Abstract
Multimodal signals facilitate communication with conspecifics during courtship, but they can also alert eavesdropper predators. Hence, signallers face two pressures: enticing partners to mate and avoiding detection by enemies. Undefended organisms with limited escape abilities are expected to minimize predator recognition over mate attraction by limiting or modifying their signalling. Alternatively, organisms with anti-predator mechanisms such as aposematism (i.e. unprofitability signalled by warning cues) might elaborate mating signals as a consequence of reduced predation. We hypothesize that calls diversified in association with aposematism. To test this, we assembled a large acoustic signal database for a diurnal lineage of aposematic and cryptic/non-defended taxa, the poison frogs. First, we showed that aposematic and non-aposematic species share similar extinction rates, and aposematic lineages diversify more and rarely revert to the non-aposematic phenotype. We then characterized mating calls based on morphological (spectral), behavioural/physiological (temporal) and environmental traits. Of these, only spectral and temporal features were associated with aposematism. We propose that with the evolution of anti-predator defences, reduced predation facilitated the diversification of vocal signals, which then became elaborated or showy via sexual selection.
Keywords: mating signals, aposematism, natural selection, sexual selection
1. Introduction
Acoustic signals of courting males are often conspicuous to eavesdropping enemies, and these males face a trade-off between attracting partners and avoiding predators [1]. Diurnal signallers are especially vulnerable to predation owing to their increased visual and acoustic detectability [2]. These individuals may avoid predation with a spectrum of strategies from crypsis to aposematism (the linking of a warning signal with a defensive strategy) [3]. Non-defended individuals usually signal from concealed places and rely on crypsis to avoid detection. If predation pressure is strong, individuals may reduce the number of signals, alter their schedule or switch communication channels [4]. By contrast, aposematic individuals (aposemes) broadcast warning signals that may be detected by predators [5]. Consequently, the evolution of aposematism, with the resulting reduction in predation risk, may enhance the diversification of mating signals and signallers. We tested this hypothesis by a phylogenetic analysis of the vocalizations of aposematic and cryptic species of the poison frog family Dendrobatidae.
Aposematism is a complex phenotype that links conspicuous signals with defence (e.g. alkaloids). The association of these traits with other ecological adaptations, including metabolic and diet specializations, characterizes the aposematic syndrome in dendrobatids [6]. Visual cues such as chromatic conspicuousness are often a warning signal to predators such as avian predators on poison frogs [7]. By contrast, acoustic cues warn predators such as bats, which avoid the ultrasound chirps from defended, nocturnal moths [8]. Although both visual and acoustic signals may alert predators, their combined effects might increase the effectiveness of anti-predator defences [9].
Some predators locate their prey by exploiting components of the prey's signal. The efficacy of visual warning signals depends on light conditions and background [5]. Conspicuous prey might be effectively cryptic if the incident light is too low [10]. By contrast, sound-oriented predators and parasites recognize long-range signals such as mating calls and when in proximity switch to visual or chemical identification [4]. For example, Corethrella midges locate male frogs by their calls and then switch to olfaction or other senses to locate the nostrils of the male, from which they obtain a blood meal [11]. Natural selection should favour individuals that avoid predator attacks by maximizing aposematic conspicuousness.
Two general types of mechanisms may explain the origin of aposematism: first, predator-related mechanisms, including prey aggregation, dietary conservatism of predators and neophobia [5] and second, traits shaped primarily by natural selection, which can then be co-opted as sexual ornaments through sexual selection [12]. Aposemes might be better at attracting mates if predators associate their mating signals with unprofitability and thus avoid courting individuals. Thus, under reduced predator pressure, aposemes may evolve more easily detected mating signals via sexual selection.
Poison frogs are a model clade for studies of the predator–prey ecology of aposematism, which evolved at least four times in this group (figure 1). However, not all dendrobatids fit the stereotype of the brightly coloured, charismatic frog. Most are cryptically coloured and rely on camouflage. By contrast, the aposematic species are visually conspicuous and defended by skin alkaloids [13] that are distasteful and at times toxic to predators (e.g. birds, crabs and snakes). Dendrobatids are mostly diurnal and use visual and acoustic signals for intraspecific communication [14]. Their vocalizations are innate and highly stereotyped, but have easily quantifiable variation among species. Within some species, females prefer males with greater calling performance [15,16]. Calling has a significant metabolic cost for male frogs [17] and aposematic dendrobatids have higher metabolic rates [6], but the relationship between acoustic signalling, aposematism and metabolic rates has not been explored in dendrobatids.
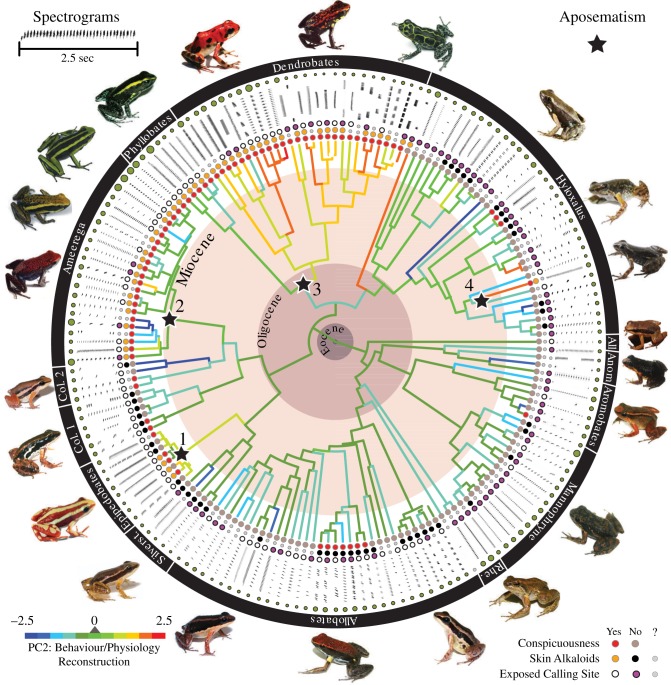
Figure 1.
