Abstract
Manipulative parasites often alter the phenotype of their hosts along multiple dimensions. ‘Multidimensionality’ in host manipulation could consist in the simultaneous alteration of several physiological pathways independently of one another, or proceed from the disruption of some key physiological parameter, followed by a cascade of effects. We compared multidimensionality in ‘host manipulation’ between two closely related amphipods, Gammarus fossarum and Gammarus pulex, naturally and experimentally infected with Pomphorhynchus laevis (Acanthocephala), respectively. To that end, we calculated in each host–parasite association the effect size of the difference between infected and uninfected individuals for six different traits (activity, phototaxis, geotaxis, attraction to conspecifics, refuge use and metabolic rate). The effects sizes were highly correlated between host–parasite associations, providing evidence for a relatively constant ‘infection syndrome’. Using the same methodology, we compared the extent of phenotypic alterations induced by an experimental injection of serotonin (5-HT) in uninfected G. pulex to that induced by experimental or natural infection with P. laevis. We observed a significant correlation between effect sizes across the six traits, indicating that injection with 5-HT can faithfully mimic the ‘infection syndrome’. This is, to our knowledge, the first experimental evidence that multidimensionality in host manipulation can proceed, at least partly, from the disruption of some major physiological mechanism.
Keywords: Acanthocephala, amphipods, multidimensionality, parasite manipulation, serotonin
1. Introduction
The concept of ‘host manipulation’ refers to the ability of some parasites to alter the phenotype of their hosts in ways that enhance their own fitness at the expense of that of infected hosts [1,2]. Numerous examples of such alterations exist, ranging from microorganisms to macroparasites [3,4]. Parasites can affect the phenotype of their hosts in various ways. In particular, parasites that rely on trophic transmission to complete their complex life cycle often induce simultaneously several phenotypic alterations in their intermediate hosts. For instance, once infective to its definitive host, the fish intestinal parasite Pomphorhynchus laevis alters several behaviours in its crustacean intermediate host Gammarus pulex, including phototaxis, geotaxis, activity, drift, refuge use and aggregation [5–10]. These behaviours are part of a general repertoire in the response of an organism to external stimuli modulating microhabitat choice and foraging (phototaxis, geotaxis, activity, drift), or are more specifically involved in the defence against predation (refuge use and aggregation). In addition, metabolic rate (MR), energy storage and immunocompetence are altered by infection [11–13].
Although earlier studies of host manipulation by parasites tended to focus on one phenotypic alteration at a time, such ‘multidimensionality’ in manipulation [14] is now receiving increasing attention [15–18]. Two points are of particular importance. The first one concerns the adaptive significance of multidimensionality in manipulation. Thomas et al. [17] argued that multidimensionality in manipulation should include only phenotypic alterations that effectively contribute to increase parasite's fitness, leaving aside simple by-products of infection with no direct consequences on the ability of the parasite to complete its life cycle. This is however a difficult task from a practical point of view, as it implies establishing a direct causal link between the increased vulnerability of infected intermediate hosts to predation by the final host and each altered phenotypic dimension, beyond what can be deduced by apparently ‘purposive’ design [19,20]. More to the point, different traits functionally involved in anti-predatory defence may act in a synergistic rather than an additive way, so that the evolution of each trait has to be examined in combination with all others [21]. A second point of importance is whether the different phenotypic dimensions that are altered by infection are independent from both historical and mechanistic point of views. One possibility is that different phenotypic dimensions have been altered in succession through evolutionary time, resulting in an ever-increasing ability of the parasite to take control of its host to its own advantage, particularly if the efficiency of each phenotypic alteration in promoting trophic transmission varies in space and time [17]. In such a case, each phenotypic alteration could correspond to the independent disruption of some particular physiological mechanism in the host. Alternatively, multidimensionality in host manipulation could result from a single major physiological effect of infection, followed by a cascade of phenotypic effects forming an infection syndrome, i.e. a series of symptoms that are all the consequences of some major physiological disruption in the infected host [16–18]. Determining which hypothesis best fits reality is of paramount importance to understand to what extent parasite-induced phenotypic alterations are truly adaptive [16].
Although the mechanisms underlying the observed phenotypic alterations remain poorly identified in most cases of host manipulation, there is some evidence that host neuromodulatory systems are targeted by manipulative parasites (see [22–25] for recent reviews, and [26,27] for pioneer studies). On the other hand, there is some evidence that genetic variants for neuromodulator biosynthesis or transporter genes can show multiple behavioural alterations. For instance, a syndrome of neural dopamine deficiency in Drosophila has been recently evidenced from its phenotypic correlates among several behavioural traits, including phototaxis, activity, startle-induced negative geotaxis and olfactory learning [28]. Because neural systems orchestrate behaviour, the joint analysis of infection syndromes and syndromes induced by neuromodulatory disruption may contribute to unravelling the mechanisms through which parasites affect host's behaviour [25].
In this study, we first address the hypothesis of a behavioural syndrome of infection in two congeneric and closely related freshwater crustacean species, G. pulex and Gammarus fossarum, used as intermediate hosts by the fish intestinal parasite P. laevis (Acanthocephala). We measured the effect of P. laevis infection on phototaxis, geotaxis, activity, refuge use and aggregation. In addition, since behaviours are modulated by energy storage and metabolism [29], which can both be altered by P. laevis infection, we recorded the MR by monitoring oxygen consumption rate. Next, we postulated that these behavioural and metabolic components of the infection syndrome might be similar to those affected by an excess of serotonin (5-HT). So far, neuropharmacological and immunocytochemical studies have evidenced the role of the serotonergic system in the modulation of phototaxis in uninfected gammarids [30] and in the reversal of phototaxis induced by P. laevis [6]. The possibility that this biogenic amine modulates other behaviours in gammarids comes from several ecotoxicological studies [31–34], and more generally from the abundant literature on crustaceans [30,35,36]. Serotonin may also be implicated in the regulation of energy balance and MR, as reported for fat storage and oxygen consumption in Caenorhabditis elegans [37], and in carbohydrate metabolism and oxygen consumption in the crustacean Daphnia magna [38]. We therefore addressed the existence of a syndrome of 5-HT excess in G. pulex through phenotypic engineering of circulating 5-HT levels in uninfected individuals [30]. The effect of 5-HT injection was measured on the same behavioural and metabolic traits as above.
In order to characterize both the ‘infection’ and the ‘injection’ syndromes, we need to appreciate to what extent one alteration in one trait was more intense than in another trait, and perform an overall comparison of the intensities of the different alterations between naturally infected, experimentally infected and 5-HT-injected gammarids [18]. The analysis of effect sizes is particularly relevant to performing such comparisons [39,40] because it produces a standardized index to quantify the size of the difference between two groups for each trait. The strength and direction of effect size are thereby comparable among traits and independent of the scale on which the variables were measured [41]. Indeed, the use of effect size as an alternative hypothesis testing methodology is increasingly used in behavioural ecology to quantify the magnitude of treatment effect on behaviour [40,41]. We therefore used effect sizes to compare the magnitude of phenotypic changes induced by P. laevis infection and by serotonin injection along several phenotypic dimensions. In addition, following recommendations from Garamszegi [39], we tested for a relationship between the effects sizes induced by the two predictor variables, i.e. experimental infection and 5-HT injection. If different mechanisms are responsible for the observed multidimensionality in host manipulation, then the effect sizes should not covary between the predictor variables. Conversely, if similar mechanisms are responsible for the observed patterns, then similar relationships should be observed for both predictor variables, and effect sizes should be positively associated across them [39] (see Cézilly et al. [18] for a general discussion on the use of effect size in the study of multidimensionality in host manipulation).
2. Material and methods
(a). Amphipod collection and maintenance
We collected G. pulex and G. fossarum from two populations located in the river Suzon (Burgundy, France, 47°24′13.91″ N, 4°53′00.54″ E) and in the river Orain (Franche-Comté, France, 46°51′50.86″ N, 5°37′35.18″ E), respectively. Acanthocephalan parasites, including P. laevis, are found only in the latter population, while Pomphorhynchus was never found in the river Suzon over the past 13 years. All gammarids were acclimatized to the laboratory environment for at least 5 days prior to experiments, in a set photoperiod (12 L : 12 D) temperature-controlled room (15 ± 1°C). Oxygenated and dechlorinated ultraviolet-treated water used for both maintenance and experiments was conditioned on river substrate to re-inoculate the microbiota (hereafter referred to as conditioned water, CW). Gammarids were maintained in large tanks filled with CW, and fed with elm and alder leaves conditioned in CW. Decaying leaves were stored dried, and subsequently rehydrated, autoclaved and conditioned upon need. Substrate from the river (stones, gravel) was also added to the tanks where gammarids were maintained and to the tanks where elm leaves were conditioned.
(b). Behavioural assays and measure of metabolic rate
The infection syndrome approach focuses on differences between groups in individual traits (infected versus uninfected; 5-HT-injected versus sham-injected), independently of potential relationships among traits at the population level. Therefore, the six traits were measured on different individuals, with the exception of laboratory-infected G. pulex and their uninfected controls owing to sample limitations.
Phototaxis was quantified through measuring the reaction to light of individual gammarids in a tube following the procedure described in Tain et al. [6]. Each amphipod was given a choice between a lighted zone and a dark zone of identical volumes, in one of 10 glass tubes filled with CW. Illumination at 850 lux was provided by 36 W solar spectra fluorescent tubes (OSRAM Lumilux-865, Molsheim, France) placed above the set of experimental glass tubes. Scan sampling of the gammarids' position was performed every 15 s during 5 min, after an initial acclimatization period of 3 min. At each step in time, each individual was scored as either 1 (present on the lighted side) or 0 (not present on the lighted side). Individual score thus varied from 0 (strongly photophobic) to 20 (strongly photophilic), with a score of 10 indicating indifference to light.
Geotaxis of an organism is a response to gravity, either positive or negative (towards the bottom or the top of the water column, respectively). Geotaxis was quantified by scoring the position of individual gammarids in a column of 35 cm height and 6 cm diameter (500 ml-graduated measuring cylinders), marked so that five zones of equal height were delimited, and filled with oxygenated CW (adapted from Cézilly et al. [5]). The inner walls of the cylinders were covered with plastic netting to offer a substrate to cling on, as available on river banks. After an acclimatization time of 3 min, the position of gammarids along the water column was recorded every 15 s for 5 min and scored from 1 (bottom) to 5 (top compartment). The cumulated geotaxis score therefore ranged from 20 to 100.
We quantified locomotor activity by recording the proportion of time spent moving. Following Dianne et al. [42], real-time automated recording of swimming activity was performed in a Petri dish filled with CW without substrate, under moderate light intensity (400 lux). After an acclimatization time of 5 min, the time spent moving above a speed threshold of 15 mm s−1 was video-recorded during 5 min using the Lighting Infrared system and an infrared camera connected to a laptop (Zebralab software, View Point, Lyon, France). Preliminary tests showed that below 15 mm s−1, moving gammarids tend to crawl rather than swim. Activity was therefore scored as the proportion of time spent swimming.
The sheltering behaviour of individual gammarids was recorded by quantifying refuge use according to Dianne et al. [42], with modifications. Briefly, individual gammarids were placed in a box filled with 300 ml CW, and offering an opaque refuge at one end, consisting of half a terracotta saucer with a 1 cm2 opening and covering about 18% of the surface. After an acclimatization time of 5 min, the gammarid position was recorded by scan sampling every 3 min during 1 h and scored as 1 (outside) or 0 (under the refuge). The cumulated score of refuge use therefore ranged from 0 (gammarid never recorded outside the refuge) to 20 (gammarid always recorded outside the refuge).
The tendency to aggregate with conspecifics was assessed following Kullman et al. [43] and Durieux et al. [10]. Briefly, a focal gammarid was given a choice between three zones of equal parts in an arena (30 × 20 × 10 cm) filled with CW, delimited by lines drawn underneath. One of the two lateral zones received an empty tea ball while the other one received a tea ball enclosing 10 conspecifics. After 10 min, the focal gammarid was introduced and confined to a wire tube placed in the central area for 2 min before being released. The tendency to aggregate with or to avoid conspecifics was estimated by scan sampling of the focal gammarid every 15 s during 5 min, and scoring its position as 1 (on the side with conspecifics), 0 (no choice, central area) or −1 (on the side without conspecifics). The cumulated score of aggregation therefore ranged from 20 (preference for the side with conspecifics) to –20 (preference for the side with no conspecifics).
MR was estimated by quantifying oxygen consumption rate using optical fluorescence-based oxygen respirometry [44] and the SensorDish device (Presens, Regensburg, Germany). The oxygen consumption rate of individual gammarids was monitored in a 3 ml-well measuring 1.7 cm in diameter, equipped with an integrated oxygen sensor spot and filled to the top with CW. Since the size of the well allowed limited movement by gammarids, our measure of MR is a slightly overestimated measure of the resting MR. Oxygen consumption rate was monitored for 20 gammarids simultaneously by using 24-well microplates (batch OD-1142–01 calibrated at 16°C). After an acclimatization time of 1 min and the sealing of the microplate with parafilm, oxygen concentration was recorded at 15 s intervals for 30 min using an oxygen meter (SDRv. 4 SensorDish Reader; Presens). Four control wells were left with no gammarids, to correct for potential microbial respiration. Each plate received both uninfected and infected gammarids, placed in random order. Respirometry measurements were performed under near darkness in the temperature-controlled room where the other behaviours were scored, at a temperature of 16 ± 1°C. Following respirometry measurements, each gammarid was blotted on a paper towel and weighed to the nearest 0.01 mg using an analytical balance (Precisa 262SMA-FR, Precisa Instruments, Bisingen, Switzerland). The calculation of individual MR must include a correction for potential microbial respiration and for gammarid's weight. We subtracted the mean value of control wells to that of experimental wells to get the amount of oxygen consumed by individual gammarids, and derived the MR (in micrograms O2 min−1) from the slope of the linear regression of this parameter on time. For statistical analysis, we corrected for gammarid's weight by using a log10–log10 relationship between MR and weight known as metabolic scaling [45]. To calculate the effect size, we used the residuals of the regression of log-transformed MR (in micrograms O2 min−1) on log-transformed fresh weight (in milligrams). However, to add clarity, the ratio of individual MR (micrograms O2 min−1) on gammarid's fresh weight (in milligrams) without transformation was used for graphical representation.
(c). Experimental infection of Gammarus pulex
Experimental infections were carried out following the procedure described in Franceschi et al. [46] and Dianne et al. [9]. Briefly, 48 h-starved males were pooled two by two in 60 ml glass dishes and allowed to feed during 48 h on a 1 cm² piece of elm leaf, on which 100 P. laevis eggs were deposited. Parasite eggs were collected from gravid females taken from the intestine of chubs from the river Vouges (Burgundy, France, 47°8′2.37″, 5°10′46.88″). A total of 240 G. pulex males were exposed to parasite eggs, while an additional 240 males were handled and maintained in the same conditions as the exposed ones to serve as controls. After exposure, gammarids were maintained as above, in large tanks with river substrate where water was partly renewed each day. Behavioural consequences of infection were investigated approximately four weeks after the parasite stage infective to the definitive host had been reached (late cystacanth stage, i.e. approximately 14 weeks after experimental infection), at which time behavioural ‘manipulation’ is fully expressed [9,46].
(d). Behavioural syndrome of 5-HT excess
The level of serotonin was transiently increased in uninfected G. pulex by the topic injection of a serotonin solution into the body cavity. Following Tain et al. [6] and Perrot-Minnot et al. [30], 1 µl of a 3.5 μg μl−1 serotonin in saline solution (crustacean Ringer) was delivered to each individual through a single injection into the body cavity, using a Hamilton syringe (701RN) with a fine needle (RN33/51/3), and a group of amphipods were ‘sham’ injected with saline as a control. Behavioural assays were conducted 30 min after injection. Serotonin concentration and time delay were adjusted based on previous studies of the effect of 5-HT on phototaxis in gammarids [6,20,30].
(e). Statistical analysis
Most distributions of behavioural data did not conform to normality, and we therefore relied on non-parametric statistics to analyse the effect of infection status and of 5-HT-injection on reaction to light, geotaxis, refuge use, aggregation and activity. For each behavioural trait, we used the Wilcoxon–Mann–Whitney U-test to compare the effect of P. laevis infection to uninfected controls, and 5-HT injection to saline-injected controls. The effects of infection and of 5-HT injection on MR compared to their respective controls were analysed using ANCOVAs, with log-transformed MR as the dependent variable, and log-transformed fresh weight and treatment as independent variables.
We estimated effect size of infection and of 5-HT injection on each trait by using the Cliff's delta [47]. The Cliff's delta was preferred to the more commonly reported Cohen's d, because this measure of effect size is robust to non-normally distributed data. Cliff delta ranges between +1 when all values of the treatment group are higher than the values of the control group and –1 when reverse is true. The more overlapping the data distributions are, the closest to zero the Cliff delta will be [48]. We estimated effect size of infection and of 5-HT injection by calculating the Cliff's delta for each phenotypic component, using the appropriate control in each experiment (uninfected G. pulex, uninfected G. fossarum and saline-injected gammarids). Median and 95% CI of the Cliff's delta were calculated using R-package ‘orddom’ v. 3.1 [48], with 10 000 bootstraps. Based on the threshold values for the Cliff's delta reported in Romano et al. [49], the magnitude of effect sizes was interpreted as negligible (less than 0.147), small (between 0.147 and 0.33), medium (between 0.33 and 0.474), or strong (more than 0.474).
Finally, we tested for a relationship between infection and injection syndromes through correlating the effect sizes of P. laevis infection and effect sizes of 5-HT injection across the six measured traits. Activity and MR were measured on an absolute scale, while reactions to light, gravity, the presence of a refuge, or of a group of conspecifics, reflected a choice by the focal gammarid. As a consequence, effect sizes on phototaxis, geotaxis, refuge use and aggregation could be defined either as a positive or a negative change, depending on how the direction of the choice was scored. For example, P. laevis-induced change in phototaxis can be scored either as increased photophily (i.e. positive effect) or decreased photophobia (i.e. negative effect size). The correlation between the effect sizes of P. laevis infection and effect sizes of 5-HT injection across the six measured traits might thus be sensitive to how these four traits out of six were defined. Therefore, we tested for a relationship between infection and injection syndromes by calculating the correlation coefficients and p-values for all the 16 possible combinations of the six traits obtained after varying the sign of effect size of one, two, three or the four choice traits simultaneously.
All statistical tests were run using the appropriate packages in R v.3.0.1 [50] and JMP (SAS), using a significance threshold of p < 0.05.
