Abstract
Animals can decrease their individual risk of predation by forming groups. The encounter-dilution hypothesis extends the potential benefits of gregariousness to biting insects and vector-borne disease by predicting that the per capita number of insect bites should decrease within larger host groups. Although vector-borne diseases are common and can exert strong selective pressures on hosts, there have been few tests of the encounter-dilution effect in natural systems. We conducted an experimental test of the encounter-dilution hypothesis using the American robin (Turdus migratorius), a common host species for the West Nile virus (WNV), a mosquito-borne pathogen. By using sentinel hosts (house sparrows, Passer domesticus) caged in naturally occurring communal roosts in the suburbs of Chicago, we assessed sentinel host risk of WNV exposure inside and outside of roosts. We also estimated per capita host exposure to infected vectors inside roosts and outside of roosts. Sentinel birds caged inside roosts seroconverted to WNV more slowly than those outside of roosts, suggesting that social groups decrease per capita exposure to infected mosquitoes. These results therefore support the encounter-dilution hypothesis in a vector-borne disease system. Our results suggest that disease-related selective pressures on sociality may depend on the mode of disease transmission.
Keywords: encounter-dilution effect, vector-borne disease, social behaviour, disease ecology, West Nile virus, American robin
1. Introduction
A ‘selfish herd’ may attract more predators owing to greater visibility of the group, but individuals ‘dilute’ their risk of predation across all other group members [1]. This hypothesis has been extended to biting insects, and risk of exposure to vector-borne disease in a concept known as the ‘encounter-dilution effect’ [2,3]. Barring an increase in the number of insects attracted to a social group, social groups decrease the per capita vector biting rate by distributing the risk of insect bites across many individuals [4,5]. Given that a host's risk of disease exposure increases with the number of insect bites received [6,7], social groups may also decrease an individual's risk of exposure to vector-borne pathogens.
Empirical evidence for the encounter-dilution effect for vector-borne pathogen transmission is mixed and largely observational, although models predict the encounter-dilution effect in vector-borne disease systems [8,9]. Comparative studies of social species and non-social congeners have found higher prevalence of malaria and arboviruses in social species of bird and primates [10–13], contrary to the predictions of this hypothesis. Conversely, studies of livestock herds and avian flocks indicate that participation in groups does decrease per capita vector biting rates [5,14,15]. Nonetheless, definitive evidence of decreased host exposure to vector-borne pathogens as a result of group formation is scant [but see 7]. Vector-borne diseases can exert significant fitness effects on hosts [16,17], resulting in selective pressure favouring gregariousness. Nonetheless, this hypothesis currently lacks empirical support.
West Nile virus (WNV) is a vector-borne pathogen transmitted by Culex spp. mosquitoes [18]. WNV is maintained in a seasonal transmission cycle between vectors and wild birds [19,20]. The American robin (Turdus migratorius, hereafter ‘robin’) is an important host species in the WNV transmission cycle owing to high competency, i.e. the ability of robins to transmit and contract the disease during interactions with vectors [21,22]. Robins form communal roosts throughout their breeding season [23,24], which coincides with the peak transmission season of WNV. Previous work suggests communal robin roosts may enable localized transmission of WNV between birds and vectors [25].
We examined communal robin roosts in the west Chicago suburbs, a WNV ‘hotspot,’ [22,26] and conducted a field experiment to test the encounter-dilution effect . We assessed the major assumption of the encounter-dilution effect, that vector abundances and infection rates were similar inside and outside of social groups, by trapping mosquitoes at roost and non-roost sites during the 2010–2012 WNV transmission seasons. By housing sentinel birds in cages inside and outside of communal roost sites in 2012, we tested the following predictions: if the encounter-dilution effect acts on vector-borne pathogen transmission, then sentinel birds in communal roosts should have lower risk of exposure to WNV than those away from communal roosts. We predicted a lower per capita vector index (i.e. the estimated number of interactions a host has with infected mosquitoes per night) for birds in communal roosts.
2. Material and methods
We located five large (200–20 000 birds) communal robin roosts in Cook County, Illinois between May and October of 2010–2012. We trapped mosquitoes inside communal roosts and in residential areas, urban parks and natural areas away from roosts. The total extent of mosquito trapping effort was 21.7 km2 in 2010, and 7.86 km2 in 2011 and 2012. Average trap density during all 3 years was at least six traps per km2 at roost and non-roost locations. We placed 66 traps inside roosts and 270 traps in non-roost areas: this equated to 140 traps total in 2010, 133 in 2011 and 63 in 2012. Vector infection rates from mosquitoes captured in 2012 were also used to estimate the per capita vector index [27], i.e. the number of infected mosquitoes encountered per host per night at roost and non-roost sites associated with the sentinel bird experiment.