Poison frog chronogram showing the diversity of mating calls. Next to each tip (species) is the first 2 s of its spectrogram. Alkaloid sequestration is indicated by coloured dots: presence (orange), absence (brown) or unknown (grey). Red dots indicate species considered conspicuous based on the total contrast score ΣSi ≥ 5 (TCS5); black dots indicate cryptic species (ΣSi < 5). White dots indicate species calling from above-ground locations or in exposed habitats; purple dots indicate calling from concealed locations. Black stars indicate origins of aposematism. Green dots indicate relative body size. Branch colours represent parsimony reconstructions of the temporal call component (PC2) using standardized units (bluish: slow rates; reddish: fast rates). All., Allobates; Ame., Ameerega; Anom., Anomaloglossus; Col., Colostethus; Rhe., Rheobates; Silverst., Silverstoneia. Support for each node is provided in electronic supplementary material, figure S1.
Using phylogenetic methods, we tested whether acoustic mating signals diversified in association with the multiple origins of aposematism. Our results support this postulate and provide evidence that aposematism is associated with increased speciation rate in dendrobatids. We propose that the origin of aposematism, with the resulting reduction in predation risk, enabled mating calls of defended species to diversify via sexual selection.
2. Material and methods
(a). Acoustic data and perching behaviour
We collected 16 657 advertisement calls from 172 species. All recordings were obtained from field collections and museum archives (electronic supplementary material, Dataset S1). The calls are characterized by single pulses with little frequency modulation (figure 1; electronic supplementary material, S1 and Dataset S1). Field recordings were digitized with a sampling rate of 16 bits at a rate of 22 or 44 kHz and filtered for background noise using a bandpass filter of 1–5 kHz. Spectrograms and power spectra were estimated using a Fast Fourier Transform (FFT) analysis using a Blackman window, 900 samples of overlap among subsequent FFTs, and 3 dB filter bandwidth of 87.5 Hz. Homology of acoustic units was assessed following a physiological definition in which the call is the sound unit produced by a cycle of trunk muscle contraction resulting in an expiratory event [18]. We used note-pulses, which have uniform temporal, spectral and taxon-specific features, as the homologous acoustic units. Temporal features and spectral properties were measured from oscillograms, spectrograms and power spectra (electronic supplementary material, figure S2 and Text). All acoustic variables were measured using RavenPro v. 1.4 [19]. We analysed 18 call variables measured from note-pulses (homologous acoustic units) as well as temperature recorded at the calling site and body size (electronic supplementary material, figure S2 and table S1). For each call variable, the mean of the individuals was used for analysis. Finally, we also qualitatively described perch (calling) site as exposed or concealed based on published and direct observations for 83 species (electronic supplementary material, Dataset S1).
(b). Alkaloid sequestration and conspicuousness variables
We compiled skin alkaloid information of 97 taxa (electronic supplementary material, table S1). Species were characterized by their ability to sequester alkaloids as state 1 (able to sequester) or 0 (unable to sequester) [6]. A species was characterized as aposematic by the presence of defensive dietary alkaloids [6] and visual conspicuousness (electronic supplementary material, tables S1 and S2).
Chromatic contrast against a natural background is considered a measurement of conspicuousness to predators [6]. Few dendrobatids have been assessed for conspicuousness using direct approaches such as total reflectance flux and models of predator perception [3,7]. Quantifying conspicuousness in life for 172 taxa using direct techniques was intractable. Therefore, we measured relative conspicuousness based on human perception of colour contrast against a leaf litter background. Some authors [20] validly criticize the quantification of colouration based on human perception. These criticisms do not necessarily invalidate our analyses because most receivers include a mixture of trichromatic conspecifics and di-, tri- and tetrachromatic predators (electronic supplementary material, S1 for discussion). All these receivers also have visual sensitivities that overlap with the human vision range (400–700 nm) and may not obtain information from the UV range [21].
As a proxy for direct approaches, we formulated a binary assessment of conspicuousness against a leaf litter background (electronic supplementary material, figure S3 and table S2). Colour descriptions of live male specimens were quantified by multiple independent human observers ( per species; electronic supplementary material, table S2). Using 11 frog skin segments, chromatic contrast (i.e. different from grey, brown and black) was scored as 1 (conspicuous) or 0 (cryptic). The total contrast score (TCS or ΣSi), which ranged from 0 (no contrast) to 11 (maximum contrast), was determined by summing all 12 binary values. To account for inter-observer variation, we used six cut-off values (ΣSi ≥ 3, ΣSi ≥ 4, … ,ΣSi ≥ 8; electronic supplementary material, figure S3) of increasing colour contrast thresholds. These thresholds ranged from liberal (a species with ΣSi ≥ 3 (TCS3) is categorized as conspicuous) to strict (ΣSi ≥ 8 (TCS8)). Thus, conspicuousness in each species was quantified under six binary variables TCS3–TCS8. For example, a given taxon with TCS7 is considered cryptic (state 0) under the threshold ΣSi ≥ 8 (TCS8); but conspicuous (state 1) under the five thresholds ΣSi ≥ 3, ΣSi ≥ 4, … ,ΣSi ≥ 7 (i.e. TCS3–TCS7). This suite of variables assesses the robustness of our results over a range of receivers and light conditions. Finally, we also determined the ability of our conspicuousness variables to predict aposematism using a joint criterion for binary classifiers as described in the electronic supplementary material, S1. We emphasize that our conspicuousness assay is not an ideal substitute for modelling perception by predators.
per species; electronic supplementary material, table S2). Using 11 frog skin segments, chromatic contrast (i.e. different from grey, brown and black) was scored as 1 (conspicuous) or 0 (cryptic). The total contrast score (TCS or ΣSi), which ranged from 0 (no contrast) to 11 (maximum contrast), was determined by summing all 12 binary values. To account for inter-observer variation, we used six cut-off values (ΣSi ≥ 3, ΣSi ≥ 4, … ,ΣSi ≥ 8; electronic supplementary material, figure S3) of increasing colour contrast thresholds. These thresholds ranged from liberal (a species with ΣSi ≥ 3 (TCS3) is categorized as conspicuous) to strict (ΣSi ≥ 8 (TCS8)). Thus, conspicuousness in each species was quantified under six binary variables TCS3–TCS8. For example, a given taxon with TCS7 is considered cryptic (state 0) under the threshold ΣSi ≥ 8 (TCS8); but conspicuous (state 1) under the five thresholds ΣSi ≥ 3, ΣSi ≥ 4, … ,ΣSi ≥ 7 (i.e. TCS3–TCS7). This suite of variables assesses the robustness of our results over a range of receivers and light conditions. Finally, we also determined the ability of our conspicuousness variables to predict aposematism using a joint criterion for binary classifiers as described in the electronic supplementary material, S1. We emphasize that our conspicuousness assay is not an ideal substitute for modelling perception by predators.
