Abstract
The objective of this study was to assess the effects of constant high ambient temperatures on meat quality, antioxidant capacity, and carnosine expression in longissimus dorsi muscle of finishing pigs. Castrated 24 male DLY (crossbreeds between Landrace×Yorkshire sows and Duroc boars) pigs were allocated to one of three treatments: constant ambient temperature at 22°C and ad libitum feeding (CON, n = 8); constant high ambient temperature at 30°C and ad libitum feeding (H30, n = 8); and constant ambient temperature at 22°C and pair-fed with H30 (PF, n = 8). Meat quality, malondialdehyde (MDA) content, antioxidant capacity, carnosine content, and carnosine synthetase (CARNS1) mRNA expression in longissimus dorsi muscle were measured after three weeks. The results revealed that H30 had lower pH24 h, redness at 45 min, and yellowness at 24 h post-mortem (p<0.05), and higher drip loss at 48 h and lightness at 24 h post-mortem (p<0.01). Constant heat stress disrupted the pro-oxidant/antioxidant balance in longissimus dorsi muscle with higher MDA content (p<0.01) and lower antioxidant capacity (p<0.01). Carnosine content and CARNS1 mRNA expression in longissimus dorsi muscle of H30 pigs were significantly decreased (p<0.01) after three weeks at 30°C. In conclusion, constant high ambient temperatures affect meat quality and antioxidant capacity negatively, and the reduction of muscle carnosine content is one of the probable reasons.
Keywords: Heat Stress, Meat Quality, Antioxidants, Carnosine, Pigs
INTRODUCTION
High ambient temperatures affect livestock production (Lin et al., 2006; Lu et al., 2007). Heat stress has negative effects on growth performance, feed intake and body weight gain (Morales et al., 2014), contributing to high mortality rates in pigs. Acute heat stress and transport stress accelerate the development of rigor mortis (McKee and Sams, 1997), which results in low pH, high L* values, and PSE—pale, soft, and exudative—meat characteristics in pigs and chickens. With the global greenhouse effect, high ambient temperatures have negatively impacted on livestock production. Recent studies have focused on the effects of acute or short-time stress on growth performance and meat quality of pigs (Pérez et al., 2002), broilers (Lu et al., 2007), and sheep (Chulayo et al., 2013). However, few studies have focused on the effects of constant heat stress on meat quality and antioxidant capacity of finishing pigs.
Several vertebrate species counteract heat-induced damage with their antioxidant defense system (Antonopoulou et al., 2013), which consists of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase. Heat stress shifts the antioxidant-free radical equilibrium towards more free radicals (Imik et al., 2012). It has been reported that heat stress is responsible for higher levels of reactive oxygen species (ROS; Lin et al., 2006) and disturbances in the pro-oxidant and antioxidant defense balance. Muscle antioxidant capacity has a positive correlation with meat quality. Oxidation in muscle tissue is evident by increased conversion of myoglobin, a red muscle pigment, to metmyoglobin, a brown pigment, and the development of rancid odors and flavors from the degradation of polyunsaturated fatty acids (Velasco et al., 2011).
Carnosine is involved in the antioxidant system. Carnosine supplementation improves the antioxidant capacity and meat quality of pigs (Ma et al., 2010). In rats exposed to chronic cold stress, carnosine supplementation improves the pro-oxidant/antioxidant balance (Kalaz et al., 2012). Higher carnosine levels in response to short-term stress have been observed in breast and thigh tissues of broilers (Manhiani et al., 2011). However, few studies have focused on the effects of carnosine on the meat quality and antioxidant capacity of longissimus dorsi (LD) muscle in finishing pigs under constant heat stress. In view of previous reports, we hypothesize that muscle carnosine is crucial for the pro-oxidant/antioxidant balance of LD muscle in finishing pigs exposed to chronic heat stress.
In this study, the effects of constant heat stress on meat quality, carnosine content and muscle oxidant status in finishing pigs were investigated. Furthermore, we proposed the hypothesis that carnosine may affect pork quality by the pro-oxidant/antioxidant balance in LD muscle of finishing pigs.
