Abstract
Although the taphonomic (post-mortem) degradation processes relevant to teeth and bones have been well described, those taking place with regards to mammalian hairs have not been characterized to the same extent. This present article describes, in detail, microscopic changes resulting from the actions of biological agents that digest and degrade hairs. The most noteworthy and prevalent agents responsible for the destruction of hair structure are fungi, which use a range of strategies to invade and digest hairs. One of the most important finds to emerge from this study is that taphonomic structures and processes can easily be interpreted by the unwary as ‘real’, or as class characteristics for a particular animal taxon. Moreover, under certain conditions, ‘taphonomic’ processes normally associated with the dead are also present on the hairs of the living. This work will improve the reliability of hair examinations in forensic, archaeological and palaeontological applications—in addition, the finding has relevance in the protection of mammalian collections susceptible to infestation. This article also addresses the popular myth that ancient peoples were often red-haired and discusses phenomena responsible for this observation. Insights gained from detailed characterization of taphonomic processes in 95 hairs from a variety of species demonstrate the range and breadth of degradative effects on hair structure and colour. Lastly, the study demonstrates that hairs often tell a story and that there is value of extracting as much morphological data as possible from hairs, prior to destructive sampling for biomolecules.
Keywords: mammalian hairs, taphonomy, forensic investigations, archaeology, microscopy
1. Introduction
Mammalian hair is one of the most frequently encountered biological materials in forensic investigations and may be associated with human remains at ancient and modern burial sites. In addition to bodily remains, mammalian hairs may also be present in artefacts such as textiles and cordage [1,2]. Hair is essentially composed of three layers, namely the outermost cuticle, an inner cortex and a central core or medulla (figure 1). The medulla consists of vacuolated cells filled with air and although they have been reported as only occurring in ‘larger’ hairs [3], medullae also occur in hairs of medium and fine diameters [4]. The cuticular scales are arranged along the hair shaft (akin to slates on a roof), with the free edges always protruding towards the tip end of the hair. During the extrusion of the hair shaft from the follicle, the hair becomes keratinized (hardens) and the cortical cells become tightly fused to the scales or cuticle. The cortex makes up the bulk of the hair shaft in most mammalian hairs and is mechanically the most significant component of the hair. It is made up of tightly packed, elongated spindle-shaped cells which are oriented parallel to the axis of the shaft. Dispersed within this densely packed structure are the melanin pigment granules, the number of which and their distribution pattern determine the colour of the hair. Hairs have their medulla centrally placed in the shaft.
Figure 1.
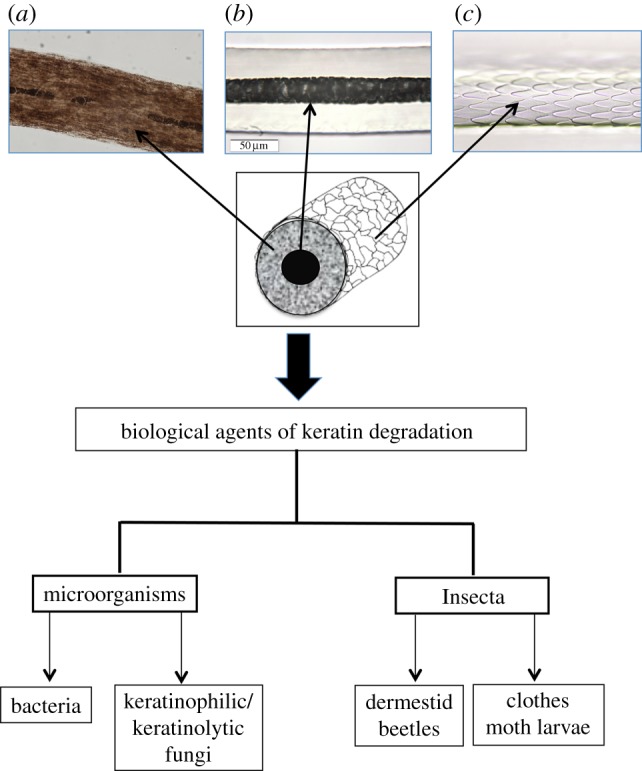
Mammalian hair structure and agents of hair degradation. (a) Pigment granules, (b) medulla and (c) cuticle scales. (Online version in colour.)
Hair can withstand the test of time, often surviving for millennia due to the resilience of the keratin biopolymer. Central to this stability and resilience of keratin is the presence of numerous disulfide bonds that form between the thiol moieties of cysteine (amino acid) residues in adjacent protein strands. These stable bonds endow hairs with the ability to withstand exposure to diverse and extreme conditions such as freezing, burial and mummification. However, despite this resilience, hair is not immune to the effects of pre-mortem or post-mortem (taphonomic) degradative processes.
Traditionally associated with palaeontology [5], the term ‘taphonomy’ is today used in a broader sense and is synonymous with post-mortem degradation processes on organic remains—whether prehistoric, ancient or contemporary in origin.
Post-mortem degradation of hairs is evident as microscopic morphological changes caused by biological agents that share the unique capability to degrade and digest keratin (figure 1). The ubiquity and unique abilities to exploit a variety of niches make microorganisms the most significant agents of hair degradation. The most prevalent of these are filamentous fungi that have the natural capacity to break down and digest keratin [6].