(a). Mosquito sampling
We estimated vector abundances and infection rates at roost and non-roost sites from 2010–2012 to verify hosts did not encounter more vectors or elevated infection rates in Culex spp. vectors (hereafter Culex) inside of communal roosts compared with outside of roosts. We deployed Centers for Disease Control and Prevention (CDC) CO2-baited light traps and infusion-baited gravid traps for one night per week from the beginning of June through to mid-October in 2010–2012. CDC carbon dioxide (CO2)-baited light traps were used to estimate abundances of host-seeking female mosquitoes of various species, as the CO2 given off by the traps simulates respiration by hosts. Infusion-baited gravid traps were used to enhance capture of Culex vectors already infected with WNV [28]. We identified all captured mosquitoes to species; an exception to this was Culex pipiens and Culex restuans, which were identified only to genus, as identification to species based on morphology is unreliable [29]. We then pooled mosquitoes based on species, unique trap identification and blood-fed status. We tested Culex pools for WNV infection following the protocols previously described [26].
(b). Assessing host risk of West Nile virus exposure
To estimate host risk of exposure to WNV at each site type, we conducted an experiment using sentinel birds. Sentinel birds (typically chickens or other galliforms) are commonly used by public health agencies to survey for transmission of vector-borne diseases such as WNV [30–32]. For this study, we used house sparrows (Passer domesticus, hereafter sparrows) as the sentinel species. We used sparrows rather than American robins, because sparrows are competent hosts for WNV [20], contribute significantly to the WNV transmission cycle in Chicago as determined from vector blood meal analysis [19], can be held in captivity with reasonable effort and unlike robins, are not protected by statute in North America.
In 2012, we selected three roost sites and three non-roost sites. The roost sites selected for this study occurred in dense stands of buckthorn or invasive Phragmites surrounded by industrial or residential areas. We selected non-roost sites based on similarity to habitats used by non-communally roosting robins observed from previous radiotelemetry work. We selected two residential sites with suitable roosting trees in residential backyards and one natural site with habitat similar to communal roosts used in the study but lacking a communal robin roost. Non-roost sites were at least 0.5 km away from any communal roost or other non-roost sites.
We captured, individually marked with coloured leg bands, and drew blood samples from free-living sparrows to screen for previous WNV exposure. After initial blood draws, we housed sparrows in flight cages at a field laboratory until screening for previous exposure was completed. We screened blood samples for WNV RNA using a quantitative RT-PCR and WNV antibodies using an inhibition ELISA as previously described [26], with one amendment being that viral RNA was extracted using the MagMAX viral RNA isolation kit (Applied Biosystems, Foster City, CA).
Birds that tested negative for WNV antibodies were transferred in groups of five to each of six field cages, three in roost sites and three in non-roost sites. Field cages were built from commercially available bird cages elevated onto four 3 m galvanized steel pipes. We coated the anchor poles with machine grease to prevent disturbance from the public or mammalian predators. Roost cages were placed in central locations within roosts, as determined by nocturnal surveys of the boundaries of the roost. Non-roost cages were placed within 1.5 m of the trunk of a suitable roost tree, within the foliage of the tree and with the top of the cage 3 m off the ground.
We deployed sentinel cages by the third week of July 2012, after which we drew blood samples by jugular venipuncture from each bird weekly for the next eight weeks. The timing of this experiment coincided with the historical period of peak WNV transmission in our study area [26]. Samples drawn from birds in field cages were tested as described above on a weekly basis. We maintained a group of WNV unexposed sparrows protected from exposure to vectors as ‘reserve’ birds. As birds housed in field cages became exposed to WNV, they were removed and replaced with unexposed birds from the reserve group. All birds were provided food and water ad libitum. Animal care, use and housing were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign, protocol no. 12047.
In 2012, we estimated the per capita vector index, the number of infected vectors encountered per host per night inside and outside of the three communal roosts used for the sentinel bird experiment. To estimate per capita biting rates of Culex on hosts, we used bird-baited traps. Bird-baited traps are modified CDC light traps that use a single bird as bait in place of CO2 and attract mosquitoes such as Culex that prefer feeding on avian hosts [33]. We constructed bird-baited mosquito traps following Emord & Morris [33]. A single sparrow was placed in the cage attached to the trap, and the trap was placed at least 20 m away from one of the sentinel bird cages for one night per week during the course of the sentinel bird study, which equated to six bird-baited trap nights per week over eight weeks. To protect bait birds from mosquitoes, all cages were lined with insect screening. Each bait bird was used in a trap only one night per week, and bait birds were kept separate from ‘reserve’ birds used in the sentinel bird experiment.
(c). Estimating vector abundance, infection rates and vector index
To describe overall Culex abundance and infection rates from 2010 to 2012, we pooled light and gravid traps into 19 spatially clustered groups; 14 outside of roosts and one inside each of five communal roosts. Spatial groups were assigned based on site type (i.e. roost or non-roost), separation by physical barriers, (e.g. major roadways) and overall habitat type (e.g. natural, residential or industrial areas). All spatial groups covered less than 2.5 km2 and had an average trap density of six gravid and light traps combined per km2. Each roost site had between two and four CDC CO2-baited light traps and two to four gravid traps each, and all traps within the same roost were assigned to the same spatial group. Vector abundance from light traps was estimated as the number of Culex captured per light trap night within each spatial group. We derived maximum-likelihood estimates of the minimum infection rate (MIR; number of infected mosquitoes per 1000 mosquitoes trapped [34]) of Culex mosquito pools from light and gravid traps with 95% confidence intervals by spatial group using the Pooled Infection Rate v. 3.0 add-in.
To assess the assumption of similar vector abundances within communal roosts and to determine whether communal roosts in our study system increased local infection rates in vectors, we compared infection rates inside and outside communal roosts from 2010 to 2012 from CDC CO2-baited light traps and gravid traps. We used Poisson regression to analyse Culex abundance and MIR in program R using the lme4 package; the response variable was either Culex per trap night or MIR, with fixed effects for site type (roost or non-roost), random effects for week nested in year and spatial group, and an offset term equal to the log of the number of traps set in a spatial group during a given week. The use of the offset term accounts for the variation in trap number set in a given week among spatial groups, allowing analysis of untransformed data [35]. For mosquito abundance estimates from bird-baited traps and the per host vector index, we used Poisson regression; we included site type as a fixed effect and a random effect for week.
To compare host encounter rates with infected vectors at roost and non-roost sites, we estimate the vector index at both site types in 2012. We calculated the weekly vector index by multiplying the per light trap night estimates of Culex abundance by the maximum-likelihood estimates of vector infection rates from Culex captured in the same light traps. To estimate the per capita vector index at roost and non-roost sites, we multiplied the MIR estimates from Culex pools from the bird-baited traps and the two closest light traps within 200 m of the bird-baited trap by the average number of Culex captured per trap night in bird-baited traps. We used MIR estimates from light traps only to estimate per host vector index, because vectors captured in light traps are actively host seeking and therefore capable of transmitting the virus upon their next blood meal. To this end, we assumed host-seeking behaviour of Culex to be similar when using bird-baited traps and light traps.
(d). Analysing host risk of West Nile virus exposure
Relative risk of sentinel bird exposure to WNV inside and outside communal roosts in 2012 was analysed using a Cox-proportional hazards mixed model in the coxme package in R. The proportional hazards model assumes the risk of an event occurring, in this case WNV infection of a given bird, is the same across all time intervals. We tested this assumption using the survival package in R, and as this assumption was not violated (p > 0.1), we used the proportional hazards model. The length of time an individual remained unexposed to WNV was analysed as dependent on a site type (roost or non-roost) with a random effect for individual birds nested within cage [36].
3. Results
(a). Vector abundance and infection rates at roost and non-roost sites
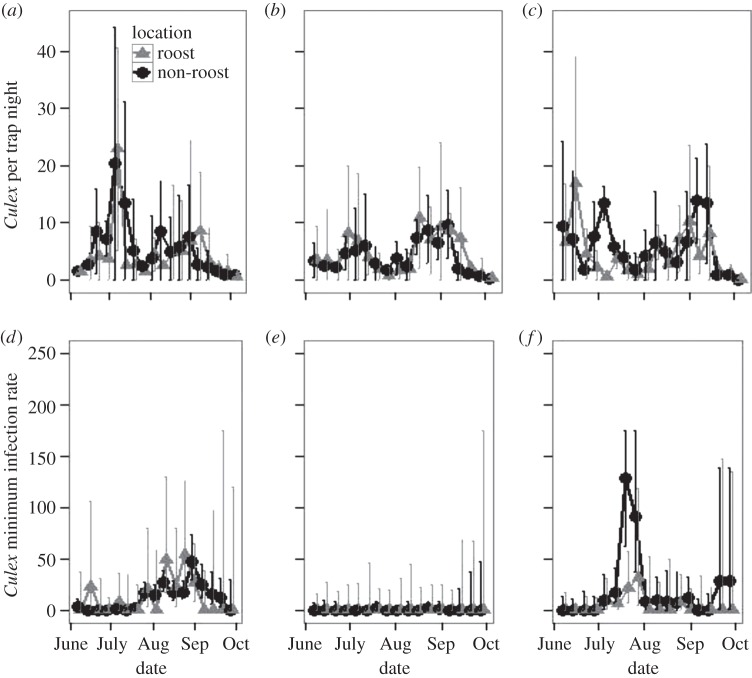
Our mosquito sampling effort totalled over 4000 trap nights of light and gravid traps across 3 years. We captured 18 158 Culex mosquitoes in light traps from 2010 to 2012, which we used to estimate abundances of host-seeking vectors inside and outside of communal roosts. With added sampling using gravid traps, we estimated MIR estimates from 2010 to 2012 using 40 761 Culex mosquitoes. Estimated Culex abundances were similar at roost and non-roost sites across all 3 years (z = 0.16, p > 0.05; figure 1a–c). Furthermore, the estimated MIR in Culex did not differ between roost and non-roost sites (z = −0.22, p > 0.05; figure 1d–f).