MATERIALS AND METHODS
Animals and experimental design
The experiment was performed in accordance with Chinese guidelines for the use of experimental animals and animal welfare (Science and Technology Ministry of China, 2006). Twenty-four castrated male DLY (crossbreeds between Landrace×Yorkshire sows and Duroc boars) pigs were selected from eight litters (body weight: 79.0±1.5 kg) from a pig breeding farm in Beijing, China, among which three pigs from each litter were allocated to one of three treatments: constant ambient temperature at 22°C and ad libitum feeding (CON, n = 8); constant high ambient temperature at 30°C and ad libitum feeding (H30, n = 8); and constant ambient temperature at 22°C and pair-fed with the diet consumed by H30 (PF, n = 8). Prior to the experiment, the animals were allowed to acclimatize at 22°C for seven days in an environmental control chamber. The experimental period lasted three weeks. During the study, the pigs were individually caged (cage dimensions: 150×80×80 cm) with 14-h light and 10-h dark cycles and 55±5% relative humidity. The animals had ad libitum access to water. Feed consisted of a fattening diet (corn-soybean meal) containing 15.73% crude protein and 13.39 MJ/kg digestible energy.
Samples and sample processing
Pigs were stunned by an electrical input (220 V, alternating current, 50 Hz for 10 s) following a 12-h overnight fast, and all the sampling processes on each carcass were completed within 15 min. Longissimus dorsi muscle tissues from the 10th thoracic vertebra of the right carcass were snap-frozen in liquid nitrogen and stored at −80°C for enzyme activity, real-time polymerase chain reaction (PCR) and high performance liquid chromatography-mass spectrometry (HPLC-MS) analyses. Longissimus dorsi muscles from the 4th and 10th thoracic vertebra of the left carcass were quickly separated and stored at 4°C in 45 min, 24 h, and 48 h for meat quality measurements.
Meat quality measurements
Longissimus dorsi samples from the 10th and 12th thoracic vertebra were used for pH and meat color measurements. The pH values were determined 45-min post-mortem (pH45 min) and 24-h and 48-h post-storage at 4°C in self-sealed plastic bags (pH24 h and pH48 h, respectively), using a portable pH meter (pH-STAR, R.Matthaus, Berlin, Germany) equipped with a spear-type electrode. At 45-min and 24-h post-mortem, meat color was measured with the Chroma meter CR-410 (Minolta, Tokyo, Japan). Color was reported in the CIE-LAB (Commission internationale de l’Éclairage color space) trichromatic system, i.e., L*, a*, and b* values (L* = lightness; a* = redness; and b* = yellowness). Meat color was expressed as the average of the three measurements.
Drip loss was determined by the filter paper method (Kauffman et al., 1986) at 24 h and 48 h post-mortem. To measure water holding capacity (WHC), four meat samples from the 4th and 6th thoracic vertebra were weighed (initial weight for drip loss). Samples were placed on a net and suspended in an inflated bag, ensuring there was no contact between the samples and the bags. Following 24 h and 48 h of storage at 4°C, two samples were removed from the bag, blotted dry, and weighed (ultimate weight). Drip loss was calculated by the following equation, drip loss (%) = (initial weight [g] − ultimate weight [g])/initial weight (g)×100%.
The shear force of samples from the 6th and 9th thoracic vertebra was measured according to Chinese agricultural standard NY/T-1180-2006. Pork chops from each sample were stored at 4°C for 24 h, individually cooked in plastic bags in a water bath (80°C) to an internal temperature of 70°C, and cooled to room temperature. After cooking, four cores (1.27 cm diameter) parallel to the longitudinal orientation of the muscle fibers were removed from each pork chop for shear force measurements. Shear force was measured with a TMS-Pro (Food Technology Corporation, Sterling, VA, USA) and expressed in Newton. The samples were sheared perpendicular to the long axis of the core.
Antioxidant indexes in longissimus dorsi muscle
Assay kits for protein, lactate, lactate dehydrogenase (LDH), malondialdehyde (MDA), SOD, and CAT were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China; corresponding catalog Numbers are A045-2, A019-2, A020-2, A003-1, A001-1, and A007-2). Muscle homogenates were centrifuged at 600×g for 10 min at 4°C to remove crude fractions, and the resulting supernatants were used for the determination of enzymatic content and activities by spectrophotometric methods using a spectrophotometer (SP752, Shanghai Spectrum, Shanghai, China). All of the assays followed the instructions of the kits. Muscle protein was measured by the method of coomassie brilliant blue and detected at 595 nm. Muscle lactate content was measured using an assay including LDH. The levels of MDA in longissimus dorsi muscle were determined with 2-thiobarbituric acid according to Placer et al. (1966) at 532 nm. The CAT was assayed with the initial rate of hydrogen peroxide decomposition at 405 nm (Aebi, 1984). The SOD activity was determined by the xanthine oxidase method described by Ōyanagui (1984). The activity of LDH was measured spectrophotometrically at 450 nm within the LDH-catalyzed reduction of pyruvate to lactate (Jurie et al., 2006). The MDA level was expressed as nanomoles per milligram of protein, and the enzyme activity was expressed as unit per milligram protein.