(c). Metabolic rate variables
In electronic supplementary material, table S1, we compiled metabolic rate parameters of 54 species of poison frogs from a previous study [6] along with our corresponding acoustic data. The variables were (i) resting metabolic rate (RMR, oxygen consumption while resting or VO2rest ml h−1), (ii) active metabolic rate (AMR) after non-sustainable exercise (oxygen consumption after forced activity or VO2active ml h−1) and (iii) mean body mass to the nearest 0.01 g of all the individuals tested. From these raw data, mass-specific metabolic rates (AMR and RMR) were estimated by dividing the metabolic rates by the body mass of each individual. The average of all conspecific rates was used as the species mass-specific metabolic rate. Finally, body mass and mass-corrected metabolic rates were transformed using natural logarithms to improve statistical distribution properties for the comparative procedures [22].
(d). Phylogenetic and comparative analyses
The phylogeny was inferred from new and published molecular data: approximately 2400 bp 12S–16S rDNA mitochondrial genes (electronic supplementary material, table S1 for GenBank numbers). The statistics of the molecular data matrix were as follows: (i) total sequence length (N = 172, 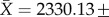
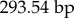 , missing cells are 13 910/414 692 or 3.35%); (ii) total sequence length per rRNA gene (12S: N = 172,
, missing cells are 13 910/414 692 or 3.35%); (ii) total sequence length per rRNA gene (12S: N = 172, 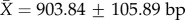 , missing cells are 3.44%; 16S: N = 172,
, missing cells are 3.44%; 16S: N = 172, 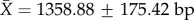 , missing cells are 4.03%); and (iii) total sequence length per tRNA gene (tVal: N = 172,
, missing cells are 4.03%); and (iii) total sequence length per tRNA gene (tVal: N = 172, 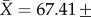
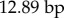 , missing cells are 7.65%). Tree estimation and nodal support were calculated under maximum-likelihood (ML) and Bayesian approaches using partitioned models. ML and Bayesian analyses gave similar tree topologies and the ML tree was used as a starting tree topology for the time-calibrated tree (electronic supplementary material, S1). The chronogram (figure 1; electronic supplementary material, S1) was determined using BEAST v. 1.5.3 [23] with five node-age constraints (electronic supplementary material, figure S1). Based on these analyses, two taxonomic changes are made: Ameerega erythromos and Colostethus jacobuspetersi as part of Hyloxalus (i.e. H. erythromos and H. jacobuspetersi; new combinations; see electronic supplementary material, S1 for details). Chronogram tree file is deposited in the TreeBASE database under the accession number 16380.
, missing cells are 7.65%). Tree estimation and nodal support were calculated under maximum-likelihood (ML) and Bayesian approaches using partitioned models. ML and Bayesian analyses gave similar tree topologies and the ML tree was used as a starting tree topology for the time-calibrated tree (electronic supplementary material, S1). The chronogram (figure 1; electronic supplementary material, S1) was determined using BEAST v. 1.5.3 [23] with five node-age constraints (electronic supplementary material, figure S1). Based on these analyses, two taxonomic changes are made: Ameerega erythromos and Colostethus jacobuspetersi as part of Hyloxalus (i.e. H. erythromos and H. jacobuspetersi; new combinations; see electronic supplementary material, S1 for details). Chronogram tree file is deposited in the TreeBASE database under the accession number 16380.
To quantify the relationship between components of aposematism and call variables, we used diversification analyses, multivariate data exploration, tests of phylogenetic signal and models of trait evolution, bivariate phylogenetic correlations, exploratory factor analyses (phylogenetic principal component analysis, PPCA) and phylogenetic logistic regressions (PLRs). Using the binary variables alkaloid sequestration and conspicuousness, we estimated the rates of speciation (λ0 and λ1), extinction (μ0 and μ1) and transition between character states (q01 and q10) using Binary State Speciation and Extinction (BiSSE) models [24]. Multivariate data explorations and variable reduction were used to narrow the dataset to 18 variables (169 taxa) that loaded on three principal components.
The PLRs were used to determine if call variables significantly predicted alkaloid sequestration and conspicuousness as dependent binary variables. Our predictors were two sets of continuous variables: the three PCs (principal components) derived from the PPCA of the call characters, and individual call variables, with body size and temperature as covariates. PLRs were performed with the PLogReg routine [25], which tests for phylogenetic signal while simultaneously performing the regressions. We applied the percentage increase in odds and the ‘divide-by-4 rule’ (i.e. β/4 where β is the regression coefficient) to determine significance of logistic regression coefficients [26]. See electronic supplementary material, S1 for an example of β/4 interpretation. Outliers were identified using standardized residuals with absolute values more than 3.0 and Cook's distance more than 1.0 as criteria. For pairwise correlations between the discrete dependent variables, we used Pagel's 1994 test for correlation of two binary characters [27]. Significance of all analyses was determined at α = 0.05, two-tailed distribution.
3. Results
We tested if acoustic courtship cues are associated with the origin of the aposematic phenotype, and if these patterns are related to species diversification. Specifically, (i) we determined whether conspicuousness and alkaloid sequestration, the components of aposematism, are correlated and show phylogenetic signal; (ii) we measured species diversification by comparing extinction, speciation and character-state transition rates between aposematic and cryptic species; (iii) we used PPCA to describe the relationship between call variables and each aposematic component; and (iv) we used PLR to determine which call variables are associated with aposematism.