Figure 1.
Variation among years in average Culex per light trap night (a) 2010 (b) 2011, and (c) 2012 by trap location, ±2 median absolute deviations. (d–f) Culex minimum infection rate maximum-likelihood estimates (d) 2010 (e) 2011, and (f) 2012 by trap location, ±95% CIs. All figures based on data collected in Chicago, Illinois.
(b). Risk of host exposure to West Nile virus and vector indices
Between July and September 2012, we placed 23 sentinel birds in cages near communal roosts and 25 in non-roost cages. Only three sentinel birds near roosts seroconverted to WNV, whereas 11 birds in non-roost cages seroconverted. At every sentinel cage, at least one sentinel bird seroconverted to WNV during the experiment; therefore, Culex did feed on sentinel sparrows even at roost cages when numerous robins were present. The risk of WNV exposure for sentinel birds caged within roosts was significantly lower than for birds caged in non-roost locations (z = −2.17, p = 0.03; figure 2).
Figure 2.
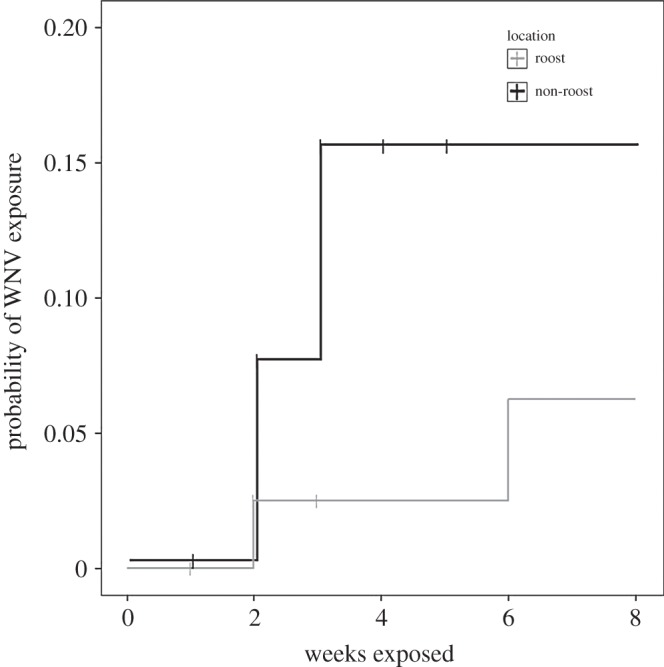
Kaplan–Meier plot of risk to an individual sentinel bird by cage location. Tick marks indicate individuals censored at that interval (e.g. owing to non-West Nile virus related mortality or escape after blood sampling). Plots are offset to ease interpretation.
Estimated per capita vector encounter rates with hosts in 2012 were consistent with results of the sentinel experiment. Vector encounter rates were significantly greater away from roosts (z = −9.568, p < 0.001). Moreover, the vector index was approximately one-third of that within communal roosts than outside of communal roosts (z = −17.4, p < 0.001; figure 3a). The average estimated per capita vector index was 1.1 infected vectors per host per night outside of roosts, which was over 40 times higher than the vector index inside roosts (0.025 infected vectors per host per night; figure 3b).
Figure 3.

All data were collected in Chicago, Illinois. (a) Average vector index, or number of infected Culex mosquitoes captured per light trap night, at roost and non-roost locations in 2012±1 standard error. (b) Average per capita vector index at roost and non-roost locations, between July and September 2012, ±1 standard error.
4. Discussion
Our results indicate that the individual risk of exposure to WNV is lower within communal roosts than outside of them. This finding supports the encounter-dilution hypothesis for transmission of vector-borne pathogens. Our data also suggest that decreased individual risk was mediated by decreased per capita interactions with infected vectors within social aggregations. Furthermore, Culex abundance and MIR did not differ between roost and non-roost sites across 3 years of observation, indicating roosts do not attract more WNV vectors than other areas, nor does host aggregation enable WNV transmission, making the action of the encounter-dilution effect possible in this vector-borne disease system.
