Abstract
Jellyfish blooms are common in many oceans, and anthropogenic changes appear to have increased their magnitude in some regions. Although mass falls of jellyfish carcasses have been observed recently at the deep seafloor, the dense necrophage aggregations and rapid consumption rates typical for vertebrate carrion have not been documented. This has led to a paradigm of limited energy transfer to higher trophic levels at jelly falls relative to vertebrate organic falls. We show from baited camera deployments in the Norwegian deep sea that dense aggregations of deep-sea scavengers (more than 1000 animals at peak densities) can rapidly form at jellyfish baits and consume entire jellyfish carcasses in 2.5 h. We also show that scavenging rates on jellyfish are not significantly different from fish carrion of similar mass, and reveal that scavenging communities typical for the NE Atlantic bathyal zone, including the Atlantic hagfish, galatheid crabs, decapod shrimp and lyssianasid amphipods, consume both types of carcasses. These rapid jellyfish carrion consumption rates suggest that the contribution of gelatinous material to organic fluxes may be seriously underestimated in some regions, because jelly falls may disappear much more rapidly than previously thought. Our results also demonstrate that the energy contained in gelatinous carrion can be efficiently incorporated into large numbers of deep-sea scavengers and food webs, lessening the expected impacts (e.g. smothering of the seafloor) of enhanced jellyfish production on deep-sea ecosystems and pelagic–benthic coupling.
Keywords: jellyfish carcasses, scavenging, deep sea, metazoans, biological pump
1. Introduction
Gelatinous zooplankton are common worldwide, constituting a total biomass in the ocean of 38.3 Tg C [1]. While recent meta-analyses show that numerous areas experience recurrent oscillations in jellyfish blooms (lasting approx. 20 years) [2], there are indications that ocean warming, over-fishing, aquaculture, eutrophication and coastal development are causing increased gelatinous zooplankton populations in many other regions [3–6]. This has caused researchers to project fundamental shifts in the biological structure and biogeochemical functioning of affected marine ecosystems, with significant environmental, societal and economic implications (such as negative effects on pelagic and benthic food webs, reductions in fisheries production and reduced tourism) [4,7,8].
Gelatinous zooplankton efficiently incorporate C and N from pelagic primary producers and secondary consumers into gelatinous biomass [9,10]. Many species are able to form large blooms that can die off episodically. As gelatinous zooplankton carcasses have high sinking speeds (1500 m d−1, [11]), they are capable of rapidly transporting nutrients from the pelagic zone to the deep sea [12–14]. Gelatinous zooplankton carcasses (jelly falls) from overlying blooms are known to accumulate at the seafloor as both small and large deposits, where they can enrich seafloor sediments in organic carbon, reduce sediment oxygen levels through smothering and alter benthic biogeochemical cycles [12–15]. Scavengers play a vital role in deep-sea ecosystems, deriving and distributing nutrients from energy-rich animal carcasses, such as dead mammals and fishes, as well as more refractory plant matter [16–19]. While some limited observations exist of some scavengers consuming jelly falls [13], many studies report little to no scavenging [12,13,15,20–22]. Moreover, the rapid scavenging activity and dense aggregations of scavengers typical for other forms of carrion and organic material (including dead fishes, whales and plant material) have never been documented at jelly falls. This has led to the paradigm that scavenging on jellyfish carcasses is limited compared with other types of carrion, although no direct scavenging studies have ever been carried out.
Here, we directly evaluated the response of deep-sea scavengers to jellyfish carcasses using the cosmopolitan coronate jellyfish species Periphylla periphylla as bait. P. periphylla is found at mesopelagic depths in several oceanic regions, including the North Atlantic and Gulf of Mexico [23,24], can form intense blooms [23], and sinks to the seafloor upon death [20]. To assess whether the scavenging responses seen using P. periphylla carcasses were typical for other jellyfish species, we also performed lander experiments using Cyanea capillata (a common scyphomedusae found in northern and southern European waters and in the North Atlantic) as bait. Scavenging of jellyfish carcasses was directly compared with scavenging of similar masses of Scomber scombrus (mackerel), a widely distributed pelagic fish. Experiments were conducted with randomized deployments of identically baited time-lapse camera landers at 1250 m depth in the Sognefjorden, Norway (see the electronic supplementary material, figure S1).
