.Hee JJ, Holway DA, Suarez AV, Case TJ. 2000. Role of propagule size in the success of incipient colonies of the invasive Argentine ant. Conserv. Biol. 14, 559–563. ( 10.1046/j.1523-1739.2000.99040.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with this manuscript have been deposited at Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.895b2/1).