Increased exposure to directly transmitted diseases has been considered a selective force acting against sociality [37], but our results support the idea that the mode of disease transmission determines the relationship between diseases and host grouping behaviour. Disease transmission models often consider how transmission responds to host density, which typically falls into one of two categories: density-dependent or frequency-dependent transmission. Diseases transmitted directly from host-to-host or through the environment (e.g. soil or water contamination) are typically density-dependent, meaning locally greater densities of hosts leads to increased disease transmission [38,39]. Transmission of vector-borne pathogens by contrast is frequency-dependent, such that the proportion of infected individuals (vectors or hosts) has more impact on disease transmission than the absolute numbers of hosts or vectors [40,41]. Further, in vector-borne disease systems where vectors are not differentially attracted to groups, vector–host interactions become directly analogous to the predator–prey interactions: larger groups result in a decreased per capita risk of interactions with a predator or infectious vector, and vectors or predators may exhibit a functional response at higher host or prey density [1–3,7,42]. In our system, increasing the number of hosts in a roost without changing the number of infected vectors decreases the ratio of infected vectors to available hosts, resulting in decreased risk of host exposure to WNV. Transmission of vector-borne pathogens thus behaves more similarly to predator–prey interactions than does the transmission of directly transmitted pathogens with respect to host grouping behaviour. Our results indicate group formation by hosts in some vector-borne disease systems can provide a selective advantage to social versus non-social individuals.
Many factors can favour gregariousness in animals, including reduced predation risk or increased foraging efficiency in groups [1,3]. Although decreased individual risk of exposure to WNV is a selective advantage to group formation in our system, it is probably not the only advantage to communal roosting. Nuisance feeding by biting insects can alter host behaviour [43], reduce fitness by increasing energy expended during behavioural defences [44,45] and reduce breeding success [46,47]. Consequently, decreased exposure to biting insects within communal roosts may benefit social individuals even in the absence of infection. Under this scenario, the selective advantages of sociality would increase in the presence of vector-borne infections owing to compounding effects of vector feeding and vector-borne infections [16,17]. At the same time, WNV is a recently introduced selective pressure in our system [19,20], whereas communal robin roosts have been documented throughout North America for over a century [23]. Therefore, it is unlikely WNV has significantly changed communal roosting behaviours in robins since its introduction to North America. Furthermore, WNV prevalence in vectors and hosts varies widely within and among years (figure 3 and [26]), and disease-related benefits of participating in a communal roost may be less pronounced during years of low WNV prevalence. Nonetheless, our results provide evidence of an emerging selective advantage to aggregation in a highly competent host species, which has important implications for future studies of emerging infectious diseases.
A potential confounder in our experimental design is vector preference for robins over house sparrows [18]. Infectious vectors could have fed on robins in communal roosts and avoided the nearby sentinel sparrows. However, sentinel bird exposure to WNV occurred at all sentinel cages throughout the duration of the experiment; therefore, Culex were attracted to and fed on sparrows even in the presence of large numbers of robins. In addition, the estimated per capita vector index, or number of infected mosquitoes encountered per host per night inside communal roosts was 0.025, compared with 1.1 away from communal roosts. Therefore, any host species using a communal roost would experience decreased risk of contact with infectious vectors. Finally, among a ranking of 25 bird species found to have been fed upon by Culex vectors in our study systems, the rate of transmission of WNV was estimated to be highest from American robins and then house sparrows [19], indicating that Culex vectors feed readily on house sparrows.
Under the original selfish herd and encounter-dilution hypotheses, more central locations within a group are more desirable owing to decreased exposure to predators [1] or biting insects [2–4]. Therefore, more favourable central positions should be held by dominant individuals or competed for among group members. Similarly, within communal roosts, more dominant individuals should seek to occupy central and more elevated positions, while relegating younger and less dominant birds to less favourable positions [48–50]. Demographic stratification within roosts may have important implications for vector-borne disease exposure, as different vector species feed at different heights within a habitat [51–55]. For example, one study found WNV infection rates in vectors were greater in the canopy than at ground level [55], and another found evidence suggesting differences in roosting positions can influence vector–host interactions [56]. While lower positions within a roost may be less favourable in terms of other potential benefits, less dominant individuals relegated to these positions may still benefit from decreased exposure to vector-borne diseases, particularly when vector feeding patterns or infection rates are vertically stratified. We placed sentinel bird cages within communal roosts at central locations based on nocturnal surveys of roost boundaries, suggesting our results are indicative of the benefits received by individual occupying preferred central locations within roosts. We did not assess the impact of host location within the roost on risk of host exposure to vector-borne diseases. Future work should examine the heterogeneity of host risk of exposure to vector-borne illnesses within groups to examine whether all members of a group benefit equally.
Acknowledgements
We thank the Village of Oak Lawn for providing field laboratory facilities, Palos Hills, the City of Chicago and private homeowners for allowing us to conduct this research. T. Thompson, D. Gohde, P. Kelly, A. Gardner, M. Gorinshteyn, S. Janairo, C. Hutter, L. Kmieciak, Z. Allison, A. Dolinski, N. Gregory and C. Hartley assisted with collecting field data. E. Boothe, A. Golnar, and L. Auckland assisted with laboratory analyses.
Ethics statement
Animal care, use and housing were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign, protocol no. 12047.
Data accessibility
Data are available at datadryad.org (doi:10.5061/dryad.fp7s8).
Funding statement
This project was supported by the National Science Foundation Ecology of Infectious Disease programme award number 0840403.
References
- 1.Hamilton WD. 1971. Geometry for the selfish herd . J. Theor. Biol. 31, 295–311. ( 10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 2.Mooring M, Hart B. 1992. Animal grouping for protection from parasites: selfish herd and encounter-dilution effects . Behaviour 123, 173–193. ( 10.1163/156853992X00011) [DOI] [Google Scholar]
- 3.Turner G, Pitcher T. 1986. Attack abatement: a model for group protection by combined avoidance and dilution . Am. Nat. 128, 228–240. ( 10.1086/284556) [DOI] [Google Scholar]
- 4.Fauchald P, Rodven R, Bardsen B, Langeland K, Tveraa T, Yoccoz NG, Ims RA. 2007. Escaping parasitism in the selfish herd: age, size and density-dependent warble fly infestation in reindeer . Oikos 116, 491–499. ( 10.1111/j.0030-1299.2007.15390.x) [DOI] [Google Scholar]
- 5.Ratti O, Ojanen U, Helle P. 2006. Increasing group size dilutes black fly attack rate in black grouse . Ornis Fennica 83, 86–90. [Google Scholar]
- 6.Dye C. 1992. The analysis of parasite transmission by bloodsucking insects . Annu. Rev. Entomol. 37, 1–19. ( 10.1146/annurev.en.37.010192.000245) [DOI] [PubMed] [Google Scholar]
- 7.Freeland WJ. 1977. Bloodsucking flies and primate poly-specific associations . Nature 269, 801 ( 10.1038/269801a0) [DOI] [PubMed] [Google Scholar]
- 8.Cummins B, Cortez R, Foppa IM, Walbeck J, Hyman JM. 2012. A spatial model of mosquito host-seeking behavior . PLoS Comput. Biol. 8, e1002500 ( 10.1371/journal.pcbi1002500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wonham MJ, Lewis MA, Renclawowicz J, van den Driessche P. 2006. Transmission assumptions generate conflicting predictions in host-vector disease models: a case study in West Nile virus . Ecol. Lett. 9, 706–725. ( 10.1111/j.1461-0248.2006.00912.x) [DOI] [PubMed] [Google Scholar]
- 10.Nunn C, Heymann E. 2005. Malaria infection and host behavior: a comparative study of neotropical primates . Behav. Ecol. Sociobiol. 59, 30–37. ( 10.1007/s00265-005-0005-z) [DOI] [Google Scholar]
- 11.Tella JL. 2002. The evolutionary transition to coloniality promotes higher blood parasitism in birds . J. Evol. Biol. 15, 32–41. ( 10.1046/j.1420-9101.2002.00375.x) [DOI] [Google Scholar]
- 12.Brown CR, Komar N, Quick SB, Sethi RA, Panella NA, Brown MB, Pfeffer M. 2001. Arbovirus infection increases with group size . Proc. R. Soc. Lond. B 268, 1833–1840. ( 10.1098/rspb.2001.1749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies C, Ayres J, Dye C, Deane L. 1991. Malaria infection-rate of Amazonian primates increases with body-weight and group-size . Funct. Ecol. 5, 655–662. ( 10.2307/2389485) [DOI] [Google Scholar]
- 14.Foppa IM, Moore J, Caillouet KA, Wesson DM. 2011. Disproportionate mosquito feeding on aggregated hosts . J. Med. Entomol. 48, 1210–1213. ( 10.1603/ME11007) [DOI] [PubMed] [Google Scholar]
- 15.Torr SJ, Prior A, Wilson PJ, Schofield S. 2007. Is there safety in numbers? The effect of cattle herding on biting risk from tsetse flies . Med. Vet. Entomol. 21, 301–311. ( 10.1111/j.1365-2915.2007.00705.x) [DOI] [PubMed] [Google Scholar]
- 16.Garvin MC, Szell CC, Moore FR. 2006. Blood parasites of nearctic–neotropical migrant passerine birds during spring trans-gulf migration: impact on host body condition . J. Parasitol. 92, 990–996. ( 10.1645/GE-758R.1) [DOI] [PubMed] [Google Scholar]
- 17.Marzal A, Bensch S, Reviriego M, Balbontin J, de Lope F. 2008. Effects of malaria double infection in birds: one plus one is not two . J. Evol. Biol. 21, 979–987. ( 10.1111/j.1420-9101.2008.01545.x) [DOI] [PubMed] [Google Scholar]
- 18.Simpson JE, Folsom-O'Keefe CM, Childs JE, Simons LE, Andreadis TG, Diuk-Wasser MA. 2009. Avian host-selection by Culex pipiens in experimental trials . PLoS ONE 4, e7861 ( 10.1371/journal.pone.0007861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamer GL, Chaves LF, Anderson TK, Kitron UD, Brawn JD, Ruiz MO, Loss SR, Walker ED, Goldberg TL. 2011. Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission . PLoS ONE 6, e23767 ( 10.1371/journal.pone.0023767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus . Emerg. Infect. Dis. 9, 311–322. ( 10.3201/eid0903.020628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. 2006. Host heterogeneity dominates West Nile virus transmission . Proc. R. Soc. B 273, 2327–2333. ( 10.1098/rspb.2006.3575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. 2009. Host selection by Culex pipiens mosquitoes and West Nile virus amplification . Am. J. Trop. Med. Hyg. 80, 268–278. [PubMed] [Google Scholar]
- 23.Brewster W. 1890. Summer robin roosts . Auk 7, 360–373. ( 10.2307/4067557) [DOI] [Google Scholar]
- 24.Howell JC. 1940. Spring roosts of the robin . Wilson Bull. 52, 19–23. [Google Scholar]
- 25.Diuk-Wasser MA, Molaei G, Simpson JE, Folsom-O'Keefe CM, Armstrong PM, Andreadis TG. 2010. Avian communal roosts as amplification foci for West Nile virus in urban areas in northeastern United States . Am. J. Trop. Med. Hyg. 82, 337–343. ( 10.4269/ajtmh.2010.09-0506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamer GL, et al. 2008. Rapid amplification of West Nile virus: the role of hatch-year birds . Vector Borne Zoonotic Dis. 8, 57–67. ( 10.1089/vbz.2007.0123) [DOI] [PubMed] [Google Scholar]
- 27.Jones RC, Weaver KN, Smith S, Blanco C, Flores C, Gibbs K, Markowski D, Mutebi J. 2011. Use of the vector index and geographic information system to prospectively inform West Nile virus interventions . J. Am. Mosq. Control Assoc. 27, 315–319. ( 10.2987/10-6098.1) [DOI] [PubMed] [Google Scholar]
- 28.Williams GM, Gingrich JB. 2007. Comparison of light traps, gravid traps, and resting boxes for West Nile virus surveillance . J. Vector Ecol. 32, 285–291. ( 10.3376/1081-1710(2007)32[285:COLTGT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 29.Harrington LC, Poulson RL. 2008. Considerations for accurate identification of adult Culex restuans (Diptera: Culicidae) in field studies. J. Med. Entomol. 45, 1–8. ( 10.1603/0022-2585(2008)45[1:CFAIOA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 30.Chuang T, Knepper RG, Stanuszek WW, Walker ED, Wilson ML. 2011. Temporal and spatial patterns of West Nile virus transmission in Saginaw County, Michigan, 2003–2006 . J. Med. Entomol. 48, 1047–1056. ( 10.1603/ME10138) [DOI] [PubMed] [Google Scholar]
- 31.Reisen WK, Carroll BD, Takahashi R, Fang Y, Garcia S, Martinez VM, Quiring R. 2009. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007 . J. Med. Entomol. 46, 139–157. ( 10.1603/033.046.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan JL, Kluh S, Madon MB, Nguyen DV, Barker CM, Reisen WK. 2010. Sentinel chicken seroconversions track tangential transmission of West Nile virus to humans in the greater Los Angeles area of California . Am. J. Trop. Med. Hyg. 83, 1137–1145. ( 10.4269/ajtmh.2010.10-0078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emord D, Morris C. 1982. A host-baited CDC trap . Mosq. News 42, 220–224. [Google Scholar]
- 34.Walter SD, Hildreth SW, Beaty BJ. 1980. Estimation of infection-rates in populations of organisms using pools of variable size. Am. J. Epidemiol. 112, 124–128. [DOI] [PubMed] [Google Scholar]
- 35.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, pp. 238–244. Princeton, NJ: Princeton University Press. [Google Scholar]
- 36.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 37.Alexander RD. 1974. The evolution of social behaviour. Annu. Rev. Ecol. Syst. 5, 325–383. ( 10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 38.Anderson RM, May RM. 1979. Population biology of infectious diseases. 1. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 39.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. 2005. Superspreading and the effect of individual variation on disease emergence . Nature 438, 355–359. ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson RMMRM. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press. [Google Scholar]
- 41.Kilpatrick AMAS. 2010. Disease ecology . Nat. Educ. Knowl. 3, 55. [Google Scholar]
- 42.Cote I, Poulin R. 1995. Parasitism and group-size in social animals: a meta-analysis . Behav. Ecol. 6, 159–165. ( 10.1093/beheco/6.2.159) [DOI] [Google Scholar]
- 43.Rohner C, Krebs CJ, Hunter DB, Currie DC. 2000. Roost site selection of great horned owls in relation to black fly activity: an anti-parasite behavior? Condor 102, 950–955. ( 10.1650/0010-5422(2000)102[0950:RSSOGH]2.0.CO;2) [DOI] [Google Scholar]
- 44.Toupin B, Huot J, Manseau M. 1996. Effect of insect harassment on the behaviour of the Riviere George Caribou . Arctic 49, 375–382. ( 10.14430/arctic1213) [DOI] [Google Scholar]
- 45.Downes CM, Theberge JB, Smith SM. 1986. The influence of insects on the distribution, microhabitat choice, and behavior of the Burwash Caribou herd . Can. J. Zool. 64, 622–629. ( 10.1139/z86-092) [DOI] [Google Scholar]
- 46.Smith RN, Cain SL, Anderson SH, Dunk JR, Williams ES. 1998. Blackfly-induced mortality of nestling red-tailed hawks . Auk 115, 368–375. ( 10.2307/4089195) [DOI] [Google Scholar]
- 47.Richner H, Oppliger A, Christe P. 1993. Effect of an ectoparasite on reproduction in great tits . J. Anim. Ecol. 62, 703–710. ( 10.2307/5390) [DOI] [Google Scholar]
- 48.Swingland IR. 1977. Social and spatial-organization of winter communal roosting in rooks (Corvus frugilegus) . J. Zool. 182, 509–528. ( 10.1111/j.1469-7998.1977.tb04167.x) [DOI] [Google Scholar]
- 49.Weatherhead PJ. 1983. Two principal strategies in avian communal roosts . Am. Nat. 121, 237–243. ( 10.1086/284053) [DOI] [Google Scholar]
- 50.McGowan A, Sharp SP, Simeoni M, Hatchwell BJ. 2006. Competing for position in the communal roosts of long-tailed tits . Anim. Behav. 72, 1035–1043. ( 10.1016/j.anbehav.2006.02.020) [DOI] [Google Scholar]
- 51.Cerny O, Votypka J, Svobodova M. 2011. Spatial feeding preferences of ornithophilic mosquitoes, blackflies and biting midges . Med. Vet. Entomol. 25, 104–108. ( 10.1111/j.1365-2915.2010.00875.x) [DOI] [PubMed] [Google Scholar]
- 52.Nasci RS, Edman JD. 1981. Vertical and temporal flight activity of the mosquito Culiseta melanura (Diptera, Culicidae) in southeastern Massachusetts . J. Med. Entomol. 18, 501–504. [DOI] [PubMed] [Google Scholar]
- 53.Savage HM, et al. 2008. Host-seeking heights, host-seeking activity patterns, and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby County TN, 2002 (Diptera: Culicidae) . J. Med. Entomol. 45, 276–288. ( 10.1603/0022-2585(2008)45[276:HHHAPA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 54.Jansen CC, Zborowski P, Ritchie SA, van den Hurk AF. 2009. Efficacy of bird-baited traps placed at different heights for collecting ornithophilic mosquitoes in eastern Queensland, Australia . Aust. J. Entomol. 48, 53–59. ( 10.1111/j.1440-6055.2008.00671.x) [DOI] [Google Scholar]
- 55.Anderson JF, Andreadis TG, Main AJ, Ferrandino FJ, Vossbrinck CR. 2006. West Nile virus from female and male mosquitoes (Diptera: Culicidae) in subterranean, ground, and canopy habitats in Connecticut . J. Med. Entomol. 43, 1019 ( 10.1603/0022-2585(2006)43[1010:WNVFFA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 56.Janousek WM, Marra PPM, Kilpatrick AM. 2014. Avian roosting behavior influences vector–host interactions for West Nile virus hosts. Parasit. Vectors 7, 399 ( 10.1186/1756-3305-7-399) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at datadryad.org (doi:10.5061/dryad.fp7s8).