In analysis (i), we used Pagel's λ [27] to test for phylogenetic signal (λ > 0) in each of the two aposematic components scored (electronic supplementary material, figure S4 and table S3). Alkaloid sequestration showed phylogenetic signal (p < 0.001), as did conspicuousness variables TCS3–TCS6 (all p < 0.001). Alkaloid-bearing species were mostly conspicuous (all TCS variables; all correlations significant at p ≤ 0.032). Thus, aposematic species tend to be closely related and not randomly distributed across the phylogeny.
In analysis (ii), we used BiSSE models [24] to estimate rates of speciation (λ), extinction (μ) and transition between alternative character states (q). Given the concern about the interpretation of BiSSE models with less than 300 terminals [28], we assessed statistical power (electronic supplementary material, S1). As determined by simulations, our sample size (172 terminals) was adequate for analyses of speciation and transition rates, but less so for extinction rates.
The speciation rate for conspicuous lineages showed a 1.36- to 2.18-fold increase over that in cryptic lineages, except for TCS6 (p < 0.05 for all; figure 2; electronic supplementary material, S4). However, for alkaloid-bearing lineages neither the speciation rate nor the extinction rate was different from that of non-defended lineages (p > 0.05 for all). Given that the speciation rate for conspicuous lineages was higher, it is surprising that defended clades did not have a high speciation rate, because conspicuousness and sequestration are generally highly correlated. This result is perhaps explainable by the large amount of missing data for alkaloid sequestration (figure 1). However, it does not alter the general conclusion that aposematic clades have a higher speciation rate. The extinction rate for conspicuous lineages was not different from that for cryptic lineages (p > 0.05 for all) except for TCS3.
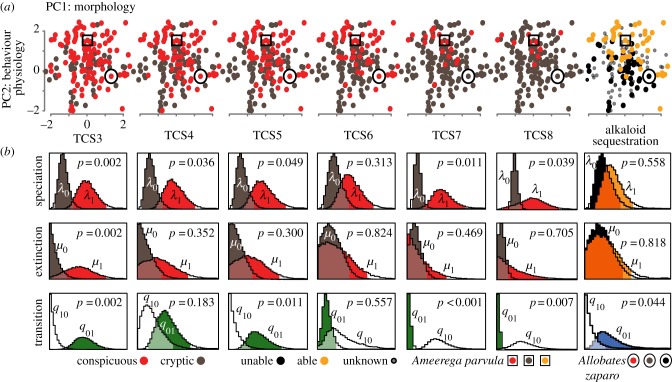
Figure 2.
Diversification analyses of aposematism in poison frogs. (a) Distribution of conspicuousness variables (TCS3–TCS8) derived from the total contrast score (TCS or ΣSi) and alkaloid sequestration ability. PC1 and PC2 represent call parameter space. Each species was coloured based on its conspicuousness and alkaloid sequestration ability. The Batesian mimic species (open circles) and the aposeme model (open squares) are also indicated. (b) Distributions of the rates of speciation–λ, extinction–μ and transition between character states–q. Alternative states are indicated by subscripts: 0 (cryptic/unable to sequester alkaloids) and 1 (conspicuous/able to sequester). The p-values refer to the probability of the null model of equal diversification rates (i.e. λ0 = λ1, μ0 = μ1 or q10 = q01).
We found that the rate of change from inability (state 0) to ability (state 1) to sequester alkaloids is 14 times higher than the reverse (q01 = 0.014 versus q10 < 0.001; p = 0.044; figure 2; electronic supplementary material, S4); essentially no reversals from the defended to the non-defended phenotype have taken place. Similarly, the transition rate from cryptic to conspicuous states (q01) and the reverse (q10) shifted from a higher rate (q01 > q10 in TCS3 and TCS5; all p ≤ 0.011) to a lower rate (q01 < q10 in variables TCS7 and TCS8; all p ≤ 0.007). This is supported by two more pieces of evidence: TCS5 was significantly correlated with alkaloid sequestration (p < 0.001), and TCS5 was also the best predictor of aposematism based only on conspicuousness (electronic supplementary material, figure S4). However, these results suggest that conclusions about rates of state change depend on how the binary state is defined, such as when large tip-ratios (more than 10 : 1) exist for change between states [28]. In summary, the extinction rates of aposematic and cryptic clades are not distinguishable, but aposematic lineages speciate more and are unlikely to revert to the cryptic/non-defended phenotype.
In analysis (iii), PPCA recovered three components describing 77.88% of the variability (electronic supplementary material, table S3): PC1 (37.46% of the variance) described body size and spectral properties such as pitch, which are related to morphology [29]; PC2 (28.34%) described temporal properties (e.g. timing of pulsed notes), which are related to behaviour/physiology (e.g. contraction of trunk muscles for sound production) and PC3 (12.08%) reflected ambient temperature. All three PCs showed phylogenetic signal (all λ > 0, all p < 0.001), indicating that call properties track phylogeny [18].
In analysis (iv), PLRs were used to determine if call components are associated with aposematism (figure 3; electronic supplementary material, table S4). The predictor variables were the three PCs, and alkaloid sequestration and conspicuousness were discrete dependent variables. PC1 (morphology) and PC2 (behaviour/physiology) predicted alkaloid sequestration (all regression coefficients at p < 0.05). All else being equal (assumed hereafter), species with larger body sizes and low-pitch calls (PC1) have a 20.2% (±6.18% s.e., p ≤ 0.024) or a 1.2-fold increase in the probability of sequestering alkaloids per each standard unit increase of their PC1 scores. Similarly, we found that species with short-duration pulses and/or pulses at faster rates (PC2) have a 39.6% (±9.70% s.e., p ≤ 0.018) or a 1.4-fold increase in their probability of sequestering alkaloids. The environmental component (PC3) was not a significant predictor (p > 0.05). Therefore, species that call at low frequencies and/or emit vocalizations at faster rates are more likely to sequester alkaloids.
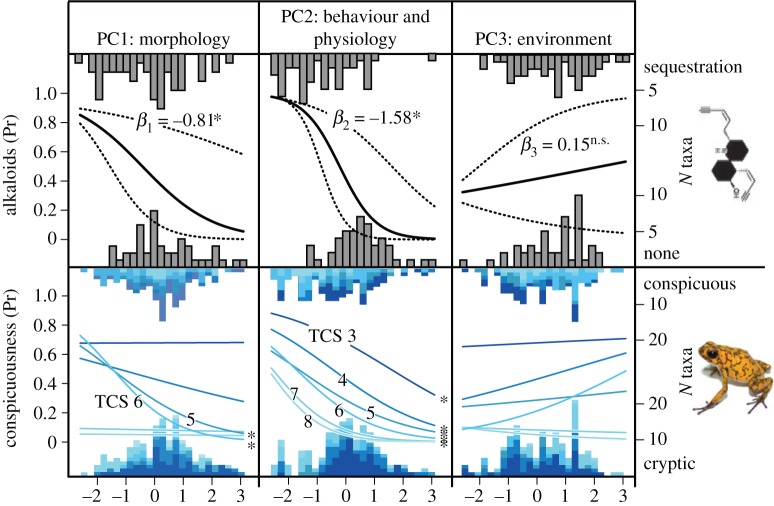
Figure 3.
Phylogenetic logistic regression curves for alkaloid sequestration (top row) and conspicuousness (bottom row). The columns correspond to the principal components PC1: morphology (body size and spectral call parameters), PC2: behaviour and physiology (temporal call parameters) and PC3: environment. In the top row, solid lines are logistic regression curves for alkaloid sequestration, with 95% CI (confidence interval; dashed lines) and β is the logistic regression coefficient. The bottom row shows logistic regression curves for each conspicuousness variable (TCS). Significant coefficients are marked with an asterisk (α = 0.05, two-tailed). Frequency histograms indicate the number of species along each principal component axis, in each category (able to sequester alkaloids or unable, conspicuous or cryptic).
Next, we investigated if PC1 was associated with conspicuousness. PC1 variables predicted conspicuousness only for variables TCS5 and TCS6 (p < 0.003 for both) but not the other variables. Species with larger body size or lower-pitched calls had approximately 1.2-fold increase (15.1 ± 5.03% s.e., p = 0.003 for TCS5 and 22.1 ± 6.15% s.e., p < 0.001 for TCS6) in the probability of being conspicuous. Thus, these results support that body size and call pitch are associated with conspicuousness, and thus aposematism.
These PLR results for PC1 further support the association between body size, metabolic rates and aposematism as found previously [6]. However, is there a relationship between call characteristics and metabolic rates in poison frogs? We tested if mass-specific metabolic rates (AMR and RMR) are correlated with the acoustic trait PCs using a phylogeny of 54 species having metabolic measurements [6] and acoustic data (electronic supplementary material, table S1). AMR and RMR were correlated with PC1 (rAMR = 0.31 and rRMR = 0.43; both p < 0.001), reinforcing the connection of metabolic rate with spectral call properties (electronic supplementary material, table S3). Interestingly, metabolic rates were not correlated with PC2 or PC3 (both p > 0.05). Given that PC2 reflects performance-related variables such as pulse rate, our results reflect the greater stamina in body wall muscles (for calling) compared with limb muscles (for locomotion) as evidenced in hylid frogs [30,31].
The correlation of alkaloid sequestration with PC2 (temporal features) that was uncovered by our PLR analyses is novel. We further examined this by examining the individual variables that contribute to PC2. After controlling for body size and temperature, we found that pulse rate was the best predictor of alkaloid sequestration. An increase of one standard unit resulted in a 36.2% (±9.55% s.e., p < 0.001) or a 1.4-fold increase in the probability of the sequestration ability. Shorter pulse duration, rise time and pulse intervals showed similar approximately 1.3-fold increases (p < 0.001 for all). Thus, individual temporal call variables (PC2) are sufficient to predict alkaloid sequestration.
The individual variables of PC2 also predicted conspicuousness (figure 3; electronic supplementary material, table S4). Species with short-duration pulses and/or faster pulse rates had a 12.0–27.1% (p < 0.05 for all conspicuousness variables) or approximately 1.2-fold increase in the probability of being conspicuously coloured. Individual temporal variables also predicted conspicuousness. After controlling for body size and temperature, pulse rate had the highest predictive ability. An increase of one standard unit of pulse rate resulted in a 13.6–23.5% (all variables p < 0.05) or approximately 1.2-fold increase in the probability of having the conspicuous phenotype. Similar results were found for other PC2-loading variables (electronic supplementary material, table S4). Thus, temporal features of mating calls are associated with conspicuous colouration.
4. Discussion
We propose two hypotheses for the connection between mating call characteristics and aposematism. Under the first, novel features of acoustic signals (shifts in spectral and temporal properties) evolved first as the primary warning component of aposematism and enhanced predator avoidance through experience with distasteful alkaloids. Visual conspicuousness (colouration) followed. However, we did not find phylogenetic or ecological support for this prediction. In a phylogenetic context, the acquisition of novel acoustic signals should precede the appearance of the visual signal (i.e. conspicuous colouration). However, we found the reverse pattern, in which changes in the vocalizations towards faster and lower-pitched calls occurred only after the appearance of conspicuous colouration (figure 1).
Our first hypothesis also makes predictions about the ecology of the warning signal. Acoustic signals facilitate social interactions, but they also increase conspicuousness to predators. A predator might perceive the mating calls of defended individuals as a warning even in the absence of a visual warning cue. Many aposematic dendrobatids have conspicuous trills, chirps and buzz calls (electronic supplementary material, Dataset S1). Similar sounds such as the buzzing of bumblebees, the rattles of venomous snakes and the chirps of distasteful moths are warning signals [32]. Thus, we might expect aposematic dendrobatids to have calls with a broader frequency content spectrum (‘harsher’) than those of non-aposematic species, which should have a narrower bandwidth or be pure tone (‘sweeter’). Comparisons between aggressive and attractive calls in birds and mammals [33] showed that these animals use harsh sounds when hostile and pure tone-like sounds when appeasing other individuals. Therefore, the calls of aposematic frogs might motivate avoidance by predators. However, we found no support for a correlation between broadband calls (measured by the bandwidth of the interquartile range) and either conspicuousness or alkaloid sequestration (both regression coefficients p > 0.05; electronic supplementary material, table S4). Thus, the first hypothesis, that the mating calls of aposematic dendrobatids are a primary warning signal, is not supported.
Our second hypothesis proposes that the evolution of aposematism, with the resulting reduction in predation risk, may have freed mating calls to evolve showy characteristics via sexual selection. This hypothesis is favoured by both phylogenetic and ecological evidence. In the evolutionary context, aposematism preceded the evolution of the diversification mating calls (figure 1). Consequently, any change in call traits occurred after aposematism was already a functional anti-predator strategy. In the ecological context, several aspects of the ecology of dendrobatid aposemes support our postulate. The diurnal activity of almost all dendrobatids includes multimodal courtships that integrate acoustic and visual displays [14]. Visually oriented predators learn to avoid aposemes, which then undergo ecological release and evolve existing calls into more conspicuous displays that attract females. Natural history information and our direct observations indicate that most non-aposemes vocalize from concealed, ground-level sites, whereas aposemes vocalize from exposed, above-ground sites. We found that exposed callers are more likely to sequester alkaloids than concealed callers (Pagel's 1994 test; electronic supplementary material, table S3, N = 83, p = 0.015). Similarly, we found that exposed callers are more likely to be conspicuous than cryptic, concealed callers (electronic supplementary material, table S3, TCS3–TCS7, N = 146, all p ≤ 0.020).
These results suggest that calling behaviour of aposemes might result in higher efficiency of acoustic transmission. In tropical frogs, males that broadcast above the ground in open environments and call with lower pitch suffer less degradation over distance [34]. These observations agree with our findings that aposemes call at a lower pitch and also from higher perching sites than do cryptic, undefended frogs. Consequently, the calls of aposematic males should be more salient to potential mates, and thus females would prefer males that call from exposed perches. Therefore, this second hypothesis is better supported and suggests that aposematism releases predator-induced constraint on the evolution of the signal and the behaviour of the signaller.
Three possible mechanisms explain how females might evaluate aposematism in mate choice. First, females select males that provide them direct benefits during courtship, such as effective predator deterrence. Second, males are selected if they provide indirect benefits to offspring such as increased viability and attractiveness derived from traits associated with aposematism. Third, males are selected due to sensory exploitation of aposematic signals by females. No experimental evidence exists for these mechanisms, but some inferences might be derived from comparison of field and laboratory studies. For example, males might gain mating advantages from female preference for conspicuous colouration, which originally evolved in a non-sexual context to warn predators. However, under certain laboratory conditions, females of Dendrobates pumilio prefer brighter and possibly more toxic males [35], while in natural conditions females prefer the closest calling male [36].
The exploitation of aposematic signals by mimics might also confer reproductive advantages to them and drive the evolution of their mating signals. Allobates zaparo, a Batesian (e.g. non-defended) mimic, derives protection from matching the reddish dorsal colour of its aposematic models Ameerega bilinguis and Ameerega parvula [37]. Interestingly, we found that A. zaparo does not acoustically resemble either model closely (figure 2a; electronic supplementary material, figure S4A), but rather is acoustically closer in call parameter space to its non-aposematic relatives within the Allobates femoralis complex (Euclidean distance: 0.809 ± 0.253 s.d.). However, calls of A. zaparo are within the range of variation of most aposemes; the PC scores of A. zaparo were not outliers in the PLR analysis (all Cook's distances ≤ 0.042). This suggests that A. zaparo might benefit from the evolution of showy mating calls as an advantage of mimicking aposematic colouration and the resulting reduced predation.
Acoustic signalling increases detectability, suggesting that predation pressure is greater on males [12] than on females, which have limited acoustic signalling (e.g. release calls). However, given that sexual dichromatism is not common in aposematic dendrobatids and the shared genetic architecture is present in males and females, predation pressure rather than mate choice is probably the main driver of visual conspicuousness. In one species (D. pumilio), females have larger quantities of alkaloids than males [38]. Hence, why may we expect aposematic females to have better defences than males? Some aspects of female ecology suggest a broader pattern of social selection. First, females are exposed to predators as they search and compete for the best partner and share with males the cost of parental care [39]. Second, the cost of aposematism might be higher if females provide defensive alkaloids directly to their offspring [40]. Third, dendrobatids engage in prolonged and interactive courtships where females search for and move towards vocalizing males [14]. This behaviour might increase females' exposure to predators compared to males', but this hypothesis needs further experimental evidence. Finally, females vigorously defend general-purpose territories, attack intruders, and may use aposematism to protect ecological resources [41]. Further research on the behavioural ecology of females rather than only phylogenetic comparative analyses (i.e. correlation does not necessarily means causation) will illuminate the sex-specific benefits and costs of aposematism.
In sum, our analyses revealed a strong association between acoustic signalling and aposematism in poison frogs. We provide two general conclusions. First, aposematic lineages speciate at a higher rate than non-aposematic lineages and rarely revert to a cryptic phenotype. A high speciation rate was found in the aposematic clades Dendrobates and Ameerega [42], which evolved aposematism independently. This is consistent with the suggestion that female preference for males with brighter visual signals in D. pumilio [35] might drive species diversification. However, this assumes intra-populational variation in brightness, which is not demonstrated in most species. Nonetheless, our results might suggest that the higher speciation rates in aposematic lineages are due to increased sexual selection once predator pressure is diminished.
Second, lower pitch (spectral properties) and faster rates in pulse-related call variables (temporal properties), as well as calling from exposed perches, are associated with multiple origins of aposematism. Consequently, our results show that dendrobatid aposemes shifted to a different type of call compared to non-aposemes. This pattern is convergent among aposematic clades; thus dendrobatid calls have become more diverse within Dendrobatidae. We proposed two hypotheses for the evolutionary shift of acoustic signals and behaviour of aposemes. First, this shift was due to the superposition of a warning function onto the pre-existing mate attraction function, which when combined with existing alkaloid defence, enhanced protection against predators. This suggests that receivers need only hear the frog to know if it is aposematic and hence unpalatable. However, our analyses do not support this postulate and it remains to be tested whether calls alone of aposemes deter predators. Second, this evolutionary shift resulted from pre-existing aposematism (alkaloid defence and visual conspicuousness), which relaxed predation pressure. The combination of low-pitch and exposed calling sites enhanced call transmission and mate attraction. This last hypothesis is supported by our data and suggests that, in general, predation exerts a strong constraint on sexual selection; aposematism removes such constraint.
Although further experiments will determine to what extent specific call traits associated with aposematism affect mate choice, our data offer evidence of the effect of predation on the evolution of signal complexity. While a similar pattern of predators driving phenotypic diversification has been demonstrated in other taxa such as Trinidadian guppies (Poecilia reticulata) and Gambusia mosquitofish [43,44]. Our results provide evidence for the interaction of signals across modalities (visual and acoustic) and across contexts (signalling to predators and to mates) to affect the evolution of communication, as well as the formation of new species in aposematic clades. Only studies of phenotypic predictors of reproductive success would be required to fully demonstrate that sexual selection via female choice is responsible for the further diversification of elaborate vocal signals in dendrobatid frogs.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
J.C.S. thanks Fonoteca Neotropical ‘Jacques Vielliard’, the Macaulay Library (Cornell University), Borror Laboratory of BioAcoustics (OSU), United States National Museum (USNM) and University of Kansas (KU) audio repositories. J.C.S. thanks N. Biani and I. Santos for their kindness and support. J.C.S. thanks N. Acosta-Buenaño, R. Bastos, R. C. Costa, C. Koch, R. M. Lehtinen and M. Read for sharing audio recordings. C.B.A. and J.C.S. thank A. Amézquita, C. Aguilar, Fundación AndígenA and Smithsonian Tropical Research Institute (STRI). We acknowledge Elicio and Italo Tapia for field assistance. We acknowledge the comments and suggestions by two anonymous reviewers. A.P.L. thanks C. Keller, C. Strussmann, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Instituto Nacional de Pesquisas da Amazonia (INPA). L.A.C. acknowledges Wikiri S.A. and the Saint Louis Zoo. J.C.S. designed and performed the research; J.C.S., M.B., C.B.A., L.A.C., L.K.E., A.P.L., D.C.C. collected data; J.C.S. wrote the first draft; M.B., C.B.A., L.A.C., L.K.E., A.P.L., D.C.C. gave comments on the early drafts; and J.C.S. and D.C.C. revised the paper.
Data accessibility
DNA sequences: KJ940454-KJ940470 (see electronic supplementary material, table S1).
Phylogenetic data: TreeBASE accession no. 16380
Funding statement
D.C.C. acknowledges NSF grant EF-0334952. J.C.S. acknowledges the NSERC-CREATE Training Program in Biodiversity Research at UBC, NSF DDIG DEB-0710033, and NESCent (NSF EF-0423641 and EF-0905606).
References
- 1.Ryan MJ, Tuttle MD. 1983. The ability of the frog-eating bat to discriminate among novel and potenitally poisonous frog species using acoustic cues. Anim. Behav. 31, 827–833. ( 10.1016/S0003-3472(83)80239-5) [DOI] [Google Scholar]
- 2.Narins PM, Grabul DS, Soma KK, Gaucher P, Hödl W. 2005. Cross-modal integration in a dart-poison frog. Proc. Natl Acad. Sci. USA 102, 2425–2429. ( 10.1073/pnas.0406407102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willink B, Brenes-Mora E, Bolanos F, Pröhl H. 2013. Not everything is black and white: color and behavioral variation reveal a continuum between cryptic and aposematic strategies in a polymorphic poison frog. Evolution 67, 2783–2794. [DOI] [PubMed] [Google Scholar]
- 4.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438. ( 10.1086/420412) [DOI] [Google Scholar]
- 5.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals & mimicry, 249 p Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Santos JC, Cannatella DC. 2011. Phenotypic integration emerges from aposematism and scale in poison frogs. Proc. Natl Acad. Sci. USA 108, 6175–6180. ( 10.1073/pnas.1010952108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maan ME, Cummings ME. 2012. Poison frog colors are honest signals of toxicity, particularly for bird predators. Am. Nat. 179, E1–E14. ( 10.1086/663197) [DOI] [PubMed] [Google Scholar]
- 8.Ratcliffe JM, Nydam ML. 2008. Multimodal warning signals for a multiple predator world. Nature 455, 96–99. ( 10.1038/nature07087) [DOI] [PubMed] [Google Scholar]
- 9.Partan SR, Marler P. 2005. Issues in the classification of multimodal communication signals. Am. Nat. 166, 231–245. ( 10.1086/431246) [DOI] [PubMed] [Google Scholar]
- 10.Endler JA, Mappes J. 2004. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547. ( 10.1086/382662) [DOI] [PubMed] [Google Scholar]
- 11.Bernal XE, Rand AS, Ryan MJ. 2006. Acoustic preferences and localization performance of blood-sucking flies (Corethrella Coquillett) to túngara frog calls. Behav. Ecol. 17, 709–715. ( 10.1093/beheco/arl003) [DOI] [Google Scholar]
- 12.Guilford T. 1988. The evolution of conspicuous coloration. Am. Nat. 131(Suppl.), S7–S21. ( 10.1086/284764) [DOI] [Google Scholar]
- 13.Saporito RA, Donnelly MA, Spande TF, Garraffo HM. 2012. A review of chemical ecology in poison frogs. Chemoecology 22, 159–168. ( 10.1007/s00049-011-0088-0) [DOI] [Google Scholar]
- 14.Hödl W, Amezquita A. 2001. Visual signaling in anuran amphibians. In Anuran communication (ed. Ryan MJ.), pp. 121–141. Washington DC: Smithsonian Institution Press. [Google Scholar]
- 15.Pröhl H. 2003. Variation in male calling behaviour and relation to male mating success in the strawberry poison frog (Dendrobates pumilio). Ethology 109, 273–290. ( 10.1046/j.1439-0310.2003.00863.x) [DOI] [Google Scholar]
- 16.Luddecke H. 2002. Male and female responses to call playback in the Andean frog Colostethus subpunctatus. Amphibia-Reptilia 23, 141–150. ( 10.1163/156853802760061787) [DOI] [Google Scholar]