3. Results
(a). Phenotypic alterations in experimentally infected Gammarus pulex and naturally infected Gammarus fossarum
Parasite load (median and quartiles) reached 2 (1–3) P. laevis in experimentally infected G. pulex and 1 (1–2) in naturally infected G. fossarum. Preliminary analysis did not reveal any effect of infection intensity on the six studied traits (all p > 0.05). Therefore, infection intensity was not included as a predictor variable in further analysis.
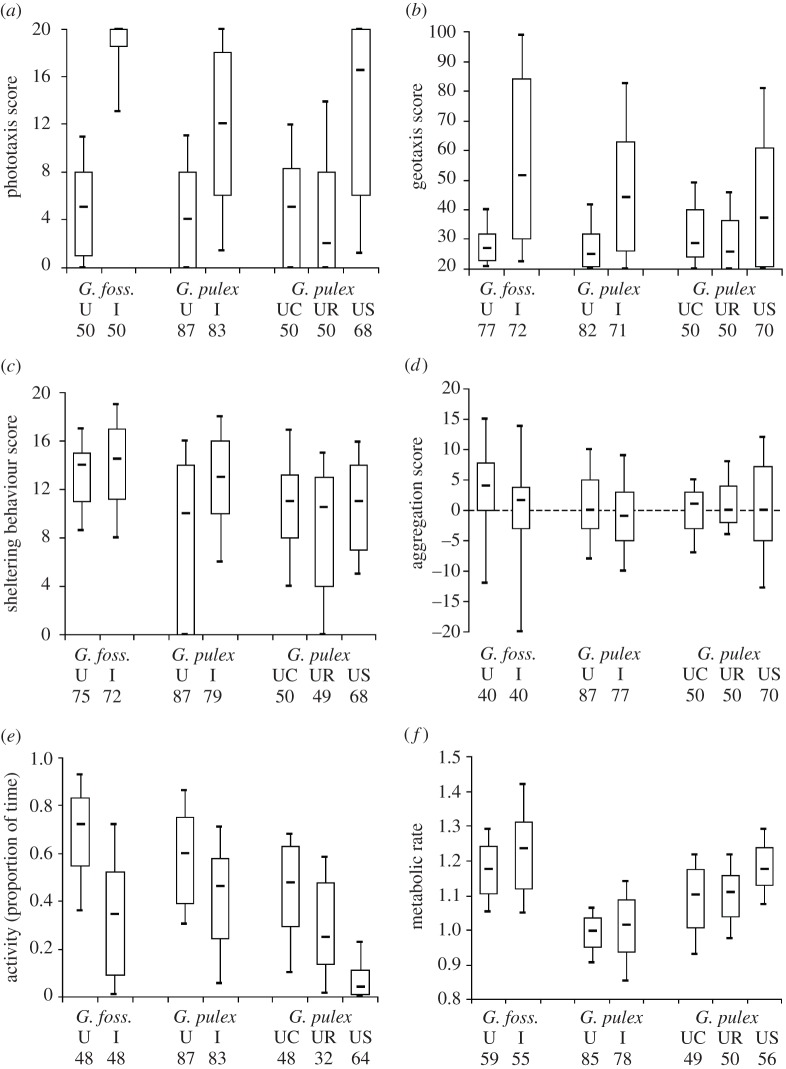
Reaction to light was significantly reversed from photophobia in uninfected gammarids to photophily in infected G. pulex (Ncontrols = 87 and Ninf = 83; Z = 6.38; p < 10−3), as well as in infected G. fossarum (Ncontrols = 50 and Ninf = 50; Z = 8.11, p < 10−3; figure 1a). Both gammarid species also tended to move up in the water column when infected, as evidenced in a significantly altered geotaxis in infected G. pulex (Ncontrols = 82 and Ninf = 71; Z = 4.81; p < 10−3) and in infected G. fossarum (Ncontrols = 77 and Ninf = 72; Z = 5.91; p < 10−3; figure 1b). Sheltering behaviour was also decreased by infection, as evidenced in a lower score of refuge use by infected G. pulex (Ncontrols = 87 and Ninf = 79; Z = 4.14; p < 10−3) and infected G. fossarum (Ncontrols = 75 and Ninf = 72; Z = 2.28, p = 0.023; figure 1c). Infection did not alter aggregation behaviour in G. pulex (Ncontrols = 87 and Ninf = 77; Z = –1.94; p = 0.052), and neither uninfected nor infected gammarids tended to avoid or aggregate with conspecifics (Wilcoxon test against the predicted no-choice score of 0, T = 308.5, p = 0.14 and T = –251, p = 0.17, respectively). By contrast, G. fossarum tended to aggregate with conspecifics more when uninfected then when infected, as evidenced in a significant effect of infection status (Ncontrols = 40 and Ninf = 40; Z = –2.2, p = 0.028; figure 1d) and in an aggregation score significantly different from 0 in uninfected gammarids (N = 40, T = 252, p < 10−3), but not in infected ones (N = 40, T = 27, p = 0.68). Activity was significantly reduced by infection, both in G. pulex (Ncontrols = 87 and Ninf = 81; Z = −3.91; p < 10−3) and in G. fossarum (Ncontrols = 48 and Ninf = 48; Z = –5.68; p < 10−3; figure 1e). Finally, MR was not significantly altered by infection in G. pulex (ANCOVA, F3,124 = 7.29, p = 0.0002; weight, t = 4.04, p < 10−3; infection status, t = −0.25, p = 0.80; interaction, t = 0.87, p = 0.39), nor in G. fossarum (ANCOVA, F3,110 = 9.32, p < 10−3; weight, t = 5.12, p < 10−3; infection status, t = −0.65, p = 0.62; interaction, t = 0.83, p = 0.22; figure 1f).
Figure 1.
Box-plot (median, first and third quartiles, and 10th percentile and 90th percentile) of the six phenotypic traits considered in the analysis of multidimensional consequences of P. laevis infection in G. fossarum and G. pulex, and of serotonin injection in G. pulex. U, uninfected; I, infected; C, uninfected non-injected controls; R, uninfected controls injected with ringer vehicule solution only; S, uninfected injected with serotonin (5-HT). Sample sizes are given below the x-axis labels. MR is expressed as the ratio of oxygen consumption in µg min−1(log10) on fresh weight in mg (log10).
The calculation of Cliff's delta indexes and their 95% CI confirmed the effects of infection with P. laevis on both G. pulex and G. fossarum (figure 2). Overall, strong (Cliff delta > 0.474) to medium (0.33 < Cliff delta < 0.474) effects of infection were observed for phototaxis, geotaxis and activity, whereas the effect was medium to small (0.147 < Cliff delta < 0.33) for both the tendency to aggregate with conspecifics and refuge use, and negligible (Cliff delta < 0.147) for MR. Furthermore, the Cliff's delta indexes of effect size calculated for each of the six phenotypic dimensions were significantly correlated between experimentally infected G. pulex and naturally infected G. fossarum in each of the 16 possible combinations of traits obtained when allowing variation in the sign of effect sizes for the four choice traits (see Material and methods). The Pearson correlation coefficient ranged from 0.83 to 0.98 and p-values from 0.04 to 0.0005. This result provides evidence for a relatively constant infection syndrome (figure 2a).
Figure 2.
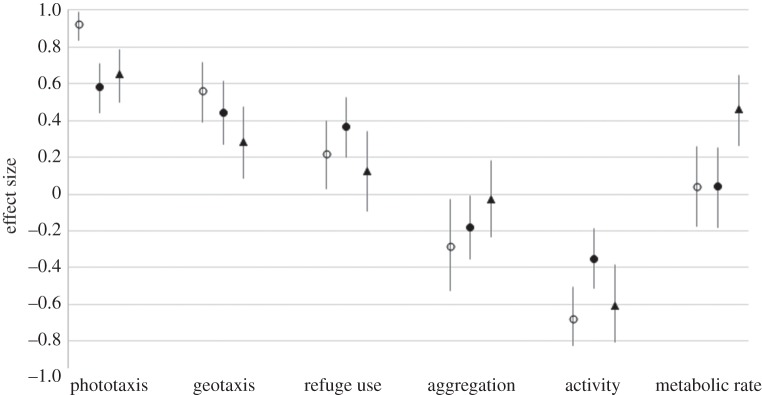
Cliff's delta indexes of effect sizes (median and interquartile) of P. laevis infection in G. fossarum (white circles), of P. laevis infection in G. pulex (black circles) and of 5-HT injection in G. pulex (black triangles), on six phenotypic components of infection or serotonin-excess syndromes (dimensions). Positive value of the effect size corresponds to an increase in the expression of the phenotypic trait in infected or 5-HT-injected gammarids, while a negative value corresponds to a decrease.
(b). Behavioural and physiological consequences of transient 5-HT increase in Gammarus pulex
Injection of the vehicle saline solution (crustacean Ringer) in uninfected G. pulex did not significantly alter most measured behaviours, compared to non-injected ones (Wilcoxon–Mann–Whitney rank-test, NSaline = 50, Nnon-injected = 50 for each behaviour; phototaxis, Z = −0.99, p = 0.32; geotaxis, Z = −1.56, p = 0.12; refuge use, Z = –0.85, p = 0.39; aggregation, Z = 0.75, p = 0.45; figure 1a–d). MR was not affected by injection of saline solution either (ANCOVA, F3, 98 = 6.14, p = 0.0007; weight, t = 3.94, p = 0.0002; treatment, t = –1.2, p = 0.23; interaction, t = 1.11, p = 0.27; figure 1f). However, saline-injected gammarids showed a significantly reduced activity compared to non-injected ones (NSaline = 31, Nnon-injected = 48, Z = −3.18, p = 0.0015; figure 1e).
Serotonin-injected gammarids were less photophobic and moved upper in the water column, compared to saline-injected ones (Wilcoxon–Mann–Whitney rank-test, N5-HT = 70, NSaline = 50 for each behaviour; phototaxis, Z = −6.1, p < 10−3; geotaxis, Z = –2.65, p = 0.008; figure 1a,b). Gammarus pulex injected with 5-HT also exhibited a lower swimming activity compared to saline-injected ones (N5-HT = 64, NSaline = 31; Z = 4.78, p < 10−3; figure 1e), and a higher MR (ANCOVA, F3,102 = 13.05, p < 10−3; weight, t = 4.86, p < 10−3; treatment, t = 4.55, p < 10−3; interaction, t = 0.82, p = 0.42; figure 1f).
By contrast, refuge use and the tendency to aggregate with conspecifics were not significantly altered by 5-HT injection (Wilcoxon–Mann–Whitney rank-test, N5-HT = 70, NSaline = 50; for each behaviour; refuge use, Z = −1.17, p = 0.24; aggregation, Z = 0.26, p = 0.80; figure 1c,d).
The calculation of Cliff's delta indexes and their 95% CI confirmed the strong effect of 5-HT-injection on phototaxis and activity, and medium to small effects on geotaxis and MR, whereas the effect was negligible for both the tendency to aggregate with conspecifics and refuge use (figure 2). More importantly, Cliff's delta indexes of effect size were significantly correlated between 5-HT-injected G. pulex and P. laevis-infected G. pulex in 12 out of the 16 possible combinations of traits obtained when allowing variation in the sign of effect sizes for the four choice traits (see Material and methods), with Pearson correlation coefficients ranging from 0.78 to 0.89 and p-values from 0.06 to 0.016. In addition, correlations between effect sizes of 5-HT-injection in G. pulex and natural infection in G. fossarum were significant in all possible comparisons, with Pearson correlation coefficient ranging from 0.86 to 0.95 and p-values from 0.03 to 0.003. These results indicate that 5-HT injection can faithfully mimic the infection syndrome (figure 2b).
4. Discussion
The syndrome approach followed here allowed comparisons between groups differing in host species infected with P. laevis, and between P. laevis infected and 5-HT injected gammarids. Interestingly, the magnitude of parasite-induced alterations in G. pulex and G. fossarum varied significantly between phenotypic dimensions. For instance, in both intermediate host species, reaction to light was strongly modified by infection as shown by the effect size, whereas infection had only a negligible effect on MR. More importantly, our results suggest that the relative magnitudes of the various phenotypic alterations are independent of the intermediate host species and/or of whether infection was natural or experimental. However, since both effects were confounded in our experimental design, it is not possible to firmly conclude from this study that infection syndrome is consistent across host species and mode of infection separately. Overall, our results provide, to our knowledge, the first evidence for the existence of an ‘infection syndrome’ as defined by Cézilly & Perrot-Minnot [16]. Our results also confirm previous findings on P. laevis induced alterations of reaction to light and refuge use in G. pulex [5–7,9]. In agreement with Durieux et al. [10] and Kullman et al. [43], uninfected G. pulex did not tend to aggregate with conspecifics in the absence of predation risk, a behaviour that remained unaffected by infection with P. laevis [10]. On the other hand, P. laevis infected G. pulex were less active compared to uninfected ones, a result contrasting with previous studies showing the reverse [51] or an absence of effect [10]. However, in these two studies, the confounding effect of the response to current [51] or to conspecifics [10], on the estimate of activity, could not be ruled out. Finally, we did not observe a significant change in MR in infected gammarids at 15°C, extending previous reports on the lack of effect of infection with P. laevis on MR at 10°C, but not at 20°C [11]. Future work, relying on quantitative measurements conducted on both naturally and experimentally infected hosts, is needed to assess to what extent infection syndromes are typical of host–parasite associations.
Uninfected G. pulex injected with serotonin exhibited several behavioural changes compared to saline-injected ones. In agreement with previous studies [6,30,31], 5-HT injection reversed the natural photophobia and decreased the locomotor activity of G. pulex. Reaction to gravity was also reversed from positive to negative in 5-HT injected gammarids, as reported in the marine amphipod Echinogammarus marinus following exposure to the serotonin-reuptake inhibitor fluoxetine [33]. Finally, serotonin injection marginally increased MR, an effect reported in another crustacean, D. magna, following exposure to fluoxetine [38]. Overall, a transient increase in serotonin in uninfected G. pulex appears to modulate the set of behaviours altered by infection with P. laevis in similar ways, with the possible exception of refuge use. Comparing the magnitude of effect sizes between infection with P. laevis and 5-HT injection confirms the similarity between the corresponding syndromes. Taken together, our results strongly suggest that multidimensionality in P. laevis-induced phenotypic alteration could stem, at least partly, from the alteration of the serotonergic system of infected G. pulex.
This main result opens the way for a refined analysis of the mechanisms of host manipulation. Indeed, the way P. laevis interferes with serotonin neuromodulation remains unknown, as it is for almost all parasite-induced changes in behaviour [23]. Parasitic manipulation evolved within the context of the alteration of other host's physiological systems critical for parasite's survival, more specifically the metabolic and immune systems [23,24]. As a consequence, no simplistic scenario can be proposed for what might be a complex and indirect set of mechanisms involved in host behavioural manipulation. However, our results suggest that it might be more difficult than previously expected [17] to establish which phenotypic dimensions altered by infection should be considered to contribute to multidimensionality in host manipulation. First, several phenotypic alterations, with different consequences for trophic transmission to final hosts, might be non-independent from a mechanistic point of view. Second, the benefits, in terms of increased trophic transmission, of multidimensionality versus unidimensionality in host manipulation remain to be established. In our study model system, the increased vulnerability to predation of G. pulex infected with P. laevis has been demonstrated, both in microcosm experiments [7,9,52], and under field conditions [8], but the traits involved in such increase have not been identified yet. Two recent studies have shown that a single altered phenotypic dimension in P. laevis infected hosts cannot explain this increased vulnerability to predation [20,52]. Since a transient increase in serotonin appears to mimic infection syndrome in a number of dimensions (this study), we would expect its injection in G. pulex to increase its vulnerability to predation. However, we have previously shown in a study designed to test the role of reversed phototaxis in vulnerability to predation, that 5-HT-injected G. pulex were not more predated upon by an unfamiliar predator than saline-injected ones [20]. Such discrepancy might be interpreted in two ways: (i) some traits, not altered by 5-HT but altered by infection albeit weakly, such as sheltering behaviour, might be responsible for such increased vulnerability to predation of infected hosts; and/or (ii) 5-HT might be involved in the increased vulnerability to predation but such an effect would not have been produced by a topic injection. This is possible if, for instance, 5-HT is involved in some long-term cognitive mechanisms implicated in anti-predatory defence, such as olfactory learning and memory (see Perrot-Minnot & Cézilly [25] for arguments). Interestingly, most of the traits measured here (refuge use, phototaxis, aggregation, activity) are risk-sensitive, i.e. exhibit a plastic response to the level of predation risk. For instance, sheltering behaviour in response to the exposure to familiar predatory fish has been shown to be important in the differential vulnerability to predation of infected and uninfected G. pulex in microcosm experiments [9]. Exposure of uninfected G. pulex to predation risk triggers increased photophobia, enhanced refuge use, aggregation towards conspecific, and decreased activity [10,21,53]. By contrast, no such plastic responses to predation risk are expressed in P. laevis infected individuals [10]. The involvement of 5-HT in the plastic response to predation risk should therefore be investigated, to test further the implication of this neuromodulator in the actual parasitic manipulation.
Although host manipulation by parasites has stimulated a large number of empirical and theoretical studies, the basic mechanisms at work still remain elusive. Results from this study suggest, however, that infection with manipulative parasites might be characterized by an infection syndrome, i.e. the simultaneous alteration of several phenotypic traits in the infected host resulting from some major physiological disruption associated with infection. The generality of the existence of infection syndromes and its consequences for the adaptive interpretation of parasite-induced phenotypic alterations remains to be assessed.
Acknowledgements
We thank Sébastien Motreuil and Aude Balourdet for their technical assistance in the field, and Mathias Galipaud and François-Xavier Dechaume-Moncharmont for advice on the use of effect size. We also are very grateful to Dan Benesh and an anonymous referee for constructive suggestions on an earlier version.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.bs910.
Funding statement
This study was funded by a research grant (grant no. 9201/AA/O/040/S00619) from the Conseil Régional de Bourgogne, the ANR ParaDiv and the Institut Universitaire de France.
References
- 1.Thomas F, Adamo S, Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Proc. 68, 185–199. ( 10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 2.Cézilly F, Thomas F, Médoc V, Perrot-Minnot M-J. 2010. Host-manipulation by parasites with complex life cycles: adaptive or not? Trends Parasitol. 26, 311–317. ( 10.1016/j.pt.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 3.Moore J. 2002. Parasites and the behavior of animals. New York, NY: Oxford University Press. [Google Scholar]
- 4.Cézilly F, Perrot-Minnot M-J, Rigaud T. 2014. Cooperation and conflict in host manipulation: interactions among macro-parasites and micro-organisms. Front. Microbiol. 5, 248 ( 10.3389/fmicb.2014.00248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cézilly F, Grégoire A, Bertin A. 2000. Conflict between co-occuring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology 120, 625–630. ( 10.1017/S0031182099005910) [DOI] [PubMed] [Google Scholar]
- 6.Tain L, Perrot-Minnot M-J, Cézilly F. 2006. Altered host behaviour and brain serotonergic activity caused by acanthocephalans: evidence for specificity. Proc. R. Soc. B 273, 3039–3045. ( 10.1098/rspb.2006.3618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaldonski N, Perrot-Minnot M-J, Cézilly F. 2007. Differential influence of two acanthocephalan parasites on the antipredator behaviour of their common intermediate host. Anim. Behav. 74, 1311–1317. ( 10.1016/j.anbehav.2007.02.027) [DOI] [Google Scholar]
- 8.Lagrue C, Kaldonski N, Perrot-Minnot M-J, Motreuil S, Bollache L. 2007. Altered drift behavior and increased vulnerability to predation in intermediate hosts infected by an acanthocephalan parasite: field evidence for adaptive manipulation. Ecology 88, 2839–2847. ( 10.1890/06-2105.1) [DOI] [PubMed] [Google Scholar]
- 9.Dianne L, Perrot-Minnot M-J, Bauer A, Rigaud T. 2011. Protection first then facilitation: a manipulative parasite modulates the vulnerability to predation of its intermediate host according to its own developmental stage. Evolution 65, 2692–2698. ( 10.1111/j.1558-5646.2011.01330.x) [DOI] [PubMed] [Google Scholar]
- 10.Durieux R, Rigaud T, Médoc V. 2012. Parasite-induced suppression of aggregation under predation risk in a freshwater amphipod. Behav. Proc. 91, 207–213. ( 10.1016/j.beproc.2012.08.002) [DOI] [PubMed] [Google Scholar]
- 11.Rumpus AE, Kennedy CR. 1974. The effect of the acanthocephalan Pomphorhynchus laevis upon the respiration of its intermediate host, Gammarus pulex. Parasitology 68, 271–284. ( 10.1017/S0031182000045789) [DOI] [PubMed] [Google Scholar]
- 12.Plaistow S, Troussard J-P, Cézilly F. 2001. The effect of the acanthocephalan parasite Pomphorhynchus laevis on the lipid and glycogen content of its intermediate host Gammarus pulex. Int. J. Parasitol. 31, 346–351. ( 10.1016/S0020-7519(01)00115-1) [DOI] [PubMed] [Google Scholar]
- 13.Cornet S, Franceschi N, Bauer A, Rigaud T, Moret Y. 2009. Immune depression induced by acanthocephalan parasites in their intermediate crustacean host: consequences for the risk of super-infection and links with host behavioural manipulation. Int. J. Parasitol. 39, 221–229. ( 10.1016/j.ijpara.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 14.Cézilly F, Perrot-Minnot M-J. 2005. Studying adaptive changes in the behaviour of infected hosts: a long and winding road. Behav. Proc. 68, 223–228. ( 10.1016/j.beproc.2004.08.013) [DOI] [PubMed] [Google Scholar]
- 15.Benesh DP, Valtonen ET, Seppälä O. 2008. Multidimensionality and intra-individual variation in host manipulation by an acanthocephalan. Parasitology 135, 617–626. ( 10.1017/S0031182008004216) [DOI] [PubMed] [Google Scholar]
- 16.Cézilly F, Perrot-Minnot M-J. 2010. Interpreting multidimensionality in parasite-induced phenotypic alterations: panselectionism versus parsimony. Oikos 119, 1224–1229. ( 10.1111/j.1600-0706.2010.18579.x) [DOI] [Google Scholar]
- 17.Thomas F, Poulin R, Brodeur J. 2010. Host manipulation by parasites: a multidimensional phenomenon. Oikos 119, 1217–1223. ( 10.1111/j.1600-0706.2009.18077.x) [DOI] [Google Scholar]
- 18.Cezilly F, Favrat A, Perrot-Minnot M-J. 2013. Multidimensionality in parasite-induced phenotypic alterations: ultimate versus proximate aspects. J. Exp. Biol. 216, 27–35. ( 10.1242/jeb.074005) [DOI] [PubMed] [Google Scholar]
- 19.Poulin R. 1995. ‘Adaptive’ changes in the behaviour of parasitized animals: a critical review. Int. J. Parasitol. 25, 1371–1383. ( 10.1016/0020-7519(95)00100-X) [DOI] [PubMed] [Google Scholar]
- 20.Perrot-Minnot M-J, Maddaleno M, Balourdet A, Cézilly F. 2012. Host manipulation revisited: no evidence for a causal link between altered photophobia and increased trophic transmission of amphipods infected with acanthocephalans. Funct. Ecol. 26, 1007–1014. ( 10.1111/j.1365-2435.2012.02027.x) [DOI] [Google Scholar]
- 21.David M, Salignon M, Perrot-Minnot M-J. 2014. Shaping the antipredator strategy: flexibility, consistency and behavioral correlations under varying predation threat. Behav. Ecol. 25, 1148–1156. ( 10.1093/beheco/aru101) [DOI] [Google Scholar]
- 22.Adamo SA. 2012. The strings of the puppet master: how parasites change host behavior. In Host manipulation by parasites (eds Hughes DP, Brodeur J, Thomas F.), pp. 36–51. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Adamo SA. 2013. Parasites: evolution's neurobiologists. J. Exp. Biol. 216, 3–10. ( 10.1242/jeb.073601) [DOI] [PubMed] [Google Scholar]
- 24.Lafferty KD, Shaw JC. 2013. Comparing mechanisms of host manipulation across host and parasite taxa. J. Exp. Biol. 216, 56–66. ( 10.1242/jeb.073668) [DOI] [PubMed] [Google Scholar]
- 25.Perrot-Minnot M-J, Cézilly F. 2013. Investigating candidate neuromodulatory systems underlying parasitic manipulation: concepts, limitations and prospects. J. Exp. Biol. 216, 134–141. ( 10.1242/jeb.074146) [DOI] [PubMed] [Google Scholar]
- 26.Helluy S, Holmes JC. 1990. Serotonin, octopamine, and the clinging behavior induced by the parasite Polymorphus paradoxus (Acanthocephala) in Gammarus lacustris (Crustacea). Can. J. Zool. 68, 1214–1220. ( 10.1139/z90-181) [DOI] [Google Scholar]
- 27.Maynard BJ, DeMartini L, Wright WG. 1996. Gammarus lacustris harboring Polymorphus paradoxus show altered patterns of serotonin-like immunoreactivity. J. Parasitol. 82, 663–666. ( 10.2307/3283801) [DOI] [PubMed] [Google Scholar]
- 28.Riemensperger T, et al. 2011. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl Acad. Sci. USA 208, 834–839. ( 10.1073/pnas.1010930108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Careau V, Thomas D, Humphries MM, Réale D. 2008. Energy metabolism and animal personality. Oikos 117, 641–653. ( 10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- 30.Perrot-Minnot M-J, Dion E, Cézilly F. 2013. Modulatory effects of the serotonergic and histaminergic systems on reaction to light in the crustacean Gammarus pulex. Neuropharmacology 75, 31–37. ( 10.1016/j.neuropharm.2013.06.028) [DOI] [PubMed] [Google Scholar]
- 31.De Lange HJ, Noordoven W, Murk AJ, Lürling M, Peeters ETHM. 2006. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 78, 209–216. ( 10.1016/j.aquatox.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 32.De Lange HJ, Peeters ETHM, Lürling M. 2009. Changes in ventilation and locomotion of Gammarus pulex (Crustacea, Amphipoda) in response to low concentrations of pharmaceuticals. Hum. Ecol. Risk Assess. 15, 111–120. ( 10.1080/10807030802615584) [DOI] [Google Scholar]
- 33.Guler Y, Ford AT. 2010. Anti-depressants make amphipods see the light. Aquat. Toxicol. 99, 397–404. ( 10.1016/j.aquatox.2010.05.019) [DOI] [PubMed] [Google Scholar]
- 34.Bossus MC, Guler YZ, Short SJ, Morrison ER, Ford AT. 2014. Behavioural and transcriptional changes in the amphipod Echinogammarus marinus exposed to two antidepressants, fluoxetine and sertraline. Aquat. Toxicol. 151, 46–56. ( 10.1016/j.aquatox.2013.11.025) [DOI] [PubMed] [Google Scholar]
- 35.Helluy S. 2013. Parasite-induced alterations of sensorimotor pathways in gammarids: collateral damage of neuroinflammation? J. Exp. Biol. 216, 67–77. ( 10.1242/jeb.073213) [DOI] [PubMed] [Google Scholar]
- 36.Fong PP, Ford AT. 2014. The biological effects of antidepressants on the molluscs and crustaceans: a review. Aquat. Toxicol. 151, 4–13. ( 10.1016/j.aquatox.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan S, Sadegh L, Elle IC, Christensen AG, Faergeman NJ, Ashrafi K. 2008. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell. Metab. 7, 533–544. (doi:101016/jcmet200804012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos B, Piña B, Barata CC. 2012. Mechanisms of action of selective serotonin reuptake inhibitors in Daphnia magna. Environ. Sci. Technol. 46, 2943–2950. ( 10.1021/es203157f) [DOI] [PubMed] [Google Scholar]
- 39.Garamszegi LZ. 2006. Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behav. Ecol. 17, 682–687. ( 10.1093/beheco/ark005) [DOI] [Google Scholar]
- 40.Nakagawa S, Cuthill IC. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. ( 10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 41.Garamszegi LZ, et al. 2009. Changing philosophies and tools for statistical inferences in behavioral ecology. Behav. Ecol. 20, 1363–1375. ( 10.1093/beheco/arp137) [DOI] [Google Scholar]
- 42.Dianne L, Perrot-Minnot M-J, Bauer A, Guvenatam A, Rigaud T. 2014. Parasite-induced alteration of plastic response to predation threat: increased refuge use but lower food intake in Gammarus pulex infected with the acanothocephalan Pomphorhynchus laevis. Int. J. Parasitol. 44, 211–216. ( 10.1016/j.ijpara.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 43.Kullmann H, Thünken T, Baldauf SA, Bakker TCM, Frommen JG. 2008. Fish odour triggers conspecific attraction behaviour in an aquatic invertebrate. Biol. Lett. 4, 458–460. ( 10.1098/rsbl.2008.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Köster M, Krause C, Paffenhöfer G-A. 2008. Time-series measurements of oxygen consumption of copepod nauplii. Mar. Ecol. Prog. Ser. 353, 157–164. ( 10.3354/meps07185) [DOI] [Google Scholar]
- 45.Glazier DS. 2005. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. Camb. Philos. Soc. 80, 611–662. ( 10.1017/S1464793105006834) [DOI] [PubMed] [Google Scholar]
- 46.Franceschi N, Bauer A, Bollache L, Rigaud T. 2008. The effects of parasite age and intensity on variability in acanthocephalan-induced behavioural manipulation. Int. J. Parasitol. 38, 1161–1170. ( 10.1016/j.ijpara.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 47.Cliff N. 1996. Ordinal methods for behavioral data analysis. Mahwah, NJ: Lawrence Erlbaum Press. [Google Scholar]
- 48.Rogmann JJ. 2013. Ordinal Dominance Statistics (orddom): an R Project for Statistical Computing package to compute ordinal, nonparametric alternatives to mean comparison (Version 3.1). Available online from http://cran.r-project.org/. [Google Scholar]
- 49.Romano J, Kromrey JD, Coraggio J, Skowronek J, Devine L. 2006. Exploring methods for evaluating group differences on the NSSE and other surveys: are the t-test and Cohen's d indices the most appropriate choices? In Annual meeting of the Southern Association of Institutional Research, 14–17 October 2006, Arlington, VA, USA. [Google Scholar]
- 50.R Core Team. R: a language and environment for statistical computing. 2013. Vienna, Austria: R Foundation for Statistical Computing.
- 51.Wellnitz TA, Giari L, Maynard B, Dezfuli B. 2003. A parasite spatially structures its host population. Oikos 100, 263–268. ( 10.1034/j.1600-0706.2003.12153.x) [DOI] [Google Scholar]
- 52.Kaldonski N, Perrot-Minnot M-J, Dodet R, Martinaud G, Cézilly F. 2009. Carotenoid-based colour of acanthocephalan cystacanths plays no role in host manipulation. Proc. R. Soc. B 276, 169–176. ( 10.1098/rspb.2008.0798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis SE, Hodel A, Sturdy T, Todd R, Weigl C. 2012. Impact of acanthocephalan parasites on aggregation behavior of amphipods (Gammarus pseudolimnaeus). Behav. Proc. 91, 159–163. ( 10.1016/j.beproc.2012.07.009) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.bs910.