Carnosine content in longissimus dorsi muscle
Carnosine extraction
Approximately 30 mg of LD muscle was transferred to Eppendorf tubes, homogenized, and extracted with 1 mL of methanol/water (50:50) for 30 min in an ultrasonic bath (Beat Ultrasonic, Zhangjiagang, China). Following centrifugation at 16,000×g for 30 min, the supernatant was passed through a 0.45-μm membrane filter. The water-extracted filtrate (supernatant) was subjected to heat treatment at 80°C for 15 min in a water bath (model no. 283, Thermo Scientific, USA), immediately cooled and centrifuged at 6,000×g for 20 min at 25±4°C to remove precipitated proteins. The resulting supernatant was collected and stored at −80°C.
Carnosine determination by HPLC-MS
Sample aliquots (5 μL) were subjected to chromatography in a C18 column (150 mm×4.6 mm; Waters Corp, Milford, MA, USA) at 0.8 mL/min and 50°C. The mobile phase consisted of 0.1% heptafluorobutyric acid+0.1% formic acid in water (A) and 0.1% heptafluorobutyric acid+0.1% formic acid in acetonitrile (B). Elution was performed during 20 min: 90% A for 5 min, 90% to 70% A for 2 min, 70% to 65% A for 3 min, 65% to 5% A for 5 min, 5% to 0% A for 0.5 min, and 90% A for 4.5 min.
Mass spectrometry was performed on a API 4000 QQQ (Applied Biosystems, Carlsbad, CA, USA) coupled to an HPLC system (LC20A, Shimadzu Instruments, Kyoto, Japan) operating in positive-ion mode. A standard curve was generated with different concentrations (5 to 1,000 mM) of pure carnosine (C9625, Sigma-Aldrich, St. Louis, MO, USA). The resulting linear regression was y = 0.000132x–3.45e−5 (r = 0.9935), of which x means carnosine concentration, y means mass spectrum response. The retention times and peak areas were analyzed by API analyst 1.5 software (LC20A, Shimadzu Instruments, Kyoto, Japan). Carnosine concentration was calculated from the standard curve.
RNA isolation and real-time polymerase chain reaction analysis
Total RNA was isolated from 20 mg skeletal muscle tissue using Ultrapure RNA extraction kit (Cat# CW0581, CWbio, Beijing, China). RNA integrity was assessed by 1% agarose gel electrophoresis, which revealed intact bands corresponding to 18S and 28S ribosomal RNA subunits. Following RNase-free DNase treatment, RNA was reverse-transcribed using HiFi-MMLV cDNA first strand synthesis kit (Cat#CW0744, CWbio, China). The gene-specific primer pairs used in this experiment are shown in Table 1. Quantitative RT-PCR was performed in duplicate in an ABI Prism 7500 apparatus (Applied Biosystems, USA). In this experiment, cDNA was amplified by PCR with gene-specific primer pairs using Ultra SYBR Mixture with ROX (#CW0956, CWbio, China). Real-time PCR data were analyzed using the delta-delta CT method, i.e., 2−ΔΔCT method (Schmittgen and Livak, 2008). The primers designed for the reference genes were based on sequences published in Genbank (http://www.ncbi.nlm.nih.gov/) and are shown in Table 1.
Table 1.
Primer sequences, product size of real-time polymerase chain reaction
| Primers | Primer sequences | GeneBank accession number | Annealing temperature (°C) | Product size(bp) |
|---|---|---|---|---|
| CARNS1 | F :CCAAGCAGAAGAGCCGTACCCA R:GGGCACAGCATAGAGGGAGGG |
XM_005660632.1 | 84.45 | 83 |
| β-actin | F:ATCCAGGCAGTGCTGTCCCTCTAC R:GGCAGCGCGTAGCCCTCATA |
XM_005670976.1 | 88.14 | 110 |
CARNS1, carnosine synthetase.
For each reference gene, gene name, GenBank accession number, primer sequences, Tm values are indicated.
Statistical analyses
Comparisons among the three experimental treatments (CON, H30, and PF) were performed with the one-way analysis of variance (SAS Institute, 2003, USA); significant means were analyzed with Duncan’s multiple range tests. Correlation analyses among meat quality index were performed by Pearson’s linear regression test (SAS Institute, 2003, USA). Statistical significance was set at p<0.05 and p<0.01.
RESULTS
Effects of constant heat stress on meat quality of longissimus dorsi muscle
The effects of constant heat exposure on pH, color, drip loss, and shear force of LD muscle are shown in Table 2 and 3. At 45 min post-mortem, H30 had higher L* values than CON and PF pigs (p<0.01), and a* values decreased (p<0.05) in H30 compared to CON. The PF had lower b* values than CON (p<0.1). At 24 h post-mortem, H30 had higher L* values (p<0.01) than CON, while the L* values of CON were higher than PF, PF had the lowest L* values (p<0.01) among all treatments. At 24 h post-mortem, H30 and PF pigs had lower b* values relative to CON (p<0.05). There were no significant differences in a* values among the treatments at 24 h post-mortem.
Table 2.
Effects of constant heat stress on meat color of longissimus dorsi muscle in finishing pigs
| Post-mortem time | Items | CON | H30 | PF | SEM | p-value |
|---|---|---|---|---|---|---|
| 45 min | L* | 44.37B | 45.73A | 44.20B | 0.29 | <0.01 |
| a* | 17.86a | 16.44b | 16.83ab | 0.37 | 0.03 | |
| b* | 5.61 | 5.04 | 4.43 | 0.35 | 0.08 | |
| 24 h | L* | 50.68B | 52.32A | 49.58C | 0.20 | <0.01 |
| a* | 21.85 | 20.70 | 21.33 | 0.38 | 0.13 | |
| b* | 12.37a | 11.38b | 11.27b | 0.27 | 0.02 |
CON, constant ambient temperature at 22°C and ad libitum feeding; H30, constant high ambient temperature at 30°C and ad libitum feeding; PF, constant ambient temperature at 22°C and pair-fed to the H30 pigs; SEM, standard error of the mean; L*, lightness (n = 8); a*, redness (n = 8); b*, yellowness (n = 8).
Treatments with different superscript capital letters in the same row are different at p<0.01.
Treatments with different superscript low letters in the same row are different at p<0.05.
Table 3.
Effects of constant heat stress on pH, drip loss and shear force of longissimus dorsi muscle in finishing pigs
| Items1 | Post-mortem time | CON | H30 | PF | SEM | p-value |
|---|---|---|---|---|---|---|
| pH value | 45 min | 6.35 | 6.37 | 6.35 | 0.13 | 0.99 |
| 24 h | 5.73a | 5.43b | 5.39b | 0.09 | 0.02 | |
| 48 h | 5.41 | 5.24 | 5.27 | 0.05 | 0.08 | |
| Drip loss (%) | 24 h | 3.49 | 3.97 | 3.64 | 0.20 | 0.25 |
| 48 h | 5.25B | 6.45A | 5.42B | 0.26 | <0.01 | |
| Shear force (N) | 48 h | 47.66B | 53.64A | 47.60B | 1.04 | <0.01 |
CON, constant ambient temperature at 22°C and ad libitum feeding; H30, constant high ambient temperature at 30°C and ad libitum feeding; PF, constant ambient temperature at 22°C and pair-fed to the H30 pigs, SEM, standard error of the mean.
pH value, n = 8; Drip loss, n = 8; Shear force, n = 6.
Treatments with different superscript capital letters in the same row are different at p<0.01.
Treatments with different superscript low letters in the same row are different at p<0.05.
There were no differences in muscle pH45 min among treatments (p>0.05). The pH24 h values of H30 and PF were significantly lower than those of CON (p<0.01); pH48 h values of H30 and PF were slightly lower than those of CON (p<0.1). The pH values of LD muscle decreased with storage up to 48 h post-mortem. In terms of WHC, H30 had higher drip loss than CON and PF at 48 h post-mortem (p<0.01). There were no significant differences in drip loss among the treatments at 24 h post-mortem. Higher drip loss was observed with increasing storage (24 h vs 48 h). Shear force values in H30 were significantly higher than those in CON and PF (p<0.01).
According to the results, constant heat stress had significant effects on meat color: high L* values at 45 min and 24 h, low a* values at 45 min, and low b* values at 24 h. Constant heat stress reduced muscle pH at 24 h and 48 h post-mortem, with similar tendencies in PF pigs, which suggests that the reduction in pH might be attributed to feed restriction. Higher drip loss at 48 h and shear force values were observed in pigs under constant heat stress.
Effects of constant heat stress on carnosine synthetase mRNA expression and carnosine content of longissimus dorsi muscle
Carnosine synthetase (CARNS1) mRNA expression in H30 of LD muscle was down-regulated compared to that in PF and CON (p<0.01; Figure 1). There was a 0.53-fold down-regulation in CARNS1 mRNA expression in H30 compared to CON in LD muscle. Carnosine content in LD muscle was 4.98 mg/g in H30, 5.62 mg/g in CON, and 5.58 mg/g in PF. As shown in Figure 2, carnosine content in H30 was significantly lower than that in CON or PF (p<0.01). The down-regulation in CARNS1 mRNA expression was in accordance with the lower carnosine content in H30.
Figure 1.
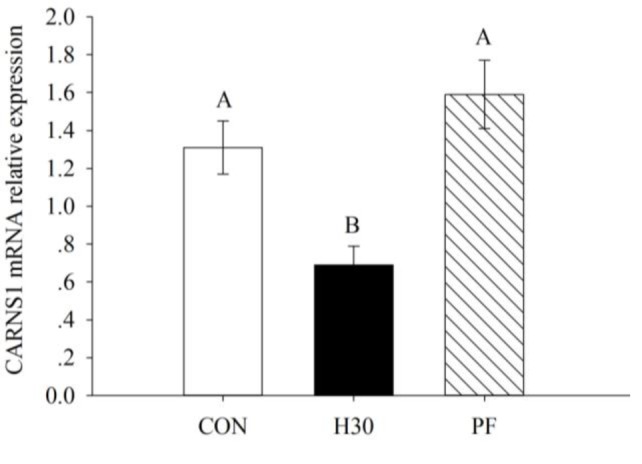
Effects of constant heat stress on carnosine synthetase (CARNS1) mRNA expression of longissimus dorsi muscle in finishing pigs. CARNS1, carnosine synthetase mRNA expression in longissimus dorsi muscle, data analyzed using the 2−ΔΔCT method, n = 8; Con, control, 22°C; H30, heat stress, 30°C; PF, pair-fed with H30, 22°C. Capital letters on columns indicate significant levels within columns (p<0.01).
Figure 2.
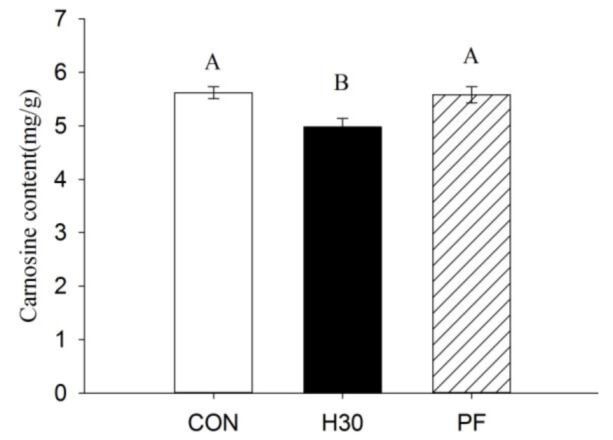
Effects of constant heat stress on carnosine content of longissimus dorsi muscle in finishing pigs. Carnosine content: carnosine content (mg/g) in longissimus dorsi muscle measured by high performance liquid chromatography-mass spectrometry, n = 8; Con, control, 22°C; H30, heat stress, 30°C; PF, pair-fed with H30, 22°C. Capital letters on columns indicate significant levels within columns (p<0.01).
Effects of constant heat stress on malondialdehyde content and enzymatic activity of longissimus dorsi muscle
The effects of constant heat stress on lactate, MDA content, LDH activity, and antioxidant enzyme activities (SOD and CAT) of LD muscle are shown in Table 4. The H30 pigs had higher muscle lactate content than CON (p<0.05). Muscle LDH activities in H30 and PF pigs were higher than those in CON (p<0.1). The MDA content of LD muscle was higher in H30 than in the other two treatments (p<0.01); MDA content in PF was higher than that in CON (p<0.01). H30 and PF pigs had lower muscle SOD activities than CON (p<0.01). The CAT activities in H30 were lower than those in CON and PF (p<0.01).
Table 4.
Effects of constant heat stress on enzyme activities of longissimus dorsi muscle in finishing pigs
| Items | CON | H30 | PF | SEM | p-value |
|---|---|---|---|---|---|
| Lactate (mm mol/g protein) | 1.02b | 1.52a | 1.32ab | 0.10 | 0.01 |
| LDH (U/mg protein) | 14.76 | 16.06 | 16.04 | 0.41 | 0.06 |
| MDA (n mol/mg protein) | 0.10C | 0.13A | 0.11B | <0.01 | <0.01 |
| SOD (U/mg protein) | 11.84A | 9.92B | 9.97B | 0.20 | <0.01 |
| CAT (U/mg protein) | 0.49A | 0.41B | 0.48A | 0.01 | <0.01 |
CON, constant ambient temperature at 22°C and ad libitum feeding; H30, constant high ambient temperature at 30°C and ad libitum feeding; PF, constant ambient temperature at 22°C and pair-fed to the H30 diets; SEM, standard error of the mean; Lactate, lactate content in longissimus dorsi muscle (n = 8); LDH, lactate dehydrogenase activity in longissimus dorsi muscle (n = 8); MDA, malondialdehyde content in longissimus dorsi muscle (n = 8); SOD, superoxide dismutase activity in longissimus dorsi muscle (n = 8); CAT, catalase activity in longissimus dorsi muscle (n = 7).
Treatments with different superscript capital letters in the same row are different at p<0.01.
Treatments with different superscript low letters in the same row are different at p<0.05.
Correlations among carnosine content, meat quality, and antioxidant enzyme activities of longissimus dorsi muscle
As shown in Table 5, 6, and 7, carnosine concentration was negatively correlated with L* values (at 45 min and 24 h post-mortem; p<0.05), drip loss at 48 h post-mortem (p<0.01) and MDA content (p<0.01), and positively correlated with CAT activity (p<0.05). The pH24 h and pH48 h values of LD muscle were positively correlated with a* and b* values at 24 h post-mortem (p<0.01). The pH48 h values of LD muscle were negatively correlated with L* values at 45 min post-mortem (p<0.05). The pH24 h values were negatively correlated with drip loss at 24 h post-mortem (p<0.01).
Table 5.
Correlation among carnosine content, MDA, SOD, CAT, and pH of longissimus dorsi muscle in finishing pigs
MDA, malondialdehyde content in longissimus dorsi muscle (n = 8); SOD, superoxide dismutase activity in longissimus dorsi muscle (n = 8); CAT, catalase activity in longissimus dorsi muscle (n = 7); LDH, lactate dehydrogenase activity in longissimus dorsi muscle (n = 8). pH, muscle pH (n = 8); CARNS1, Carnosine: carnosine content in longissimus dorsi muscle (n = 7–8 [correlation between carnosine and CAT, n = 7; otherwise n = 8]).
0.01<p<0.05;
p<0.01.
Correlation analysis was performed by a Pearson’s linear regression test.
Table 6.
Correlation among carnosine content, drip loss, meat color, and shear force of longissimus dorsi muscle in finishing pigs
| Index | Drip loss 24 h | Drip loss 48 h | L* 45 min | a* 45 min | b* 45 min | L* 24 h | a* 24 h | b* 24 h | Shear force |
|---|---|---|---|---|---|---|---|---|---|
| Carnosine | −0.32 | −0.58** | −0.45* | 0.15 | −0.22 | −0.50* | 0.18 | −0.02 | −0.42 |
Drip loss (n = 8); L*, lightness (n = 8); a*, redness (n = 8); b*, yellowness (n = 8); Shear force (n = 6); Carnosine, carnosine content in longissimus dorsi muscle (n = 6–8 [correlation between carnosine and shear force, n = 6; otherwise n = 8]).
0.01<p<0.05;
p<0.01.
Correlation analysis was performed by a Pearson’s linear regression test.
Table 7.
Correlation among malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and drip loss, meat color, shear force of longissimus dorsi muscle in finishing pigs
| Index | Drip loss 24 h | Drip loss 48 h | L* 45 min | a* 45 min | b* 45 min | L* 24 h | a* 24 h | b* 24 h | Shear force |
|---|---|---|---|---|---|---|---|---|---|
| pH45 min | −0.16 | −0.37 | −0.34 | −0.21 | 0.12 | −0.08 | −0.40 | −0.21 | 0.24 |
| pH24 h | −0.52** | −0.25 | −0.26 | 0.31 | 0.11 | −0.11 | 0.66** | 0.63** | −0.06 |
| pH48 h | −0.30 | −0.22 | −0.40* | 0.48* | 0.23 | −0.17 | 0.61** | 0.58** | 0.02 |
| MDA | 0.26 | 0.53** | 0.48* | −0.31 | −0.19 | 0.69** | −0.37 | −0.32 | 0.73** |
| SOD | −0.11 | −0.38 | −0.43* | 0.40 | 0.16 | −0.12 | 0.19 | 0.47* | −0.27 |
| CAT | −0.34 | −0.40 | −0.59** | 0.37 | 0.18 | −0.64** | 0.41 | 0.26 | −0.62* |
Drip loss, n = 7–8 (correlation between drip loss and CAT, n = 7; otherwise n = 8); L*, lightness, n = 7–8 (correlation between L* and CAT, n = 7; otherwise n = 8); a*, redness, n = 7–8 (correlation between a* and CAT, n = 7; otherwise n = 8); b*, yellowness, n = 7–8 (correlation between b* and CAT, n = 7; otherwise n = 8); MDA, malondialdehyde content in longissimus dorsi muscle, n = 6–8 (correlation between MDA and shear force, n = 6; otherwise n = 8); SOD, superoxide dismutase activity in longissimus dorsi muscle, n = 6–8 (correlation between SOD and shear force, n = 6; otherwise n = 8); CAT, catalase activity in longissimus dorsi muscle, n = 5–7 (correlation between CAT and shear force, n = 5; otherwise n = 7); pH, n = 6–8 (correlation between pH and shear force, n = 6; otherwise n = 8).
0.01<p<0.05;
p<0.01.
Correlation analysis was performed by a Pearson’s linear regression test.
Muscle MDA content had significant positive correlations with drip loss at 48 h (p<0.01), L*values at 24 h, and shear force (p<0.01). The SOD activities were negatively correlated with L* values at 45 min and positively correlated with b* values at 24 h post-mortem (p<0.05), while CAT activities were negatively correlated with L* values (at 45 min and 24 h post-mortem; p<0.01) and shear force (p<0.05).
DISCUSSION
Effects of constant heat stress on meat quality of longissimus dorsi muscle in finishing pigs
Effects of constant heat exposure on pork quality, carnosine content and oxidant capacity of LD muscle in finishing pigs were investigated in the study. To distinguish the direct effects of high ambient temperature from the effects caused by a decrease in feed-intake, a pair-fed treatment at 22°C ambient temperature was included in this study. The heat-exposed pigs had higher L* values and lower a* and b* values than CON. H30 pigs had higher L* values than PF and CON at 45 min and 24 h post-mortem, revealing the direct effects of high ambient temperature on the muscle lightness. Lower a* and b* values were observed in H30 compared with CON, no significantly differences were observed among H30 and PF, suggesting that the decreased feed-intake in H30 pigs might have contributed to the differences in meat color. Küchenmeister et al. (2000) found more pale pork muscle (higher L* values) in the summer than in the winter. Pre-slaughter exercise decreased a* values compared to non exercised pigs, indicated that pre-slaughter stress affect pork color by less redness (Rosenvold et al., 2003).
Meat color is attributed to carboxymyoglobin, deoxymyoglobin, oxymyoglobin and metmyoglobin. In the presence of oxygen, Myoglobin (Mb, purplish-red color) is converted into the red-colored oxymyoglobin (MbO2), which is gradually oxidized into the dull brown-colored metmyoglobin. These three compounds are very sensitive to oxidation (Mancini et al., 2005). Thus, the results of higher L* values and lower a* and b* values in H30 pigs may be on account of muscle oxidative status under constant heat stress.
It was found that H30 and PF pigs had the same tendency of decreased muscle pH values at 24 h and 48 h post-mortem, suggesting that the phenomenon may be due to the reduction of feed-intake under constant heat stress. The lower pH24 h and pH48 h values in H30 are in accordance with the results obtained in pigs under per-slaughter or chronic heat stress (Salmi et al., 2012; