Abstract
The challenges of maintaining cohesion while making collective decisions in social or aggregating insects can result in the emergence of a leader or leaders. Larval aggregations of the steel-blue sawfly Perga affinis forage nocturnally, and some larvae lead the aggregation on foraging trips more often than expected by chance. We investigated the relationship between these leader and follower roles by comparing the weight and growth of individual larvae with different roles. Our observations reveal no significant difference between the growth of leaders and followers, suggesting that the role of leadership may not provide direct foraging benefits. However, by experimentally manipulating the social structure of larval aggregations, we found that individuals within aggregations that comprise a mixture of leaders and followers enjoy higher growth rates than those in aggregations comprising a single behavioural type. These data demonstrate, for the first time, individual benefits to maintaining a balance of leader and follower roles within larval aggregations, and highlight the importance of considering the perspectives of both leaders and followers when investigating the evolutionary significance of this behavioural variation within animal groups.
Keywords: collective movement, followers, larval aggregation, leadership, consensus decision-making
1. Introduction
In order to retain the benefits of aggregated living, individuals must move together—a complex decision when individuals do not benefit equally [1] or when individual resource requirements differ [2]. The conflicts and congruencies of interest associated with collective movement [3] can be at least partially overcome with the emergence of a leader who can initiate and steer the behaviour of the grouped followers. Leaders are generally positioned at the front of group movements, not only to initiate that movement, but also to guide direction [4]. This paradigm of leaders and followers can function in either an unshared or a shared fashion (also termed despotic or democratic consensus) [4,5]. A typical unshared structure occurs where dominant individuals lead the group [6,7], and followers comply with decisions even when the relative cost to themselves is high [4,8]. By contrast, leaders within a shared consensus system depend on the inclination of the majority to follow, and thus such roles can be temporally variable [9].
The emergence and persistence of leaders and followers in group-living animals that lack an obvious means for establishing hierarchy are poorly understood. Because followers in a shared system may choose whether or not to follow a certain individual, they can influence both collective movements and which individuals become leaders [10]. In some shared systems, individuals emerge as leaders because they are more informed, or have knowledge that is beneficial to the timing or direction of movement [11,12]. Such individual information can be crucial for a group when relocating or foraging, with informed individuals leading, or guiding, the uninformed [13]. Alternatively, individuals that are more highly motivated may be more likely to initiate movement and become leaders [1,14]. For example, fish that occupy the front positions of shoals may be nutritionally challenged and are thus more motivated to forage [15], whereas females in energetically demanding reproductive states tend to lead foraging movements in several group-living mammals [16,17].
While these studies highlight the role of the leader and the motivations behind the individuals who assume leadership, they provide little insight into why followers would allow such social arrangements to persist. More specifically, studies have not investigated the benefits to followers of allowing others to initiate and lead collective movements. Such analyses require experimental manipulation of animal groups, which are currently lacking.
Insects that aggregate as larvae are helpful model species to explore these questions because their activities are typically synchronized, and individuals generally maintain close contact with conspecifics [18]. Nevertheless, even in such seemingly homogeneous groups, individuals can exhibit behavioural differences, and collective decision-making can be strongly influenced by the behaviour of a few individuals [19]. Perga affinis sawfly larvae are endemic to Australia and form social groups that are maintained throughout their seven-month larval period [18]. They readily amalgamate with other groups of Perga, irrespective of instar or species, to create huge aggregations of individual larvae that can defoliate a Eucalyptus tree within a few days [20]. These larval groups are nocturnally active, forming stationary ‘resting clusters’ during the day and beginning the movement out to forage at dusk [21]. This movement to the outer leaves of the tree is slow, and individuals maintain constant contact with each other as the shape of the aggregation elongates (see the video in the electronic supplementary material). Larvae feed in groups on adjacent leaves overnight and regroup into a new cluster before dawn. Observations of P. dorsalis (a closely related species) suggest that around 20% of larvae consistently inhabit peripheral positions as the group moves out to forage, indicating the presence of leaders [22]. However, the characteristics, if any, shared by these leaders (and their followers), and the benefits of leadership roles in foraging decisions, are unknown.
These features of sawflies allow for large scale, experimental investigations of the fitness consequences of leadership. Here, we use P. affinis larvae to explore the differences between leaders and followers at the individual level as well as how leaders influence the collective performance of the group. Thus, we investigate whether the growth of an individual correlates with its role (leader or follower) within the aggregation. Using this information, we then manipulate the ratios of leaders and followers within aggregations to determine how this impacts individual fitness. Thus, we can consider the consequences of structured roles for both followers and leaders.
2. Methods
Field experiments were conducted at a eucalypt plantation within the Phillip Island Koala Conservation Centre, Victoria, Australia (GPS: −38.49657, 145.220131), during the winter of 2011. The trees had been planted three years previously in a grid pattern approximately 1 m apart and included Eucalyptus ovata and Eucalyptus viminalis. Observations were conducted between 16.00 and 21.00, as the timing of the single foraging movement of each larval aggregation varied considerably each night.
(a). The presence of leaders
In order to confirm that larvae within the experimental population behave as consistent leaders (as observed in P. dorsalis [22]), ten P. affinis sawfly aggregations with a range of 20–28 larvae per aggregation (average = 23.1, n = 10) were identified. A minimum of 20 individuals per aggregation was set, because aggregations with fewer larvae generally have high mortality and low growth rates [23]. Individuals from all aggregations were removed from their trees and individually weighed (0.001–30 g portable diamond scales, YC Scale Co.). They were then painted with an individual colour combination using enamel paints (Revell). The paint was applied to the dorsal surface of the larvae, below the head and roughly one quarter of the length of the larvae. Two lines of different coloured paint were used (left and right) in order to distinguish between individual larvae. Colours were chosen arbitrarily. Larvae were then positioned randomly within their original aggregation and replaced on their original tree.
Each aggregation was allowed to recover for two nights before monitoring began. There were two monitoring periods, one of 15 and one of 14 nights, a length of time that minimized the number of larvae that moulted and thus lost their identifying colours during the monitoring period. At the end of the first monitoring period, all surviving larvae were removed from their trees, weighed, repainted and replaced. The second monitoring period commenced two days later. During each monitoring period, we recorded the identity of those larvae that were leading this movement (using a torch to distinguish colours) within each aggregation. Leaders were defined as the three individuals at the front of the moving aggregation (which contracts into a single line), as these larvae most clearly displayed the leadership characteristics of aiding the initiation, encouragement and direction of aggregation movement. This definition of leadership follows Weinstein [22], except we did not include larvae at the rear of the aggregation because they did not appear to play an active role in the movement of the aggregation (L.K.H. 2011, personal observation). In rare instances, when an aggregation splits into multiple directions, the first larva in each direction was recorded as a leader. Where the aggregation moved in two directions, the third larva was identified from the largest of the two aggregations. At the end of the second monitoring period, all larvae were removed from their trees and weighed again, allowing us to calculate individual weight gain (represented as percentage growth) within each of the monitoring periods.
Although the primary purpose of this treatment group was to determine the presence of leaders and followers within the experimental population, the data were collected concurrently with the social manipulation experiment below, allowing us to use this treatment as a natural control. For convenience, we refer to this treatment group as the control group.
(b). Leadership consequences for leaders and followers
Ten aggregations of at least 60 larvae per aggregation (average 71.4, n = 10) were located, and each individual was removed, measured, individually painted and replaced as above. The aggregations were left undisturbed for two days, and during the first monitoring period (15 days), we again identified the leaders at the front of the foraging movement each night. However, owing to the larger number of larvae within these aggregations, we recorded the front five larvae as leaders of the aggregation. When an aggregation moved in multiple directions to forage, the front larvae in each direction were deemed leaders (up to a maximum of 10 individuals per aggregation). In order to determine a gradation of larvae most likely to lead the aggregation consistently (for the later purpose of separating leaders and followers), the first five larvae were allocated a number according to their proximity to the front of the moving aggregation, with the front larva given five points, the second four and so forth. These numbers were then summed over the 15-day monitoring period. Larvae with higher accumulated scores within the aggregation were deemed leaders (or larvae more likely to display leadership), whereas larvae with the lowest scores were deemed followers (or larvae least likely to lead).
At the end of the monitoring period, we divided each aggregation into three equally sized groups (of approx. 20 larvae each): (i) Leaders—one-third of the individuals in the aggregation that were deemed to have led the aggregation the most frequently over the first monitoring period; (ii) Mixed—this group simulated the naturally occurring combination [21], in which 20% of the aggregation comprised larvae that led the aggregation often, with the remaining individuals having never, or rarely, led; and (iii) Followers—larvae that never led the aggregation or led the least over the monitoring period. Aggregations were divided into two rather than three smaller groups (leaders and followers only) if they suffered high mortality during the first monitoring period, resulting in fewer than 48 larvae per aggregation. Where division of an aggregation required the separation of individuals on the same score, priority was given to larvae that led the highest number of different nights over those that led fewer nights but in higher positions.
These new aggregations were returned to separate similarly sized trees of the same species from which they were sourced (excluding the source tree), with the observer blind to the treatment of each aggregation. The second monitoring period was conducted as above, noting the first three larvae leading the foraging movement each night and weighing each individual at the beginning and end of each period to determine overall percentage weight gain.
(c). Analysis
We examined whether some larvae led the aggregation more than expected by creating null distributions through computer simulations of the field experiment control group. Three individuals were randomly chosen from each aggregation for each night that aggregation was observed in the field, using a macro in Microsoft Excel (v. 14.3.7). A simulated distribution of the number of nights each individual larva would be expected to lead was then produced by conducting 1000 repeat simulations. We thus generated null distributions for the mean number of larvae expected to lead 0, 1, 2, 3, 4, 5, 6 and 7 nights, assuming the null hypothesis (that leading among larvae is random) to be true. By comparing these null distributions with our observed field data, we could calculate the probability that our observed result differed from this null hypothesis. For these analyses, we included and compared only the number of individuals within each aggregation that did not moult during monitoring (on average 46% during the first period and 83% during the second period), in order to ensure we had accurate information on leadership over the period observed.
We used general linear-mixed models, with aggregation identity included as a random factor, in order to investigate whether variation in initial weight and percentage growth are good predictors for individual leadership within the control groups. General linear-mixed models were also used to determine whether percentage growth varied across all four treatment groups (control, leaders, mixed and followers) after manipulation. Data were analysed using the statistical package JMP v. 10.0.0 (SAS Institute Inc. 2012). Aggregations with a survival rate of less than 85% over the monitored period were excluded from analyses in order to minimize any effects of aggregation size.
For original data associated with this study, see the electronic supplementary material.
3. Results
(a). Evidence for leadership
There was significant variation in the number of nights individuals spent leading within the control aggregations. The numbers of larvae that never led or led frequently (for five nights or more) were significantly higher than expected by the null models (p-values < 0.001, = 0.043 and = 0.001 respectively; table 1). On the other hand, the number of larvae observed to lead on one night only was significantly lower than expected (p-value < 0.001; table 1).
Table 1.
The observed number of larvae within the experiment leading x number of nights compared with the mean and standard deviation of the generated null distribution models. The probability of the observed number being greater or lesser than that expected by the null hypothesis is calculated, with p-values < 0.05 shown in italics.
| no. nights led | observed number of larvae | null mean number of larvae | null standard deviation | p-value observed > expected | p-value observed < expected |
|---|---|---|---|---|---|
| 0 | 35 | 22.526 | 3.688 | <0.001 | 1 |
| 1 | 26 | 41.504 | 4.839 | 1 | <0.001 |
| 2 | 33 | 37.039 | 5.318 | 0.795 | 0.25 |
| 3 | 16 | 21.581 | 3.971 | 0.945 | 0.096 |
| 4 | 13 | 9.009 | 2.688 | 0.098 | 0.952 |
| 5 | 6 | 2.793 | 1.513 | 0.043 | 0.987 |
| 6 | 5 | 0.659 | 0.797 | 0.001 | 1 |
| 7 | 1 | 0.135 | 0.356 | 0.130 | 0.995 |
(b). Benefits to individual leaders
Within the control colonies, neither initial larval weight (F1,48 = 0.030, p = 0.863; figure 1) nor the percentage growth over the first monitoring period (F1,48 = 0.508, p = 0.480; figure 2) was associated with the frequency with which an individual led their aggregation. This was also the case in the second monitoring period (initial larval weight: F1,90 = 2.424, p = 0.123, figure 1; percentage growth: F1,90 = 1.356, p = 0.248; figure 2).
Figure 1.
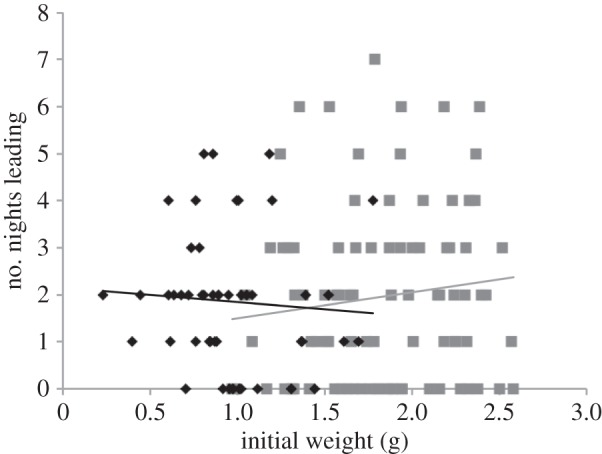
The initial weight of larvae within the control groups by the number of nights they led during the first (black diamonds, n = 49) and second (grey squares, n = 90) monitoring periods.
Figure 2.
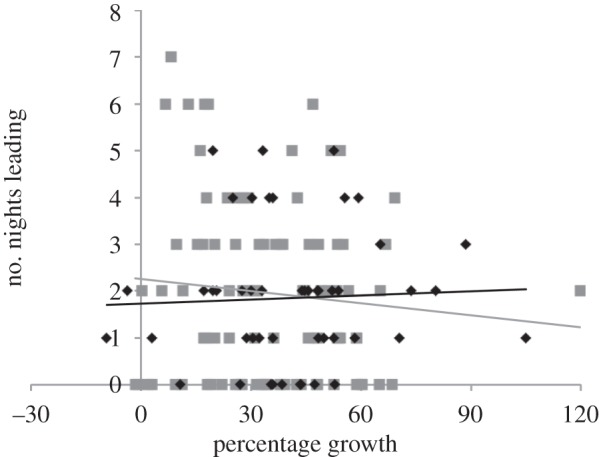
The percentage growth of larvae within the control groups by the number of nights they led during the first (black diamonds, n = 49) and the second (grey squares, n = 90) monitoring periods.
In the second monitoring period, the initial weight of a larva was not associated with the frequency of leading in either the leader groups (F1,93 = 0.116, p = 0.734; figure 3a) or the mixed groups (F1,78 = 0.327, p = 0.569; figure 3b). However, weight significantly predicted the frequency of leading in the follower groups, with larger larvae leading the aggregation more often (F1,119 = 10.229, p = 0.002; figure 3c). There was no significant association between the frequency of leading and percentage growth in any of the experimental groups (leaders F1,93 = 0.857, p = 0.357; mixed F1,78 = 0.925, p = 0.339; followers F1,119 = 0.688, p = 0.409).
Figure 3.
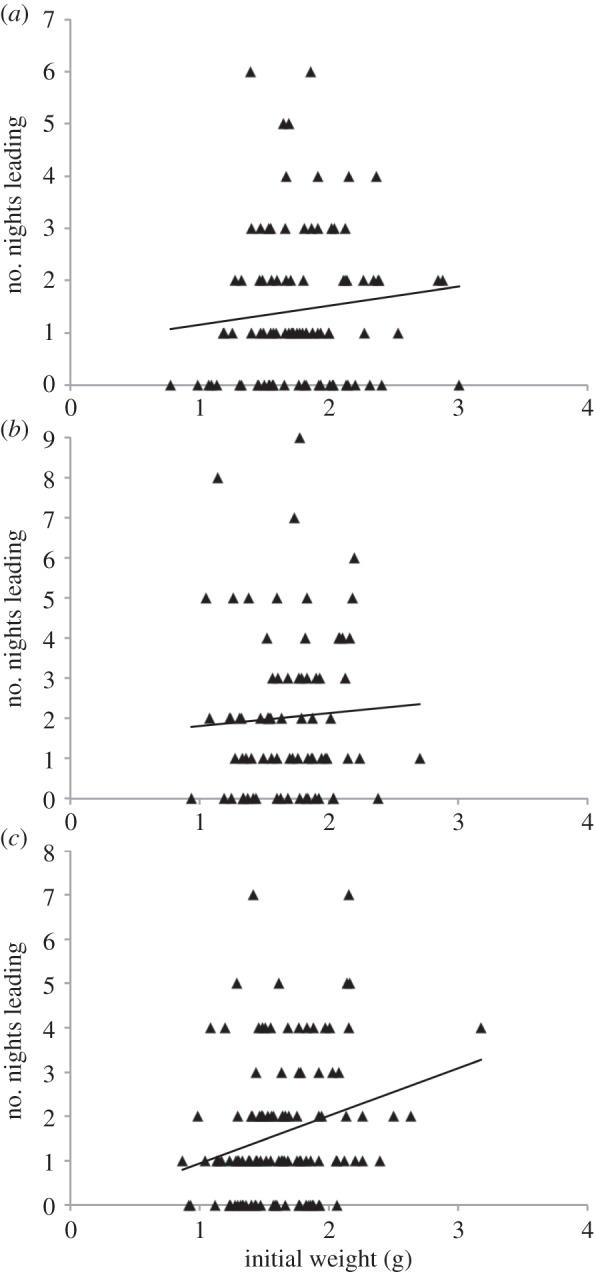
The weight of each larvae measured at the start of the second monitoring period by the number of times that larvae led the aggregation. Depicted are the raw data from (a) the groups of leaders (n = 93), (b) the mixed groups (n = 78) and (c) the follower groups (n = 119).
(c). Benefits of leadership to the aggregation
There was a significant difference in the percentage growth between the treatment groups (GLMM: F3,380 = 9.24, p < 0.001). A post hoc Tukey's test showed that the mixed group had a significantly higher percentage growth than either the leaders only or the followers only groups. The percentage growth of individuals in the control group did not differ significantly from the other three groups (figure 4).
Figure 4.
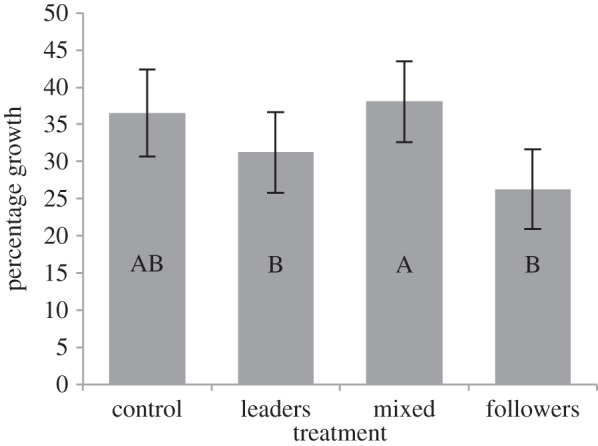
The percentage growth of the four different treatment groups over the second monitoring period (14 days). Data presented are the least-square means ± s.e. from the general linear-mixed model. Columns not displaying the same letter are significantly different. From left to right, n = 90, n = 93, n = 78, n = 119 for each treatment.
4. Discussion
There is behavioural polyethism in P. affinis sawfly larvae, with aggregations comprising both leaders and followers that show considerable temporal consistency. Similar patterns have been reported for larvae of the congeneric P. dorsalis [22]. Manipulation of the ratio of leaders to followers in field aggregations of P. affinis revealed that individuals in aggregations comprising a mixture of leaders and followers enjoyed higher percentage growth than those in aggregations comprising all leaders or all followers. However, we found no evidence that individual leaders benefit from their behaviour: individuals that led more frequently were neither heavier nor showed higher levels of growth than those that led less frequently. Nevertheless, while leaders and followers do not differ in growth patterns, leaders may acquire other benefits, such as lower predation or access to nutrients that assist in immune function.
In species where leadership is determined consensually, it is critical to consider the perspective of following individuals, because a leadership role does not exist without them: followers must benefit by choosing to follow particular individuals who become, as a consequence, the leader. In P. affinis, both leaders and followers had significantly higher weight gain in aggregations that contained a mix of leaders and followers, presumably because followers benefit from having a leader. These data are consistent with theoretical models [24], which predict that pairs of either two leaders or two followers are less capable of reaching a decision than a combination of both. The degree of behavioural divergence in P. affinis could similarly influence the ability of a group to make collective decisions effectively, and thus explain why aggregations preserve a mixture of leaders and followers and why followers allow others to direct decision-making.
Within despotic animal groups, dominant, leading individuals are usually larger and stronger, yet these physiological differences are much less common in democratic groups. In fact, the distinction between leaders and followers in such cases is often linked to more subtle characteristics [25,26]. In P. affinis, the extent to which an individual led was not linked to its size, nor did the leadership role influence the growth of that individual in the majority of treatment groups. Significantly, foraging was initiated by the heaviest larvae only when identified leaders were removed (in the followers treatment), which may reflect a difference in the resource requirements between follower individuals. The resource requirements of animals are linked with body size, and can strongly influence foraging decisions: differing resource requirements motivating an individual's choice to lead are supported by both theoretical [1,14,27] and empirical studies [15,28–30]. In bees, larger workers are not only more likely to forage, they also start to forage earlier in life [31]. If differences in individual nutritional need or motivation of individuals maintains the distinction between leaders and followers in P. affinis, then these differences are not reflected in the relative size or percentage growth of leading larvae. It seems more likely that other mechanisms sustain this behavioural distinction, such as experience or familiarity with the environment [11,32].
Individual differences can have clear consequences for the collective behaviour of group-living animals, and a few atypical members may influence collective decisions [19,33–36]. These differences can lead to behavioural polyethism, with individuals displaying fixed behavioural differences while still maintaining group cohesion. The primary focus of studies has been on how polyethism evolved, with much less known about why this social arrangement is maintained. Here, we empirically demonstrate that sustaining an aggregation of both leaders and followers is beneficial to the growth of each individual within that aggregation. Regardless of whether an individual is a leader or a follower, functioning within a social dynamic that contains both behavioural types is clearly favourable.
Supplementary Material
Acknowledgements
We thank the Phillip Island Koala Conservation Centre for use of their premises. We are also grateful to our field assistants Eunice Tan, Tamara Johnson and Jacob Lawrence who helped with data collection, Natalie Hodgkin and Ross Holmberg for accommodation and Phil Hodgkin for advice on data analysis. The comments of two reviewers helped sharpen the manuscript.
Funding statement
Research was supported by a Holsworth Wildlife Research Endowment and an Australian Postgraduate Scholarship (to L.K.H.).
References
- 1.Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. 2003. Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434. ( 10.1038/nature01630) [DOI] [PubMed] [Google Scholar]
- 2.Goymann W, Wingfield JC. 2004. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 67, 591–602. ( 10.1016/j.anbehav.2003.08.007) [DOI] [Google Scholar]
- 3.Jeanson R, Dussutour A, Fourcassié V. 2012. Key factors for the emergence of collective decision in invertebrates. Front. Neurosci. 6, 121 ( 10.3389/fnins.2012.00121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King AJ, Cowlishaw G. 2009. Leaders, followers and group decision-making. Commun. Integr. Biol. 2, 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conradt L, Roper TJ. 2007. Democracy in animals: the evolution of shared group decisions. Proc. R. Soc. B 274, 2317–2326. ( 10.1098/rspb.2007.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King AJ, Douglas C, Huchard E, Isaac NJ, Cowlishaw G. 2008. Dominance and affiliation mediate despotism in a social primate. Curr. Biol. 18, 1833–1838. ( 10.1016/j.cub.2008.10.048) [DOI] [PubMed] [Google Scholar]
- 7.Peterson RO, Jacobs AK, Drummer TD, Mech LD, Smith DW. 2002. Leadership behaviour in relation to dominance and reproductive status in gray wolves, Canis lupus. Can. J. Zool. 80, 1405–1412. ( 10.1139/z02-124) [DOI] [Google Scholar]
- 8.Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature 421, 155–158. ( 10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- 9.Petit O, Bon R. 2010. Decision-making processes: the case of collective movements. Behav. Process. 84, 635–647. ( 10.1016/j.beproc.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 10.King AJ. 2010. Follow me! I'm a leader if you do; I'm a failed initiator if you don't? Behav. Process. 84, 671–674. ( 10.1016/j.beproc.2010.03.006) [DOI] [PubMed] [Google Scholar]
- 11.McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. 2011. Leadership in elephants: the adaptive value of age. Proc. R. Soc. B 278, 3270–3276. ( 10.1098/rspb.2011.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeley TD, Buhrman SC. 1999. Group decision making in swarms of honey bees. Behav. Ecol. Sociobiol. 45, 19–31. ( 10.1007/s002650050536) [DOI] [Google Scholar]
- 13.Pillot MH, Gautrais J, Gouello J, Michelena P, Bon R. 2010. Moving together: incidental leaders and naïve followers. Behav. Process. 83, 235–241. ( 10.1016/j.beproc.2009.11.006) [DOI] [PubMed] [Google Scholar]
- 14.Conradt L, Krause J, Couzin ID, Roper TJ. 2009. ‘Leading according to need’ in self-organizing groups. Am. Nat. 173, 304–312. ( 10.1086/596532) [DOI] [PubMed] [Google Scholar]
- 15.Krause J, Hoare D, Krause S, Hemelrijk CK, Rubenstein DI. 2000. Leadership in fish shoals. Fish Fish. 1, 82–89. ( 10.1111/j.1467-2979.2000.tb00001.x) [DOI] [Google Scholar]
- 16.Barelli C, Boesch C, Heistermann M, Reichard UH. 2008. Female white-handed gibbons (Hylobates lar) lead group movements and have priority of access to food resources. Behaviour 145, 965–981. ( 10.1163/156853908784089243) [DOI] [Google Scholar]
- 17.Fischhoff IR, Sundaresan SR, Cordingley J, Larkin HM, Sellier M-J, Rubenstein DI. 2007. Social relationships and reproductive state influence leadership roles in movements of plains zebra, Equus burchellii. Anim. Behav. 73, 825–831. ( 10.1016/j.anbehav.2006.10.012) [DOI] [Google Scholar]
- 18.Costa JT. 2006. The other insect societies. Cambridge, MA: The Belknap Press of Harvard University Press. [Google Scholar]
- 19.Dussutour A, Nicolis SC, Despland E, Simpson SJ. 2008. Individual differences influence collective behaviour in social caterpillars. Anim. Behav. 76, 5–16. ( 10.1016/j.anbehav.2007.12.009) [DOI] [Google Scholar]
- 20.Carne PB. 1962. The characteristics and behaviour of the saw-fly Perga affinis affinis (Hymenoptera). Aust. J. Zool. 10, 1–34. ( 10.1071/ZO9620001) [DOI] [Google Scholar]
- 21.Macdonald J, Ohmart CP. 1993. Life history strategies of Australian Pergid sawflies and their interactions with host plants. In Sawfly life history adaptions to woody plants (ed. Wagner MR, Raffa KF.), pp. 485–502. Cambridge, MA: Academic Press. [Google Scholar]
- 22.Weinstein P, Maelzer DA. 1997. Leadership behaviour in sawfly larvae Perga dorsalis (Hymenoptera: Pergidae). Oikos 79, 450–455. ( 10.2307/3546887) [DOI] [Google Scholar]
- 23.Carne PB. 1969. On the population dynamics of the eucalypt-defoliating sawfly Perga affinis affinis Kirby (Hymenoptera). Aust. J. Zool. 17, 113–141. ( 10.1071/ZO9690113) [DOI] [Google Scholar]
- 24.Johnstone RA, Manica A. 2011. Evolution of personality differences in leadership. Proc. Natl Acad. Sci. USA 108, 8373–8378. ( 10.1073/pnas.1102191108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauchamp G. 2000. Individual differnces in activity and explorationinfluence leadership in pairs of foraging zebra finches. Behaviour 137, 301–314. ( 10.1163/156853900502097) [DOI] [Google Scholar]
- 26.Harcourt JL, Ang TZ, Sweetman G, Johnstone RA, Manica A. 2009. Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252. ( 10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]
- 27.Sueur C, Deneubourg JL, Petit O, Couzin ID. 2010. Differences in nutrient requirements imply a non-linear emergence of leaders in animal groups. PLoS Comput. Biol. 6, e1000917 ( 10.1371/journal.pcbi.1000917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furrer RD, Kunc HP, Manser MB. 2012. Variable initiators of group departure in a cooperative breeder: the influence of sex, age, state and foraging success. Anim. Behav. 84, 205–212. ( 10.1016/j.anbehav.2012.04.034) [DOI] [Google Scholar]
- 29.McClure M, Ralph M, Despland E. 2011. Group leadership depends on energetic state in a nomatic collective foraging caterpillar. Behav. Ecol. Sociobiol. 65, 1573–1579. ( 10.1007/s00265-011-1167-5) [DOI] [Google Scholar]
- 30.Sueur C, MacIntosh AJJ, Jacobs AT, Watanabe K, Petit O. 2013. Predicting leadership using nutrient requirements and dominance rank of group members. Behav. Ecol. Sociobiol. 67, 457–470. ( 10.1007/s00265-012-1466-5) [DOI] [Google Scholar]
- 31.Brian AD. 1952. Division of labour and foraging in Bombus agrorum Fabricius. J. Anim. Ecol. 2, 223–240. ( 10.2307/1959) [DOI] [Google Scholar]
- 32.Stroeymeyt N, Franks NR, Giurfa M. 2011. Knowledgeable individuals lead collective decisions in ants. J. Exp. Biol. 214, 3046–3054. ( 10.1242/jeb.059188) [DOI] [PubMed] [Google Scholar]
- 33.Dyer JRG, Johansson A, Helbing D, Couzin ID, Krause J. 2009. Leadership, consensus decision making and collective behaviour in humans. Phil. Trans. R. Soc. B 364, 781–789. ( 10.1098/rstb.2008.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reebs SG. 2000. Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 59, 403–409. ( 10.1006/anbe.1999.1314) [DOI] [PubMed] [Google Scholar]
- 35.Bode NW, Franks DW, Wood AJ. 2012. Leading from the front? Social networks in navigating groups. Behav. Ecol. Sociobiol. 66, 835–843. ( 10.1007/s00265-012-1331-6) [DOI] [Google Scholar]
- 36.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