- 17.Bucher TL, Ryan MJ, Bartholomew GA. 1982. Oxygen consumption during resting, calling, and nest building in the frog Physalaemus pustulosus. Physiol. Zool. 55, 10–22. [Google Scholar]
- 18.Erdtmann L, Amezquita A. 2009. Differential evolution of advertisement call traits in dart-poison frogs (Anura: Dendrobatidae). Ethology 115, 801–811. ( 10.1111/j.1439-0310.2009.01673.x) [DOI] [Google Scholar]
- 19.Charif RA, Waack AM, Strickman LM. 2010. Raven Pro 1.4. Ithaca, NY: Cornell Lab of Ornithology. [Google Scholar]
- 20.Bennett ATD, Cuthill IC, Norris KJ. 1994. Sexual selection and the mismeasure of color. Am. Nat. 144, 848–860. ( 10.1086/285711) [DOI] [Google Scholar]
- 21.Lyytinen A, Alatalo RV, Lindstrom L, Mappes J. 2001. Can ultraviolet cues function as aposematic signals? Behav. Ecol. 12, 65–70. ( 10.1093/oxfordjournals.beheco.a000380) [DOI] [Google Scholar]
- 22.Lanfear R, Welch JJ, Bromham L. 2010. Watching the clock: studying variation in rates of molecular evolution between species. Trends Ecol. Evol. 25, 495–503. ( 10.1016/j.tree.2010.06.007) [DOI] [PubMed] [Google Scholar]
- 23.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 ( 10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character's effect on speciation and extinction. Syst. Biol. 56, 701–710. ( 10.1080/10635150701607033) [DOI] [PubMed] [Google Scholar]
- 25.Ives AR, Garland T. 2010. Phylogenetic logistic regression for binary dependent variables. Syst. Biol. 59, 9–26. ( 10.1093/sysbio/syp074) [DOI] [PubMed] [Google Scholar]
- 26.Gelman A, Hill J. 2006. Data analysis using regression and multilevel/hierarchical models, 1st edn, 648 p New York, NY: Cambridge University Press. [Google Scholar]
- 27.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete data. Proc. R. Soc. Lond. B 255, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 28.Davis MP, Midford PE, Maddison W. 2013. Exploring power and parameter estimation of the BiSSE method for analyzing species diversification. BMC Evol. Biol. 13, 38 ( 10.1186/1471-2148-13-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cocroft RB, Ryan MJ. 1995. Patterns of advertisement call evolution in toads and chorus frogs. Anim. Behav. 49, 283–303. ( 10.1006/anbe.1995.0043) [DOI] [Google Scholar]
- 30.Wells KD. 2007. The ecology and behavior of amphibians, 1400 p Chicago, IL: University of Chicago Press. [Google Scholar]
- 31.Schwartz JJ, Rahmeyer KM. 2006. Calling behavior and the capacity for sustained locomotory exercise in the Gray Treefrog (Hyla versicolor). J. Herpetol. 40, 164–171. ( 10.1670/208-05a.1) [DOI] [Google Scholar]
- 32.Jablonski PG, Cho HJ, Song SR, Kang CK, Lee SI. 2013. Warning signals confer advantage to prey in competition with predators: bumblebees steal nests from insectivorous birds. Behav. Ecol. Sociobiol. 67, 1259–1267. ( 10.1007/s00265-013-1553-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morton ES. 1977. Occurrence and significance of motivation structural rules in some bird and mammal sounds. Am. Nat. 111, 855–869. ( 10.1086/283219) [DOI] [Google Scholar]
- 34.Kime NM, Turner WR, Ryan MJ. 2000. The transmission of advertisement calls in Central American frogs. Behav. Ecol. 11, 71–83. ( 10.1093/beheco/11.1.71) [DOI] [Google Scholar]
- 35.Maan ME, Cummings ME. 2009. Sexual dimorphism and directional sexual selection on aposematic signals in a poison frog. Proc. Natl Acad. Sci. USA 106, 19 072–19 077. ( 10.1073/pnas.0903327106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meuche I, Brusa O, Linsenmair KE, Keller A, Pröhl H. 2013. Only distance matters—non-choosy females in a poison frog population. Front. Zool. 10, 1–16. ( 10.1186/1742-9994-10-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darst CR, Cummings ME. 2006. Predator learning favours mimicry of a less-toxic model in poison frogs. Nature 440, 208–211. ( 10.1038/nature04297) [DOI] [PubMed] [Google Scholar]
- 38.Saporito RA, Donnelly MA, Madden AA, Garraffo HM, Spande TF. 2010. Sex-related differences in alkaloid chemical defenses of the dendrobatid frog Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. J. Nat. Prod. 73, 317–321. ( 10.1021/np900702d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weygoldt P. 1987. Evolution of parental care in dart poison frogs (Amphibia: Anura: Dendrobatidae). Z. Zool. Syst. Evol. 25, 51–67. ( 10.1111/j.1439-0469.1987.tb00913.x) [DOI] [Google Scholar]
- 40.Stynoski JL, Torres-Mendoza Y, Sasa-Marin M, Saporito RA. 2013. Evidence of maternal provisioning of alkaloid-based chemical defenses in the strawberry poison frog Oophaga pumilio. Ecology 95, 587–593. ( 10.1890/13-0927.1) [DOI] [PubMed] [Google Scholar]
- 41.Pröhl H. 2005. Territorial behavior in dendrobatid frogs. J. Herpetol. 39, 354–365. ( 10.1670/162-04A.1) [DOI] [Google Scholar]
- 42.Santos JC, Coloma LA, Summers K, Caldwell JP, Ree R, Cannatella DC. 2009. Amazonian amphibian diversity is primarily derived from late Miocene Andean lineages. PLoS Biol. 7, e56 ( 10.1371/journal.pbio.1000056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berglund A, Bisazza A, Pilastro A. 1996. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399. ( 10.1111/j.1095-8312.1996.tb01442.x) [DOI] [Google Scholar]
- 44.Langerhans RB, Layman CA, Shokrollahi AM, DeWitt TJ. 2004. Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58, 2305–2318. ( 10.1111/j.0014-3820.2004.tb01605.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: KJ940454-KJ940470 (see electronic supplementary material, table S1).
Phylogenetic data: TreeBASE accession no. 16380