Fungi that can attack hair are described as ‘keratinophilic’ and ‘keratinolytic’ and these descriptors have been used interchangeably despite clear distinctions between their meanings. Keratinolytic describes a group of microorganisms capable of decomposing and digesting keratin through the production of boring hyphae (fungal structures with appendages capable of penetrating hairs) and subsequent enzymatic digestion of keratin [7,8], while ‘keratinophilic’ describes fungal species capable of only using easily degraded substances such as partially decomposed keratin or materials related to keratin [7,9]. Species from each of these fungal types mainly reside primarily in soils (geophilic) with variable distribution patterns that are dependent on a variety of factors, one of which includes a preference for co-location with mammals [10,11]. Keratinolytic fungi represent an ecologically significant group of fungi that recycle one of the most abundant and highly stable mammalian proteins on Earth—keratin.
The aim of this study was to microscopically examine ancient and modern mammalian hairs in order to document the effects of biological degradation commonly regarded as taphonomic (occurring after death). An additional aim was to demonstrate that accurate interpretation of mammalian hair morphology is technically demanding; as such it is important that forensic, archaeological and other practitioners are aware of potential artefacts in structure, colour and composition that can occur in hairs both pre- and post-mortem. An appreciation for what information can be gained from studying the effects of biological degradation of hair will ensure that they are recorded and imaged prior to destructive analyses.
2. Material and methods
(a). Materials
Approximately 450 hairs, from a variety of species, were examined and 95 hairs were chosen as exemplars of the spectrum of taphonomic characteristics—these were examined in detail. These exemplar hairs included extinct megafaunal hairs and ancient human hairs that were the subject of previous studies [12–14], others were modern human and animal hairs. Table 1 provides further details in relation to each of the samples examined. The ages cited in relation to hairs relating to the ‘Somerton Man’, unidentified female, Norse Kal male, Borum Eshøj male, polar bear and Asian red-cheeked squirrel were provided on documentation accompanying the samples.
Table 1.
Sources, age and depositional environments of hairs examined in this study.
| hair sources | hair type | age of specimens | depositional environment |
|---|---|---|---|
| woolly mammoth (Jarkov) | coarse | 20 380 ± 140 BP | permafrost |
| woolly mammoth (Yukagir) | coarse | 18 560 ± 50 BP | permafrost |
| woolly mammoth (M25) | coarse | 5930 ± 2700 | permafrost |
| woolly rhino (Churapcha) | coarse | 19 500 ± 120 BP | permafrost |
| woolly mammoth (Yuka) | coarse | 39 440–38 850 BP | permafrost |
| human (Saqqaq male) | scalp | 4044 ± 31 BP | permafrost |
| human (Norse Kal male) | scalp | ca fourteenth century | wrapped in cloth and buried in soil |
| Borum Eshøj ‘young man’ | scalp | ca fourteenth century | oak coffin buried in barrow |
| Aboriginal (male) | scalp | 100 years | stored in office drawer |
| ‘Somerton Man’ historic unsolved homicide-unidentified male | chest | 60 years | removed from plaster cast of upper torso |
| historic unsolved homicide-unidentified female | scalp | 30 years | remains found in a tropical rainforest |
| Asian red-cheeked squirrel (D. rufigenis) | base of tail | 9 years | curated mammalian collection (M32566) |
| polar bear (U. maritimus) | flanks, belly | 7 years | zoo specimen part of curated mammalian collection (M10983) |
Hairs were selected on the basis of microscopic morphological features that best articulated the effects of biodegradation or environmental alterations.
(b). Methods
To ensure that each hair sample was comprehensively examined and to maximize the amount of morphological data obtainable, the following methodologies were adhered to:
— initial examinations with low magnification (6–40×) stereo/dissecting microscopy. These enabled gross morphological features to be recorded, e.g. colour, length and profile, and enabled selection of hairs for further, more detailed examinations;
— detailed examinations conducted at higher magnifications using transmitted visible light microscopy (TVLM) at 100–400×. This enabled internal and external morphological traits to be examined along the entire length of the shaft;
— scanning electron microscopy (SEM) was used to examine surface artefacts or details, in much greater detail than possible with TVLM.
(c). Stereomicroscopy
Samples were examined with a Wild M3Z Type-S stereomicroscope (Heerbrugg, Switzerland).
(d). Transmitted visible light microscopy
Hairs were mounted using Safe-T-Mounting permanent mounting medium (FRIONINE Pty Ltd, refractive index approx. 1.52). Hairs were mounted between conventional glass microscope slides and coverslips (0.17 mm thick). Microscopy was performed on Leica DME compound transmitted visible light microscope equipped with UPLFL20× semi-apochromatic, UPLANO40× apochromatic objectives. Images were acquired with a Q imaging camera and associated software.
(e). Scanning electron microscopy
Each hair was affixed to double-sided adhesive tape attached to a 12.6 mm diameter aluminium stub then coated with a 90 nm layer of gold in a Balzers Union Ltd sputter coater (Liechtenstein) before being examined and photographed in a Philips XL20 scanning electron microscope (the software for image capture is part of the microscope operating software).
(f). Scale cast pattern
Scale cast patterns were produced in accordance with the methodology of Brunner & Coman [15]. Briefly, a coverslip was coated with clear nail polish and the hair was placed on the wet polish; once hardened, the hair was removed leaving an impression of the cuticle in the nail polish.