2. Material and methods
(a). Field study
To quantitatively assess scavenging on jellyfish carcasses at the deep-sea floor, baited camera deployments were conducted over a two-week period in October 2012 at a water depth of 1250 m in the Sognefjorden, Norway (see the electronic supplementary material, figure S1). A randomized sampling design was used in which 14 lander deployments of two identical landers were made at randomly selected stations along the central axis of the fjord (see the electronic supplementary material, figure S1). Landers were deployed at least 2 km apart to reduce interactions from bait odour plumes that, based on seafloor current measurements, would travel a maximum of 100 m over an experimental period of 18 h. Landers were designed to minimize flow artefacts associated with the frames. Each lander was equipped with an Ocean Imaging Systems camera and strobe system (camera settings: ISO 200, f-stop 8.0 and a 1/25–1/60s exposure). Two different jellyfish species (P. periphylla and C. capillata) and a fish (S. scombrus) were used as bait in the experiments. P. periphylla jellyfish were initially caught in September 2010 at 400 m depth in Lurefjorden, western Norway (060°41.7N; 005°08.5E) using a MOCNESS system and then frozen. These jellyfish were thawed before deploying as bait on four separate lander deployments. Freshly sampled P. periphylla were also used as bait on four different lander deployments. These were collected in the Lurefjorden in October 2012 by dip-net sampling at night. C. capillata were collected in the summer of 2010 by a dip-net in Vestrepolen, western Norway and frozen. Thawed C. capillata baits were deployed on each camera lander once (n = 2 deployments). Intact S. scombrus were purchased frozen from a local fisherman in October 2012 and deployed four separate times in a thawed condition on the camera landers. Prior to deployment, all baits were standardized to 316 ± 10 g wet weight (mean ± standard error, n = 14) and cable-tied to a bait plate situated 1.5 m in front of each camera. To standardize the odour plume from the thawed baits, each jellyfish bait deployment comprised five to six jellyfish, whereas fish bait deployments were made up of a single mackerel cut up into five to six sections that were identical in size to each thawed jellyfish. Baited landers were deployed at the seafloor for 18 h with photographs taken every 2.5 min. After 18 h on the seafloor, each lander was recalled to the surface, and the photographs downloaded to a computer. Environmental characteristics at the seafloor were measured on three lander deployments using a small Aanderaa Seaguard RCM acoustic single point current meter with oxygen, conductivity and temperature sensors attached to one of the lander systems. Bottom waters were well oxygenated (204 ± 0.1 µmol l−1, mean ± s.e., n = 3), and had a temperature and salinity of 7.4 ± 4 × 10−5 °C (mean ± s.e., n = 3) and 35 ± 3.8 × 10−4 (mean ± s.e., n = 3), respectively. To later identify scavengers attending each bait in the baited camera photographs, traps baited with fish and jellyfish baits were deployed twice at 1250 m depth for 3 h.
(b). Analysis of scavenger abundances in photographs
We assessed the abundance of each scavenger type at each bait every 5 min over each 18 h lander deployment. To do this, all Myxine glutinosa (Atlantic hagfish), Munida tenuimana (galatheid crab) and decapod shrimp attending the bait (defined as on the bait plate) were counted in every second photograph using the cell-counter plugin in ImageJ (v. 1.44, National Institute of Health). This plugin allowed annotation of each image, which facilitated quality control. Owing to the large abundances of Orchomenella obtusa (lysianassid amphipod) in each image, the maximum abundance and time of maximum abundance of O. obtusa were estimated through time for each deployment using a custom-built macro in ImageJ to estimate the area of amphipods, and linear regression modelling to calibrate the ‘amphipod area’ against abundances measured in randomly selected images (see the electronic supplementary material, Methods).
(c). Analyses of scavenging rates and bait removal times
Wet-weight consumption rates of the baits were determined from the wet weight of the carcass and the time between the arrival of the lander at the seafloor and the disappearance of the tissue of the bait from view (i.e. removal time). Owing to the large abundance of amphipods on the S. scombrus baits, which partially obscured the view of the bait, the scavenging data calculated from the S. scombrus treatments are the maximum consumption rates and minimum removal times. The concentrations of carbon, nitrogen and gross energy (GE) in each bait type were determined using standard carbon, hydrogen, nitrogen and bomb-calorimetry analysis at a commercial laboratory (NOFIMA, Bergen, Norway). These data were used together with the wet weight of the baits and removal times to calculate the rates of C, N (g d−1) and GE consumption (kJ d−1).
(d). Statistical analysis
To ensure a balanced statistical design, scavenging metrics (e.g. maximum abundance of scavengers, bait consumption rates) were only compared statistically between the P. periphylla and S. scombrus treatments (n = 4 for each treatment). Differences in the maximum abundance of M. glutinosa, M. tenuimana and decapod shrimp scavengers in the P. periphylla and S. Scombrus experiments were analysed using generalized-linear models (GLMs) based on Poisson distributions, or quasi-Poisson distributions if datasets were overdispersed. Because the maximum number of O. obtusa in each experiment was modelled and datasets contained no zero values, significant differences in the maximum abundance of O. obtusa in the S. scombrus and P. periphylla experiments were assessed using a parametric ANOVA test. The time of maximum abundance for each scavenger was compared across baits using ANOVA or non-parametric analogues if datasets failed to meet parametric assumptions. Removal times, and wet weight, C, N and GE consumption rates were compared between treatment types using ANOVA, or non-parametric Kruskal–Wallis tests if datasets were non-normal and/or heteroscedastic. An alpha level of 0.05 was chosen as the criterion for statistical significance. All data were analysed using the computer programming language R [25].