3. Results and discussion
(a). Fungal invasions and digestion of hairs
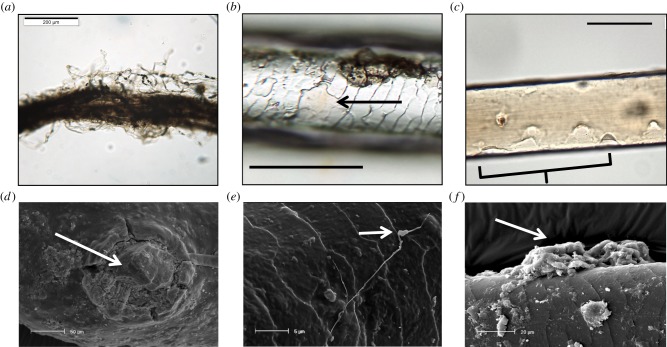
We observed that the prequel to fungal infiltration of the hair is the envelopment of the structure by mycelia (a mat of filamentous fungal threads or hyphae) (figure 2); this is a process that takes place irrespective of whether the attacking fungi are keratinophilic or keratinolytic [16]. Hyphae on the woolly mammoth hair (figure 2a), unlike the hair itself, may not be thousands of years old but a reflection of modern fungal invasion; either post-excavation or post-thaw. Keratinolytic fungi produce eroding fronds and penetrating/perforating organs that attack the cuticle by lifting or eroding the protective cuticle or scales (figure 2b,c). Perforating or penetrating organs (figure 2d) may be connected to filamentous hyphae [17] (figure 3e) or produced from a mycelial mass (figure 2f) [16,18].
Figure 2.
Fungal invasion of hairs. (a) Woolly Mammoth (Jarkov) hair engulfed by hyphae, (b,c) partial removal of cuticle (arrow) and dissolution of cuticle (bracketed) on woolly mammoth (Jarkov) hair, (d) SEM image of a penetrating organ (arrow) embedded in a woolly rhino hair. (e) SEM image of a lateral fungal hypha with an eroding frond (arrow), (f) mycelial mass (arrow) on shaft of woolly mammoth (M10) hair. Scale bars: a = 200 μm, b,c = 100 μm, d = 50 μm, e = 5 μm, f = 20 μm. (Online version in colour.)
Figure 3.
Fungal penetration of hair shafts. (a,b) Thin hyphae invading ancient human scalp hair (Saqqaq) and woolly mammoth (Yukagir) hair (lateral fronds are circled). (c) Lateral hyphae in woolly rhino hair. (d) Extensive mass of lateral hyphae in polar bear flank hair. (e) ‘Needle’ (i) and ‘fissure’ (ii) type damage, to the shaft of woolly mammoth (Yukagir) hair. (f) Surface fungal ‘bloom’ on M26 woolly mammoth hair. Scale bar: a = 50 μm, b = 100 μm, c = 100 μm, d = 200 μm, e = 200 μm, f = 100 μm. (Online version in colour.)
Once inside the hair shaft, keratinolytic digestion of hairs resulted in the following distinctive and varied morphological features:
— thin thread-like hyphae (figure 3a,b), some of which spread laterally (figure 3b–d),
— deeply penetrating or perforating organs (‘borers'), which bore into the cortex (figure 3e; electronic supplementary material, figures S1a–d and figure S2a–c), and
— fungal ‘blooms', which appear to feed at a superficial level with negligible penetration into the cortex (figure 3f and electronic supplementary material, figure S2d–f).
In some instances, the high number and concentration of fungal hyphae present in the cortex may be so severe as to cause the shaft to bulge and distort (electronic supplementary material, figure S3).
We also observed apparent fungal preferences in relation to their modus operandi in their destruction of hairs, which are discussed in detail in the ensuing sections.
(b). Fungal infiltration: from the outside in
Fungal hyphae engulf the hair shaft (figure 2a) and eventually compromise the cortical structure such that it disintegrates, as illustrated in the electronic supplementary material, figure S4a,b. The efficacy with which the keratinolytic fungi invade and ultimately destroy hairs is mostly due to the production of keratinase—an enzyme that specifically dismantles keratin [7,19].
Human scalp hairs from a 4000-year-old Palaeo-eskimo [12] and hairs from woolly rhino [14] each showed superficial fungal invasion by thin hyphae. These hyphae grew transversely along the hair axis and some produced hyphae that travelled laterally (figure 3a,b). DeGaetano et al. [20] also reported similar thread-like fungal structures in human scalp hairs from the buried remains relating to a forensic investigation. The thin hyphae observed here and by DeGaetano et al. [20] closely resemble those produced by Curvularia ramosa [16] and Alternaria spp. [21]; these mildly keratinophilic fungi are environmental in origin and are found in soil and decaying plant matter [22]. However, Shaw & Vanderwyk [23] identified the presence of Alternaria spp. on the scalp hair of healthy subjects. The finding that these fungal species colonize the hair of the living and not just the dead has implications for forensic science. For example, a person is missing and their hairs are found at the premises of a suspect; the hairs exhibit fungal structures. Hitherto, it would be concluded that the missing person is dead and that the suspect has been involved with moving the body after it had been buried in soil or leaf litter; a search for a clandestine grave would begin and police would question the suspect as to their involvement in moving the body. In reality, the missing person might still be alive, albeit with an infection of fungus.
(c). Fungal infiltration: from the inside out
Penetrating hyphae, once inside the cortex, in some instances, appear to preferentially invade the medullary canal rather than digesting the cortex (electronic supplementary material, figure S5a), an observation previously noted by Kanbe & Tanaka [17]. It appears that fungal hyphae might find it easier to digest vacuolated medullary canals in preference to the much denser cortex. In essence, the fungus digests the hairs from the inside out. We observed this type of destruction in hairs from the woolly mammoth ‘Yuka’. This medulla to cuticle invasion is clearly visible as finger-like or stellate projections emanating from the medulla and penetrating the cortex towards the cuticle (electronic supplementary material, figure S5b).
(d). Keratinolytic fungal invasion of hairs on the living
Keratinolytic fungal pathogens (dermatophytes) are capable of invading hairs of living humans and animals (non-humans) [24,25] as well as those associated with the dead. Dermatophytes may be divided into three broad epidemiological groups [26]:
(i) Geophilic: these fungi live in the soil; some species may invade skin, hair or nails of animals and humans following contact with the soil.
(ii) Zoophilic: these fungi are primarily parasitic on animals.
(iii) Anthropophilic: these fungi are primarily parasitic on humans.
Ecological sites inhabited by keratinolytic (and keratinophilic) fungi are diverse and favour areas that are frequented by humans and/or animals, e.g. playgrounds, stables and zoological gardens [11,27]. Lewin et al. [28] investigated a reasonably common phenomenon that involved healthy polar bears (Ursus maritimus) kept in zoological gardens undergoing a ‘greening’ of their hair. In addition to algae (the source of the green coloration), images also show ‘stellate’ medullae and what appear to be fungal bores on the surface of the shaft. We also noted stellate medullae in belly hairs of polar bears kept in a zoo (electronic supplementary material, figure S5c). An additional phenomenon was noted in several of the polar bear hairs we examined, which was manifested as the complete hollowing out of the medullary canal (electronic supplementary material, figure S5d). However, based on the data here, it is possible that Lewin et al. [28] may have misinterpreted the stellate medullae as normal characteristics of hairs, rather than features arising from fungal attack. Whether this fungal invasion occurs in polar bears in the wild, as well as in captivity, has yet to be determined.
Brunner & Coman [15] and Brunner & Triggs [29] depict stellate medullae in hairs from an Australian marsupial—the common wombat (Vombatus ursinus). These authors reported this medulla type as a genetically determined, class characteristic for this species and therefore a suitable taxon-specific marker. However, based on the findings of this study, we believe that this type of medulla is likely due to the actions of keratinolytic fungi. The invasion most likely results from repeated and continuous exposure to geophilic keratinolytic fungi; wombats lead a largely subterranean existence. This observation demonstrates that misinterpretation of fungal artefacts can result in erroneous identification of hairs in faunal research applications and forensic investigations.
The presence of keratinolytic fungi and keratinophilic fungi on hairs that have been buried may reveal information in relation to the season or time of year interment occurred. Rowe [30, p. 347] states ‘the presence of the (fungal) tunnels is consistent with the burial taking place at a time of the year when it was warm enough and there was sufficient moisture available for the growth of the fungi’.
The presence of these fungi in mammalian hairs most likely reflects burial in warm conditions. This is important in an archaeological context as an indication of the season of death may be inferred. In addition to indicating the season of death, which has obvious importance to an investigation, the observation of fungal tunnelling can have other relevance. Firstly, fungal tunnelling may preclude the positive identification of a hair as being human in origin and prevents its comparison against hair known to be from a missing person. Secondly, if hairs exhibiting fungal tunnelling are found associated with a suspect, the implication is that the suspect has been in contact with hairs (and possibly therefore a body) that have been buried.
(e). Keratinolytic bacteria
Compared to research into the biodegradation of keratin through fungal invasions, there appears to be a relative paucity of work conducted on the effects of keratinolytic bacteria. This may reflect the general lack of recording of or recognition of the effects bacteria may exert on hairs, or difficulty in culturing bacterial colonies for identification purposes. Instances in which bacteria have compromised the structure of hairs are presented and discussed below.
(f). Keratinolytic bacterial invasion of hair shafts
Appleyard [31] found that bacterial activity on wool fibres created numerous small pits on the surface of hairs, which were similar to pits found in extinct megafauna hairs in this study (electronic supplementary material, figure S6a). Rod-like bacilli were found in tail hairs taken from an Asian red-cheeked squirrel (Dremomys rufigenis) from a curated natural history collection (electronic supplementary material, figure S6b), in which the devastation of internal medulla structures is obvious in comparison to a healthy, unaffected hair (electronic supplementary material, figure S6c). The medulla of the infected hair has been digested resulting in a ‘hollowed out’ cortex (electronic supplementary material, figure S6b). The hollowed out medulla compromises the structural integrity of the shaft, to the extent that the affected hairs collapse under the pressure of obtaining the cross sections (electronic supplementary material, figure S6d). In comparison to fungal invasions, bacterial invasion of hairs may appear to be significantly less pronounced and superficial [32]. However, bacterial invasions of mammalian hairs have the potential to devastate curated skins and specimens. Appleyard [31] also observed that bacterial invasion of wool fibres not only occurred in detached fleece, but also occurred on the fleece of living animals. In relation to humans hairs, Shelley & Miller [33] identified and recorded the destructive nature of pathogenic corynebacteria colonies on axillary (armpit) hairs of otherwise healthy humans.
(g). Post-mortem banding
Post-mortem banding (PMB) is a post-mortem degradation process that has received attention over the years due to its potential relevance in forensic investigations. The most recent studies conducted by Koch et al. [34] suggest that PMB formation is accelerated in warm and humid conditions but retarded in colder ones. Biodegradation appears to occur solely in roots that are actively growing (anagen and catagen roots) at the time of death, rather than in roots in which growth has ceased (telogen). The preferential invasion of anagen and catagen roots is attributed to these roots being softer and more easily degraded, unlike telogen roots that are bulbous and hardened through the keratinization process [34]. Although the precise mechanism responsible for PMB of hairs remains unknown, Linch & Prahlow [35] suggest that microbial and autolytic activities may be of importance.
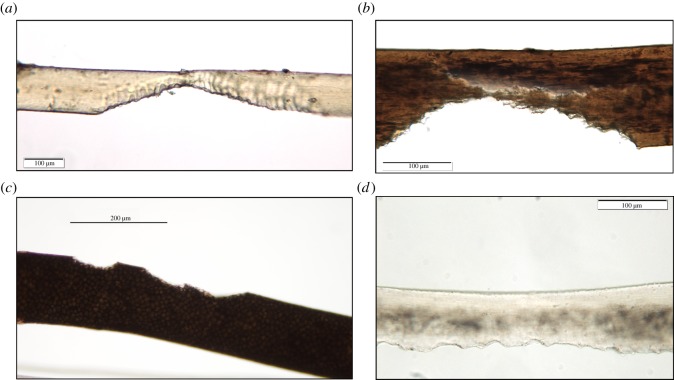
PMB is observable by TVLM as a darkened band at the proximal (root) end of the hair (figure 4a,b). This phenomenon has been observed not only in ancient hairs that are hundreds, or even thousands of years old [14], but is also observed in contemporary hairs [34] as illustrated by the examples in figure 4. PMB occurs irrespective of the environment in which the hairs or remains were deposited. This is evident from the examples shown in figure 4, in which the depositional environments ranged from warm and humid (tropical) to frozen soil (permafrost). As the decomposition advances, the root will eventually break off at the darkened area and leave the proximal end with a pointed or a brush-like appearance (figure 4c,d). This loss of the band and root may clarify the erroneous assertion of Wilson [36, p. 134] who states ‘ … postmortem root banding … is not noted in older hairs from archaeological remains'.
Figure 4.
PMB. (a) ‘Somerton man’ chest hair. (b) Pointed root of scalp hair from a murder victim. (c) Pointed root of Norse Kal male scalp hair. (d) Brush root of woolly mammoth (Jarkov) hair. Scale bar: a = 50 μm, b = 200 μm, c = 100 μm, d = 500 μm. (Online version in colour.)
Koch et al. [34] concluded that due to extensive environmental variables, PMB cannot be used to determine post-mortem interval (PMI), but it can determine whether an individual was dead when the hair was pulled or detached from the scalp. This determination can be instrumental in forensic investigations. For example, Tafaro [37] was able to unequivocally associate a suspect with a body that was undergoing decomposition; a finding that was instrumental in eliciting a confession to murder from the suspect.
(h). Natural or acquired hair colour?
Archaeological hair samples, human and animal, frequently exhibit red coloration [14,38]. Post-mortem or post-depositional colour change to hairs may be attributed to a number of factors such as photo-degradation of pigment granules in sunlight [39] or the oxidation of melanin pigment granules over millennia [40]. Microscopical examination of a 100-year-old tress of scalp hair from an Aboriginal youth [13] revealed hairs that most likely showed the effects of photo-degradation of pigment granules. Pigment granules in the oldest part of the hair (tip end or distal shaft) were significantly lighter in colour in comparison to the younger part of the hair (electronic supplementary material, figure S7a). In an ante-mortem context, other factors can impact on bleaching of pigment granules (e.g. chemicals), but in the case of the Aboriginal youth [13] it is reasonable to assume that the older parts of the hair have had more exposure to sunlight.
Wolfram et al. [41] noted that while all pigmented hair lightens when exposed to sunlight, the affect was most notable at low latitudes and in high humidity environments. Rowe [30], in his study of biodeterioration of hair colour, observed that exposure of white, unpigmented dog hair to relatively high humidity resulted in the development of a distinct yellow–orange coloration. Further investigation of these hairs revealed the presence of brown fungal hyphae and spores on the surface of the hair; a phenomenon also seen in extinct megafauna hairs that caused the shaft of the hairs to become orange/red in colour [14]. Although the chemical reactions and their effects on hair pigments undoubtedly account for some of the red coloration of hairs, they cannot be the sole cause. Krefft [42] found that hairs devoid of pigmentation also exhibited this red coloration, which he attributed to the alteration of the chemical structure of keratin, as demonstrated in animal ancient and modern animal hairs (electronic supplementary material, figure S7b,c). Irrespective of the cause, red hair on or near remains should be thoroughly investigated to determine if the colour is natural or acquired. Naturally red (ginger) scalp hair, unlike brown or black hair, usually shows medial (central) distribution of pigment granules within the shaft (electronic supplementary material, figure S7d).
(i). Insect damage
Curated mammalian hair collections represent important and valuable scientific resources. Mammalian furs from these collections are often used for species identification of animal hairs of unknown origin. Structural integrity of these collections may be compromised not only by keratin-digesting fungi and bacteria but also by the action of insects. The implications of this destruction are that these exemplar hairs are no longer reliable as comparative resources from which unknown animal hairs may be identified.
The most commonly encountered insects able to use keratin are larvae of clothes moths (Tineidae) and carpet beetles (Dermestidae) [31]. Although these larvae are usually associated with the invasion and destruction of museum and other curated mammalian specimens, they may also affect forensic and archeological hair samples as illustrated in figure 5a–d.
Figure 5.
Insect larvae damage. (a) Woolly rhino coarse hair, (b) Borum Eshøj ‘young man’ scalp hair. (c) Red deer (Cervus elaphus) dorsal hair. (d) Chest hair from deceased ‘Somerton Man’. Images (a,b,d) show cuspate bite marks caused by larval mandibles. Scale bars: a,b,d = 100 μm, c = 200 μm. (Online version in colour.)
(j). Taphonomy of hair and survival of DNA
Hair has, in recent years, gained more prominence as a substrate for ancient genomic analyses. Extraction of DNA from hair is often preferable to drilling bone as it has been reported to contain a higher proportion of endogenous DNA relative to contaminating microorganisms [43]. The presence of nDNA in the roots of ‘modern’ hairs is beyond the scope of this study. DNA preservation in hair shafts is less well established and more relevant to hair discovered in archaeological/palaeontological and in some forensic contexts as a result of taphonomic or physical severance of the root. While mtDNA is present in abundance in the hair shafts, nDNA appears to be present at significantly lower, and often variable, levels [44]. Some researchers propose nDNA is present within the outer layer (cuticle) of the hair shaft [45], while others challenge this hypothesis by proposing that nDNA is present within the cortex and in the cuticle [46].
Irrespective of the location of DNA within hair shafts, assessment of hairs for their suitability for molecular analyses should take into account the depositional environment from which the hairs were removed, rather than the elapsed time since they were deposited. Burger et al. [47, p. 1722] also agree with this proposition stating, ‘Recent publications indicate that environmental conditions have more influence on DNA preservation than does time’ and they conclude that there is no general correlation between the age of the sample and the preservation of DNA. Bengtsson et al. [3] illustrate this premise by demonstrating successful extraction of DNA in ancient hairs (permafrost), some of which exceed the limit of radiocarbon dating; however, attempts to detect DNA in hairs from considerably younger ‘bog bodies' failed. Perhaps the moist, bog environments accelerated DNA degradation to a much higher rate than that exerted by storage in permafrost. Alternatively, bog environments may contaminate hairs with powerful PCR inhibitors, e.g. humic acids, phenolics or tannins. It is unclear without further research, if morphological features (e.g. microbial attack) on hair shafts are a predictor of the abundance of preserved DNA.
4. Conclusion
This study is, to our knowledge, the first to describe holistically the factors responsible for the biodegradation of mammalian hairs. Of significance is the finding that degradation processes previously reported as wholly post-mortem also occur in hairs from living mammals. Our data also reveal that taphonomic processes are not well understood and we found internal structures resulting from fungal infiltrations that have previously been interpreted as class characteristics upon which taxonomic identifications have been made. Collectively, this study provides key information for fields where mammalian hairs play a central role including, but not limited to, palaeontology, archaeology, ancient DNA studies, forensic investigations and conservation biology.
Unlike other degradative effects on hairs, we propose that PMB is the only hair modification process that unequivocally takes place after death and then only in catagen and anagen hairs. This finding can be of use in cases involving missing persons, mass disasters or even when analysing hair in archaeological/palaeontological contexts. Although PMB cannot determine PMI, it may, however, help investigators to reconstruct events and corroborate (or refute) statements and theories. In archaeological specimens, the presence of PMB indicates that the environment in which the remains decomposed were sufficiently conducive for this phenomenon to occur.
This study demonstrates the importance of familiarity and expertise in the recognition of normal and abnormal microscopical characteristics of mammalian hairs; an ideal that is discussed in greater depth by Tridico et al. [48]. Microscopical examination of hairs for post-mortem degradation may reveal the types of causative agents and conditions at the time of burial or death. There is much to be gained from examining hairs with transmitted light microscopy prior to more destructive techniques, such as molecular analyses or electron microscopy. The consequences of not performing detailed transmitted light microscopical examinations on hairs prior to more destructive analyses are irremediable damage to the hair and the likely loss of critical or key pieces of information or evidence.
Acknowledgements
It is with gratitude and appreciation that we thank the following individuals and their collaborators for granting permission to examine hairs used in this study: Prof. Eske Willerslev (Saqqaq and Aboriginal), Prof. Tom Gilbert (extinct megafauna), ‘The Rise’ funded by the European Research Council under the European Union's Seventh Framework programme (FP/2007-2013)/ERC grant agreement no. 269442 (Norse Kal), National Museum of Denmark (Borum Eshøj young man), Det (retired) G. Feltus (Somerton Man), Commonwealth Scientific and Industrial Research Organisation Australian National Wildlife Collection, Canberra, Australia, and United States Fish and Wildlife Forensics Laboratory, OR, USA, for the provision of hairs from the Asian red-cheeked squirrel and polar bear (respectively).
Ethics statement
All hairs collected and analysed in this study were ethically obtained and permission sought and granted. For the most part, samples were from museum [14] and/or reference collections established over many years by SRT. In relation to the ancient human Palaeo-eskimo and aboriginal hairs, permissions are documented in previous publications [12,13].
Funding statement
M.B. was funded by an Australian Research Council Future Fellowship (FT0991741).
References
- 1.Appleyard HM. 1978. Guide to the identification of animal fibres. Leeds, UK: Wool Industries Research Association (WIRA). [Google Scholar]
- 2.Rowe WF. 2010. Extreme degradation of human hair by keratinophilic and kertinolytic fungi. Microscope 58, 115–119. [Google Scholar]
- 3.Bengtsson CF, Olsen ME, Brandt LO, Bertelsen MF, Willerslev E, Tobin DJ, Wilson AS, Gilbert MT. 2012. DNA from keratinous tissue. Part I: hair and nail. Ann. Anat. 194, 17–25. ( 10.1016/j.aanat.2011.03.013) [DOI] [PubMed] [Google Scholar]
- 4.Teerink BJ. 1991. Hair of west European mammals: atlas and identification key, p. 221 New York, NY: Cambridge University Press. [Google Scholar]
- 5.Lyman RL. 2010. What taphonomy is, what it isn't, and why taphonomists should care about the difference. J. Taphonomy 8, 17. [Google Scholar]
- 6.Kunert J. 1989. Growth of keratinolytic and non-keratinolytic fungi on human hairs. A physiological study. Acta Univ. Palacki Olomuc Fac. Med. 122, 25–38. [PubMed] [Google Scholar]
- 7.Blyskal B. 2009. Fungi utilizing keratinous substrates. Int. Biodeterioration Biodegradation 63, 631–653. ( 10.1016/j.ibiod.2009.02.006) [DOI] [Google Scholar]
- 8.Marchisio VF. 2000. Keratinophilic fungi: their role in nature and degradation of keratinic substrates. In Biology of dermatophytes and other keratinophilic fungi (eds Kushwaha RKS, Guarro J.), pp. 86–92. Bilbao, Spain: Revista Iberoamericana de Micología. [Google Scholar]
- 9.Sharma RS, Rajak RC. 2003. Keratinophilic fungi: nature's keratin degrading machines! Resonance 8, 28–40. ( 10.1007/BF02837919) [DOI] [Google Scholar]
- 10.Deshmukh SK. 1985. Isolation of dermatophytes and other keratinophilic fungi from soil of Mussoorie (India). Mykosen 28, 98–101. ( 10.1111/j.1439-0507.1985.tb02102.x) [DOI] [PubMed] [Google Scholar]
- 11.Garg AP, Gandotra S, Mukerji KG, Pugh GJF. 1985. Ecology of keratinophilic fungi. Proc. Indian Acad. Sci (Plant Sci.) 94, 149–163. [Google Scholar]
- 12.Rasmussen M, et al. 2010. Ancient human genome sequence of an extinct Palaeo-Eskimo. Nature 463, 757–762. ( 10.1038/nature08835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen M, et al. 2011. An Aboriginal Australian genome reveals separate human dispersals into Asia. Science 334, 94–98. ( 10.1126/science.1211177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tridico SR, Rigby P, Kirkbride KP, Haile J, Bunce M. 2014. Megafaunal split ends: microscopical characterisation of hair structure and function in extinct woolly mammoth and woolly rhino. Q. Sci. Rev. 83, 68–75. ( 10.1016/j.quascirev.2013.10.032) [DOI] [Google Scholar]
- 15.Brunner H, Coman B. 1974. The identification of mammalian hairs. Melbourne, Australia: Inkata Press. [Google Scholar]
- 16.English MP. 1963. The saprophytic growth of keratinophilic fungi on keratin. Mikologia lekarska 2. [Google Scholar]
- 17.Kanbe T, Tanaka K. 1982. Ultrastructure of the invasion of human hair in vitro by the keratinophilic fungus Microsporum gypseum. Infect. Immunol. 38, 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English MP. 1968. The developmental morphology of the perforating organs and eroding mycelium of dermatophytes. Sabouraudia 6, 218–227. ( 10.1080/00362176885190421) [DOI] [PubMed] [Google Scholar]
- 19.Oyeka CA. 2000. Trichophyton mentagrophytes a keratinophilic fungus. In Biology of dermatophytes and other keratinophilic fungi (eds Kushwaha RKS, Guarro J.), pp. 60–65. Bilbao, Spain: Revista Iberoamericana de Micología. [Google Scholar]
- 20.DeGaetano D, Kempton JB, Rowe WJ. 1992. Fungal tunneling of hair from a buried body. J. For. Sci. 37, 1048–1052. [PubMed] [Google Scholar]
- 21.English MP. 1965. The saprophytic growth of non-keratinophilic fungi on keratinized substrata, and a comparison with keratinophilic fungi. Trans. Br. Mycol. Soc. 48, 219 ( 10.1016/S0007-1536(65)80088-2) [DOI] [Google Scholar]
- 22.Thomma BP. 2003. Alternaria spp.: from general saprophyte to specific parasite. Mol. Plant Pathol. 4, 225–236. ( 10.1046/j.1364-3703.2003.00173.x) [DOI] [PubMed] [Google Scholar]
- 23.Shaw TS, Vanderwyk RW. 1967. The human scalp as a habitat for molds. J. Soc. Cosmet. Chem. 18, 563–568. [Google Scholar]
- 24.English MP, Bayley JA. 1978. Dermatophytes in a population of bank voles and woodmice. Mycopathologia 66, 67–71. ( 10.1007/BF00429595) [DOI] [PubMed] [Google Scholar]
- 25.Papini R, Nardoni S, Ricchi R, Mancianti F. 2008. Dermatophytes and other keratinophilic fungi from coypus (Myocastor coypus) and brown rats (Rattus norvegicus). Eur. J. Wildl. Res. 54, 455–459. ( 10.1007/s10344-008-0170-2) [DOI] [Google Scholar]
- 26.Ellis D. 2014. Mycology online. Adelaide, Australia: University of Adelaide. [Google Scholar]
- 27.Deshmukh SK, Verekar SA. 2006. The occurrence of dermatophytes and other keratinophilic fung from the soils of Himachal Pradesh (India). Czech Mycol. 58, 8. [Google Scholar]
- 28.Lewin RA, Robinson PT, Paul A, Yamanaka G. 1981. The algae of green polar bears. Phycologia 20, 303–314. ( 10.2216/i0031-8884-20-3-303.1) [DOI] [Google Scholar]
- 29.Brunner H, Triggs B, Ecobyte Pty Ltd. 2002. Hair ID: an interactive tool for identifying Australian mammalian hair, 1st edn Melbourne, Australia: CSIRO Publishing. [Google Scholar]
- 30.Rowe WE. 1997. Biodegradation of hairs and fibers. In Forensic taphonomy of human remains (eds Haglund WD, Sorg MH.), pp. 337–351. Boca, CA: CRC Press. [Google Scholar]
- 31.Appleyard HM. 1972. Identification of biological damage to fibres. WIRA Rep. 164, 11. [Google Scholar]
- 32.McBride ME, Freeman RG, Knox JM. 1970. Keratinophilic activity in species of Corynebacterium. Can. J. Microbiol. 16, 1024–1025. ( 10.1139/m70-173) [DOI] [PubMed] [Google Scholar]
- 33.Shelley WB, Miller MA. 1984. Electron microscopy, histochemistry, and microbiology of bacterial adhesion in trichomycosis axillaris. J. Am. Acad. Dermatol. 10, 1005–1014. ( 10.1016/S0190-9622(84)80325-4) [DOI] [PubMed] [Google Scholar]
- 34.Koch SL, Michaud AL, Mikell CE. 2013. Taphonomy of hair: a study of postmortem root banding. J. Forensic Sci. 58(Suppl. 1), S52–S59. ( 10.1111/j.1556-4029.2012.02271.x) [DOI] [PubMed] [Google Scholar]
- 35.Linch BS, Prahlow JA. 2001. Post mortem microscopic changes observed at the human hair proximal end. J. Forensic Sci. 1, 15–20. [PubMed] [Google Scholar]
- 36.Wilson AS. 2008. The decomposition of hair in the buried environment. In Soil analysis in forensic taphonomy chemical and biological effects of buried human remains (eds Tibbett M, Carter DO.), pp. 123–151. Boca Raton, FL: CRC Press. [Google Scholar]
- 37.Tafaro J. 2000. The use of microscopic postmortem changes in anagen hair roots to associate questioned hairs with known hairs and reconstruct events in two murder cases. J. Forensic Sci. 45, 495–499. [PubMed] [Google Scholar]
- 38.Wilson AS. 2001. Yesterday's hair—human hair in archaeology. Biologist 48, 225–236. [PubMed] [Google Scholar]
- 39.Lee WS. 2010. Hair photoaging. In Aging hair (eds Trueb RM, Tobin DJ.), pp. 123–133. Heidelberg, Germany: Springer. [Google Scholar]
- 40.Lister A, Bahn P. 2007. Mammoths giants of the ice age, p. 192 Los Angeles, CA: University of California Press. [Google Scholar]
- 41.Wolfram LJ, Dika E, Maibach HI. 2006. Hair anthropology. In Ethnic skin and hair (eds Berardesca E, Lévêque J-L, Maibach HI.), pp. 55–78. London, UK: Informa Healthcare. [Google Scholar]
- 42.Krefft MDS. 1969. About postmortem changes in the struture and color of hairs and other keratin-containing dermal substances. Arch. Fuer Kriminol. 143. [Google Scholar]
- 43.Gilbert MTP, et al. 2007. Whole-genome shotgun sequencing of mitochondria from ancient hair shafts. Science 317, 1927–1930. ( 10.1126/science.1146971) [DOI] [PubMed] [Google Scholar]
- 44.Butler JM. 2010. Fundamentals of forensic DNA typing. New York, NY: Academic Press. [Google Scholar]
- 45.McNevin D, Wilson-WIide L, Robertson J, Kyd J, Lennard C. 2005. Short tandem repeat (STR) genotyping of keratinised hair. Part 1. Review of current status and knowledge gaps. Forensic Sci. Int. 153, 237–246. ( 10.1016/j.forsciint.2005.05.006) [DOI] [PubMed] [Google Scholar]
- 46.Amory S, Keyser C, Crubezy E, Ludes B. 2007. STR typing of ancient DNA extracted from hair shafts of Siberian mummies. Forensic Sci. Int. 166, 218–229. ( 10.1016/j.forsciint.2006.05.042) [DOI] [PubMed] [Google Scholar]
- 47.Burger J, Hummel S, Hermann B, Henke W. 1999. DNA preservation: a microsatellite-DNA study on ancient skeletal remains. Electrophoresis 20, 1722–1728. () [DOI] [PubMed] [Google Scholar]
- 48.Tridico SR, Houck MM, Kirkbride KP, Smith ME, Yates BC. 2014. Morphological identification of animal hairs: myths and misconceptions, possibilities and pitfalls. Forensic Sci. Int. 238 ( 10.1016/j.forsciint.2014.02.023) [DOI] [PubMed] [Google Scholar]