3. Results
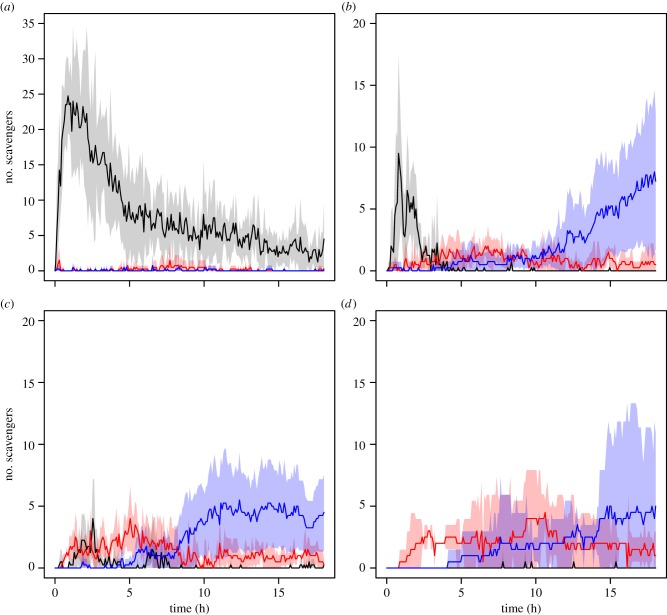
In our experiments, the scavenger response to thawed and fresh P. periphylla and thawed S. scombrus carcasses was extremely rapid, with the first scavengers arriving in large numbers within minutes of the landers reaching the seafloor (figures 1 and 2; see the electronic supplementary material, movies S1–S3). All bait treatments attracted the same diverse scavenger assemblage typical of the NE Atlantic bathyal zone, including the Atlantic hagfish, M. glutinosa; the galatheid crab, M. tenuimana; the lysianassid amphipod O. obtusa; as well as an unidentified decapod shrimp (likely Pontophilus norvegicus; figures 1a–c and 2a–c and table 1). In terms of scavenger numbers, the maximum abundance of M. glutinosa was significantly higher (116–760%) in the S. scombrus treatments than in the P. periphylla jellyfish experiments (GLM, p < 0.0001, figure 1a–c and table 1; see the electronic supplementary material, table S3). However, lysianassid amphipods were by far the most abundant scavengers in both the P. periphylla and S. scombrus experiments, and attained statistically similar maximum densities (ANOVA test, p = 0.602) in both the two P. periphylla and S. scombrus experiments (figure 2a–c and table 1; see the electronic supplementary material, table S3). The maximum abundance of decapod shrimp was significantly higher in both the thawed and fresh P. periphylla compared with the mackerel experiments (GLM, p = 0.009, figure 1a–c and table 1; see the electronic supplementary material, table S3). Significantly higher maximum abundances of M. tenuimana were also found in the fresh P. periphylla experiments compared with at the mackerel bait (GLM, p = 0.012, figure 1a–c and table 1; see the electronic supplementary material, table S3).
Figure 1.
Mean number of scavengers in the S. scombrus (a), P. periphylla (thawed and fresh) (b and c, respectively) and C. capillata (d) experiments as a function of time at the seafloor. Black lines and grey shading denote mean number of M. glutinosa ± 95% CIs; red line and shading denote mean number of M. tenuimana ± 95% CIs, and blue line and shading denote mean number of decapod shrimp ± 95% CIs. Note different y-axis scales.
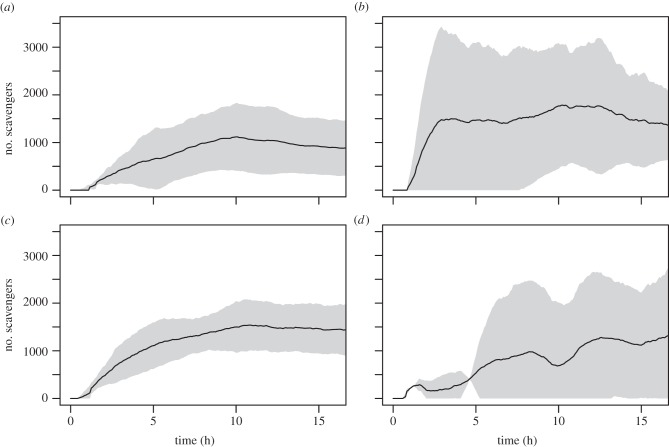
Figure 2.
Estimated mean number of Orchomenella obtusa in the S. scombrus (a), P. periphylla (thawed and fresh) (b and c, respectively) and C. capillata (d) experiments as a function of time at the seafloor. Black lines and grey shading denote mean number ± 95% CIs.
Table 1.
Maximum number of scavengers (mean ± s.e.) and the time (mean ± s.e.) at which the maximum abundance was reached at the different baits (min). The number of replicates (n) totals 4 for the P. periphylla and S. scombrus treatments, and 2 for the C. capillata treatment.
| bait | scavenger | maximum number |
time of maximum number (min) |
||
|---|---|---|---|---|---|
| mean | s.e. | mean | s.e. | ||
| S. scombrus (thawed) | M. glutinosa | 29.5 | 3.5 | 115.0 | 61.2 |
| M. tenuimana | 2.0 | 1.0 | 238.8 | 129.2 | |
| O. obtusa | 1609.5 | 273.5 | 712.5 | 108.2 | |
| decapod shrimp | 1.3 | 0.6 | 118.1 | 92.9 | |
| P. periphylla (thawed) | M. glutinosa | 12.8 | 2.6 | 102.5 | 36.0 |
| M. tenuimana | 3.5 | 0.3 | 286.3 | 121.8 | |
| O. obtusa | 2024.7 | 1135.9 | 623.8 | 257.0 | |
| decapod shrimp | 9.0 | 3.1 | 781.3 | 238.9 | |
| P. periphylla (fresh) | M. glutinosa | 6.3 | 0.9 | 193.8 | 67.1 |
| M. tenuimana | 5.8 | 0.9 | 218.8 | 35.3 | |
| O. obtusa | 1136.7 | 361.0 | 501.3 | 68.0 | |
| decapod shrimp | 6.5 | 2.3 | 570.6 | 194.5 | |
| C. capillata (thawed) | M. glutinosa | 1.0 | 0.0 | 610.0 | 142.5 |
| M. tenuimana | 5.0 | 1.0 | 600.0 | 37.5 | |
| O. obtusa | 1625.5 | 282.3 | 1272.5 | 4.3 | |
| decapod shrimp | 6.0 | 3.0 | 867.5 | 100.0 | |
Our experiments indicated somewhat different patterns of scavenger succession in the jellyfish versus mackerel experiments. In the mackerel experiments, two waves of scavengers were identified: a rapid increase in M. glutinosa abundance followed by a broad peak in O. obtusa for the duration of the 18-h experiments (figures 1a and 2a). By contrast, three waves of scavengers were observed in the P. periphylla experiments: hagfish abundance peaked quickly and then declined after approximately 3.5 h (figure 1b–c); as hagfish declined, M. tenuimana and O. obtusa increased in numbers at the bait plate, most likely feeding on small pieces of jellyfish tissue left over from ‘sloppy feeding’ by hagfish (figures 1b–c and 2b–c); finally, after 7–10 h, decapod shrimp increased in abundance (figure 1b–c). The time when scavengers reached their maximum abundance was not significantly different between treatments (table 1 and see the electronic supplementary material, table S3).
An analysis of the removal times of the different baits revealed no significant difference across treatments (ANOVA test, p = 0.488; table 2). Assuming a linear loss of material through time, mean wet-weight consumption rates of the different baits showed no statistically significant difference between any treatment (Kruskal–Wallis test, p = 0.199; table 2). In order to assess the importance of the jellyfish to the scavenger food web, we converted all wet-weight consumption rate data into C, N and GE consumption rates (table 2). We observed significantly lower rates of C, N and GE uptake into the scavenger food web in the fresh P. periphylla experiments relative to the S. scombrus studies (Kruskal–Wallis test, p = 0.007, table 2). However, no significant difference in C, N and energy consumption rates was detected between the thawed P. periphylla and S. scombrus treatments (p > 0.05, table 2), although the non-parametric statistical test used was conservative. This suggests that freezing and thawing of jellyfish carrion may increase C, N and energy consumption rates, perhaps by rupturing cells and enhancing the release of olfactory cues. The higher C, N and energy consumption rates in the fish relative to the fresh P. periphylla experiments was most likely caused by the significantly higher abundance of M. glutinosa at the mackerel bait (figure 1a and table 1; see the electronic supplementary material, table S3) responding to the 29- to 40-fold higher C, N and GE content in mackerel compared with fresh P. periphylla (C: 56.8 ± 0.8 versus 1.7 ± 0.2 g C (mean ± s.e., n = 4); N: 9.5 ± 0.1 versus 0.3 ± 0.0 g N (mean ± s.e., n = 4); GE: 2778.7 ± 37.4 versus 69.9 ± 7.0 kJ (mean ± s.e., n = 4) for S. scombrus and fresh P. periphylla treatments, respectively).
Table 2.
Mean bait removal times (±s.e., n = 4) and wet weight, C, N and gross energy (GE) consumption rates (±s.e., n = 4) calculated for the different baits. The removal time was the time between the lander arriving at the seafloor and the time when no more bait was visible on the bait plate. Consumption rates were calculated from the known wet weight, C, N and energy content of the baits (derived from the wet weight of the bait and C, N and GE content per unit wet weight) and the removal times. p-values from statistical tests are shown and treatments that differed significantly (p < 0.05) are shown in the significant multiple comparisons row.
| removal time (min) | wet-weight consumption (g d−1) | C consumption (g C d−1) | N consumption (g N d−1) | energy consumption (kJ d−1) | |
|---|---|---|---|---|---|
| statistical test | ANOVA | Kruskal–Wallis | Kruskal–Wallis | Kruskal–Wallis | Kruskal–Wallis |
| statistical results | f2,9 = 0.788 p = 0.488 |
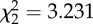 p = 0.199
p = 0.199 |
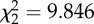 p = 0.007
p = 0.007 |
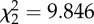 p = 0.007
p = 0.007 |
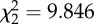 p = 0.007
p = 0.007 |
| significant multiple comparisons | S. scombrus > P. periphylla (fresh) | S. scombrus > P. periphylla (fresh) | S. scombrus > P. periphylla (fresh) | ||
| bait treatment | |||||
| S. scombrus | 421.9 ± 102.8 | 1388.3 ± 328.6 | 233.3 ± 55.2 | 39.0 ± 9.2 | 11413.3 ± 2701.6 |
| P. periphylla (thawed) | 288.8 ± 80.5 | 1934.6 ± 464.0 | 48.5 ± 11.6 | 8.7 ± 2.1 | 2107.4 ± 505.5 |
| P. periphylla (fresh) | 395.6 ± 46.0 | 1124.8 ± 290.0 | 6.7 ± 1.7 | 1.3 ± 0.3 | 278.8 ± 71.9 |
The scavenger responses to C. capillata were within the range of that for fresh P. periphylla (table 2). The mean bait removal time for C. capillata was 803.8 ± 321.3 min (mean ± range, n = 2), which corresponded to a wet-weight consumption rate of 713.7 ± 268.2 g d−1. While these second experiments provided further evidence for unexpectedly rapid consumption of gelatinous material at the deep-sea floor, the scavenger response at the C. capillata bait differed from that at both P. periphylla baits by an almost a complete absence of hagfish (figures 1b–d and 3b–d and table 1; see electronic supplementary material, movies S2–S4).
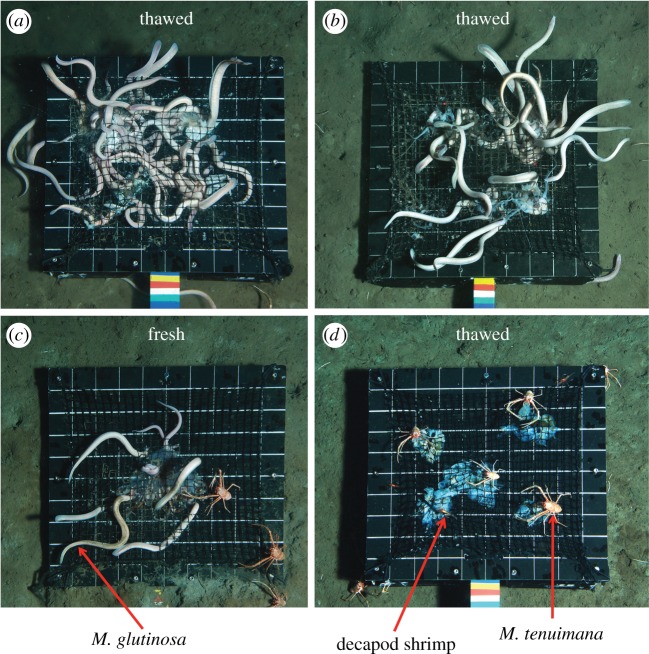
Figure 3.
Myxine glutinosa scavengers swarming at the S. scombrus bait (a). M. glutinosa voraciously feeding on thawed P. periphylla bait (b). M. glutinosa and M. tenuimana feeding on a single fresh P. periphylla carcass (c). M. tenuimana and decapod shrimp feeding on thawed C. capillata bait (d). The black bait plate is 50 × 50 cm with gridlines separated by 5 cm.
4. Discussion
Scavenging is a key process in marine ecosystems, controlling the entry of energy from carrion falls into various food-web components (e.g. microbial decomposers versus higher trophic levels) as well as the persistence time and standing stock of carrion at the seafloor. Although numerous observations of jelly falls have been made in recent years, the dynamics of scavenging on deep-sea jelly falls (such as the number of scavengers, carrion consumption rates) and the difference in scavenging on jelly falls compared with other, more protein-rich, carrion parcels (e.g. fish carcasses) have remained unexplored. In our study, jelly falls rapidly attracted dense aggregations of scavengers (maximum abundances of scavengers exceeding more than 1000 animals), and scavengers typical of the NE Atlantic bathyal zone consumed dead jellyfish within a matter of hours, with very little time for microbial decomposition. The jellyfish species used in this study efficiently prey on microzooplankton and fish larvae in the pelagic zone, and sink to the seafloor upon death [9,10,20]. They therefore represent a direct link between primary producers and secondary consumers in the pelagic zone and demersal and benthic fauna at the seafloor [14]. The rapid consumption rates measured in the jellyfish treatments in this study suggest that jellyfish carcasses may not regularly accumulate to form the large deposits that have been seen in other regions such as the Ivory Coast [13] and in the Gulf of Oman [12]. If these results are typical for other deep-sea areas, our results show that the role of dead gelatinous zooplankton in transporting pelagic production to the seafloor may be easily overlooked and their role in the biological C-pump may be underestimated. Extensive microbial degradation and low scavenging activity were reported at large, deep-sea jelly-fall deposits (jelly-detritus cover 100% of the seafloor) in the Gulf of Oman [12] and at large deep-sea pysosome deposits discovered off Ivory Coast [13] and the continental slope off the eastern US coast [21,22]. The difference between our study and these other observations could be related to differences in the sizes of the jelly-fall deposits between studies, with high concentrations of dissolved inorganic carbon (DIC), noxious sulfide and ammonium produced at large jelly-fall deposits deterring scavengers from feeding [14,26,27]. However, the small size of jelly falls in this study does not appear to be the main reason for the rapid scavenging activity observed as comparatively little or a complete lack of scavenging has also been observed at small gelatinous deposits in the deep Gulf of Oman (approx. 5 carcasses m−2, [12]), off the Ivory Coast (approx. 5 carcasses m−2 [13]), the abyssal NE Atlantic (less than 1 carcass m−2, [15]) and a Norwegian fjord (0.01 carcasses m−2, [20]). Previous studies have shown that scavenging activity can change as a function of season, with scavengers being more active during and immediately following the seasonal particulate organic matter (POM) enrichment of the benthos [28]. This study was undertaken around the time of the autumn POM pulse [29], in contrast to the Gulf of Oman [12] and Ivory Coast observations [13], which were made during the winter [12] or early spring [13] (i.e. before the springtime POM pulse). Thus, seasonal changes in scavenging activity may also partially explain the much greater scavenging rates observed in this study relative to other published observations. Clearly, further investigations using different quantities of dead jellyfish deployed at the seafloor at different times of year are needed to understand the main factors driving scavenging processes at jelly falls.
No significant differences in the diversity of scavengers, bait removal times and wet-weight consumption rates were found between jellyfish and fish carrion in this study, suggesting that scavenging dynamics at jelly falls may be similar to other types of carrion. In fact, based on the time for complete removal of the baits, mean wet-weight consumption rates for thawed P. periphylla (1934.6 ± 464.0 g wet weight d−1, table 2) were within the same order of magnitude as rates reported from the Arabian Sea and Arctic Ocean for tuna (2690 g d−1) [30], and trout, turbot and mackerel (2600 g d−1) carcasses [31], respectively. Nevertheless, some differences in scavenging dynamics were observed. Jellyfish falls attracted higher abundances of invertebrates (including galatheids and decapod shrimp) per unit carrion mass compared with energy-rich fish carrion, which attracted higher abundances of vertebrate scavengers. Moreover, the P. periphylla carcasses attracted three scavenger successional stages relative to the S. scombrus experiments, where only two were observed. The different scavenger succession at the jellyfish carcasses may be related to the lower energy content of the P. periphylla carrion (6.7 and 16.7 kJ g dry weight−1 for the fresh and thawed P. periphylla baits, respectively) relative to S. scombrus (26.4 kJ g dry weight−1) as scavenger composition and succession do change as a function of the biochemical content of carrion (e.g. at whale falls, [32]). We found selective feeding by hagfish on the energy-rich gonadal tissue of fresh P. periphylla carcasses prior to the consumption of other tissues (see the electronic supplementary material, movie S3). These observations suggest that energy content may be a major factor driving the temporal succession of scavengers at jellyfish carcasses. While this study showed, for the first time, that the scavenging process at jellyfish and fish carrion is similar in many aspects, it also revealed that carrion of different species of jellyfish can attract different scavenger assemblages. The exact reason for the lack of M. glutinosa at the C. capillata baits relative to the P. periphylla experiments is unclear. However, some decapod crustaceans are more resistant to highly toxic nematocysts and jellyfish mucus compared with certain fishes [33]. While the nematocysts of C. capillata were probably destroyed during the freezing process, the difference in scavenger diversity observed between the C. capillata and P. periphylla carcass treatments could be related to differences in tissue toxicity between species, as C. capillata produces highly toxic mucus [34] that may be particularly noxious to fish [33].
5. Conclusion
Efficient predation pressure imposed by jellyfish in the pelagic zone [7,9,10] and their fast sinking speeds (as carcasses, [11]) means that jellyfish blooms can effectively incorporate pelagic C into gelatinous biomass and make it rapidly available to seafloor communities when jellyfish blooms senesce. If jellyfish carrion deposits are extensive, they may alter seafloor biogeochemistry over large areas (through smothering) [12–14] as well as benthic boundary layer hydrodynamics, with concomitant impacts on many other benthic processes (e.g. recruitment, [14]). The removal of carcasses by scavenging is thus one of the main processes regulating the effect of jelly falls on the benthic environment. However, until now, no direct quantitative scavenging studies have ever been carried out in the deep sea to directly assess scavenging dynamics at jelly falls, or identify differences in scavenging dynamics relative to more protein-rich fish carrion. Using two different and widely distributed jellyfish species, we have shown that dense scavenger assemblages (more than 1000 scavengers) composed of species characteristic of the NE Atlantic bathyal zone rapidly gather and consume jellyfish carcasses at the deep-sea floor, with many aspects of the scavenging process mirroring processes observed at fish carcasses of similar mass. Moreover, our results show that jellyfish carrion may not always accumulate at the seafloor to form large deposits, and could therefore be easily overlooked as a source of nutrition to deep-sea benthic and demersal fauna. If these observations are typical for other deep-sea habitats, they suggest that gelatinous zooplankton carcasses may be far more important in sustaining deep-sea benthic and demersal communities than has been previously thought [12–15,20,35]. This potential oversight could have significant consequences for estimating energy and carbon transfer from gelatinous pelagic food webs to metazoan biomass in the deep sea. Ultimately, our study has shown a tight link between the surface and seafloor ecosystems, including a potentially more direct pathway for surface production to enter benthic food webs. In particular, the energy contained in gelatinous bodies can rapidly attract large numbers of scavengers, and be rapidly incorporated into deep-sea food webs, lessening expected impacts of enhanced jellyfish production on deep-water fisheries and pelagic–benthic coupling [8]. Thus, changes to biological communities and biogeochemical cycling in the deep ocean resulting from overlying jellyfish blooms, and the resulting societal and economic consequences, could be lower than those currently being forecasted [8,36,37].
Supplementary Material
Acknowledgements
We thank L. Pedersen, F.C. De Leo, A.F. Sweetman and A.C. Budarf for assistance on the research cruise. We thank A. Brodin and T.A. Krakeli at NOFIMA, Norway for carrying out the C, N and GE measurements on the jellyfish and mackerel baits. We thank T. Horton at the National Oceanography Centre, UK for assistance with the O. obtusa identifications. We thank L.A. Levin and one anonymous reviewer whose comments helped improve the manuscript, the University of Hawaii at Manoa and National Oceanography Center, UK for sharing equipment, and the Norwegian Research Council for providing funding to A.K. Sweetman to carry out this research (Norwegian Research Council grant no. 196699/S40).
Data accessibility
The database for all data and electronic supplementary videos from this article are publicly available at Dryad (http://datadryad.org/): doi:10.5061/dryad.90kt3.
References
- 1.Lucas C, et al. 2014. Gelatinous zooplankton biomass in the global ocean: geographic variation and environmental drivers. Glob. Ecol. Biogeog. 23, 701–714. ( 10.1111/geb.12169) [DOI] [Google Scholar]
- 2.Condon RH, et al. 2013. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl Acad. Sci. USA 110, 1000–1005. ( 10.1073/pnas.1210920110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Link JS, Ford MD. 2006. Widespread and persistent increase of Ctenophora in the continental shelf ecosystem off NE USA. Mar. Ecol. Prog. Ser. 320, 153–159. ( 10.3354/meps320153) [DOI] [Google Scholar]
- 4.Purcell JE, Uye SI, Lo WT. 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Mar. Ecol. Prog. Ser. 350, 153–174. ( 10.3354/meps07093) [DOI] [Google Scholar]
- 5.Utne-Palm AC, et al. 2010. Trophic structure and community stability in an overfished ecosystem. Science 329, 333–336. ( 10.1126/science.1190708) [DOI] [PubMed] [Google Scholar]
- 6.Purcell JE. 2012. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annu. Rev. Mar. Sci. 4, 1.1–1.27. ( 10.1146/annurev-marine-120709-142751) [DOI] [PubMed] [Google Scholar]
- 7.Condon RH, Steinberg DK. 2008. Development, biological regulation, and fate of ctenophore blooms in the York River estuary, Chesapeake Bay. Mar. Ecol. Prog. Ser. 369, 153–168. ( 10.3354/meps07595) [DOI] [Google Scholar]
- 8.Condon RH, Steinberg DK, del Giorgio PA, Bouvier TC, Bronk DA, Graham WM, Ducklow HW. 2011. Jellyfish blooms result in a major microbial respiratory sink of carbon in marine systems. Proc. Natl Acad. Sci. USA 108, 10 225–10 230. ( 10.1073/pnas.1015782108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fancett MS. 1988. Diet and prey selectivity of scyphomedusae from Port Phillip Bay, Australia. Mar. Biol. 98, 503–509. ( 10.1007/BF00391541) [DOI] [Google Scholar]
- 10.Youngbluth MJ, Båmstedt U. 2001. Distribution, abundance, behavior and metabolism of Periphylla periphylla, a mesopelagic coronate medusa in a Norwegian fjord. Hydrobiology 451, 321–333. ( 10.1023/A:1011874828960) [DOI] [Google Scholar]
- 11.Lebrato M, Mendes PJ, Steinberg DK, Cartes JE, Jones BM, Birsa LM, Benavides R, Oschlies A. 2013. Jelly biomass sinking speed reveals a fast carbon export mechanism. Limnol. Oceanogr. 58, 1113–1122. ( 10.4319/lo.2013.58.3.1113) [DOI] [Google Scholar]
- 12.Billett DSM, Bett BJ, Jacobs CL, Rouse IP, Wigham BD. 2006. Mass deposition of jellyfish in the deep Arabian Sea. Limnol. Oceanogr. 51, 2077–2083. ( 10.4319/lo.2006.51.5.2077) [DOI] [Google Scholar]
- 13.Lebrato M, Jones DOB. 2009. Mass deposition event of Pyrosoma atlanticum carcasses off Ivory Coast (West Africa). Limnol. Oceanogr. 54, 1197–1209. ( 10.4319/lo.2009.54.4.1197) [DOI] [Google Scholar]
- 14.Lebrato M, et al. 2012. Jelly-falls historic and recent observations: a synthesis to drive future research directions. Hydrobiology 690, 227–245. ( 10.1007/s10750-012-1046-8) [DOI] [Google Scholar]
- 15.Roe HSJ, Billett DSM, Lampitt RS. 1990. Benthic/midwater interactions on the Madeira Abyssal Plain; evidence for biological transport pathways. Prog. Oceanogr. 24, 127–140. ( 10.1016/0079-6611(90)90025-W) [DOI] [Google Scholar]
- 16.Britton JC, Morton B. 1994. Marine carrion and scavengers. Oceanogr. Mar. Biol. Annu. Rev. 32, 369–434. [Google Scholar]
- 17.Fleury AG, Drazen JC. 2013. Abyssal scavenging communities attracted to Sargassum and fish in the Sargasso Sea. Deep-Sea Res. I 72, 141–147. ( 10.1016/j.dsr.2012.11.004) [DOI] [Google Scholar]
- 18.Jeffreys RM, Lavaleye MSS, Bergman MJN, Duineveld GCA, Witbaard R, Linley T. 2010. Deep-sea macrourid fishes scavenge on plant material: evidence from in situ observations. Deep-Sea Res. I 57, 621–627. ( 10.1016/j.dsr.2010.01.007) [DOI] [Google Scholar]
- 19.Jeffreys RM, Lavaleye MSS, Bergman MJN, Duineveld GCA, Witbaard R. 2011. Do abyssal scavengers use phytodetritus as a food resource? Video and biochemical evidence from the Atlantic and Mediterranean . Deep-Sea Res. I 58, 415–428. ( 10.1016/j.dsr.2011.02.002) [DOI] [Google Scholar]
- 20.Sweetman AK, Chapman AC. 2011. First observations of jelly-falls at the seafloor in a deep-sea fjord. Deep-Sea Res. I 58, 1206–1211. ( 10.1016/j.dsr.2011.08.006) [DOI] [Google Scholar]
- 21.Cacchione DA, Rowe GT, Malahoff A. 1978. Submersible investigation of outer Hudson submarine canyon. In Sedimentation in canyons, fans and trenches (eds Stanley DJ, Kelling G.), pp. 42–50. Stroudsburg, PA: Dowden, Hutchinson and Ross. [Google Scholar]
- 22.Wiebe PH, Madin LP, Haury LR, Harbison GR, Philbin LM. 1979. Diel vertical migration by Salpa aspera and its potential for large-scale particulate organic matter transport to the deepsea. Mar. Biol. 53, 249–255. ( 10.1007/BF00952433) [DOI] [Google Scholar]
- 23.Lucas CH. 2009. Biochemical composition of the mesopelagic coronate jellyfish Periphylla periphylla from the Gulf of Mexico. J. Mar. Biol. Assoc. UK 89, 77–81. ( 10.1017/S0025315408002804) [DOI] [Google Scholar]
- 24.Sørnes TA, Aksnes DL, Båmstedt U, Youngbluth MJ. 2007. Causes for mass occurrences of the jellyfish Periphylla periphylla: a hypothesis that involves optically conditioned retention. J. Plankton Res. 29, 157–167. ( 10.1093/plankt/fbm003) [DOI] [Google Scholar]
- 25.R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 26.Titelman J, Riemann L, Sørnes TA, Nilsen T, Griekspoor P, Båmstedt U. 2006. Turnover of dead jellyfish: stimulation and retardation of microbial activity. Mar. Ecol. Prog. Ser. 325, 43–58. ( 10.3354/meps325043) [DOI] [Google Scholar]
- 27.Treude T, Smith CR, Wenzhofer F, Carney E, Bernadino AF, Hannides AK, Kruger M, Boetius A. 2009. Biogeochemistry of a deep-sea whale fall: sulfate reduction, sulfide efflux and methanogenesis. Mar. Ecol. Prog. Ser. 382, 1–21. ( 10.3354/meps07972) [DOI] [Google Scholar]
- 28.Priede IG, Bagley PM, Smith KL., Jr 1994. Seasonal change in activity of abyssal demersal scavenging grenadiers Coryphaenoides (Nematonurus) armatus in the eastern North Pacific Ocean. Limnol. Oceanogr. 39, 279–285. ( 10.4319/lo.1994.39.2.0279) [DOI] [Google Scholar]
- 29.Wassmann P. 1984. Sedimentation and benthic mineralization of organic detritus in a Norwegian Fjord. Mar. Biol. 83, 1432–1793. ( 10.1007/BF00393088) [DOI] [Google Scholar]
- 30.Janssen F, Treude T, Witte U. 2000. Scavenger assemblages under differing trophic conditions: a case study in the deep Arabian Sea. Deep-Sea Res. II 47, 2999–3026. ( 10.1016/S0967-0645(00)00056-4) [DOI] [Google Scholar]
- 31.Premke K, Klages M, Arntz WE. 2006. Aggregations of Arctic deep-sea scavengers at large food falls: temporal distribution, consumption rates and population structure. Mar. Ecol. Prog. Ser. 325, 121–135. ( 10.3354/meps325121) [DOI] [Google Scholar]
- 32.Smith CR, Baco AR. 2003. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 41, 311–354. [Google Scholar]
- 33.Shanks AL, Graham WM. 1988. Chemical defense in a scyphomedusa. Mar. Ecol. Prog. Ser. 45, 81–86. ( 10.3354/meps045081) [DOI] [Google Scholar]
- 34.Lassen S, Helmholz H, Ruhnau C, Prange A. 2011. A novel proteinaceous cytotoxin from the northern Scyphozoa Cyanea capillata (L.) with structural homology to cubozoan hemolysins. Toxicon 57, 721–729. ( 10.1016/j.toxicon.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 35.Smith KL, Ruhl HA, Kahru M, Huffard CL, Sherman AD. 2013. Deep ocean communities impacted by changing climate over 24 years in the abyssal northeast Pacific Ocean. Proc. Natl Acad. Sci. USA 110, 19 838–19 841. ( 10.1073/pnas.1315447110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynam CP, Gibbons MJ, Axelsen BE, Sparks CAJ, Coetzee J, Heywood BG, Brierley AS. 2006. Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 16, R492–R493. ( 10.1016/j.cub.2006.06.018) [DOI] [PubMed] [Google Scholar]
- 37.Richardson AJ, Bakun A, Hays GC, Gibbons MJ. 2009. The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 24, 312–322. ( 10.1016/j.tree.2009.01.010) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The database for all data and electronic supplementary videos from this article are publicly available at Dryad (http://datadryad.org/): doi:10.5061/dryad.90kt3.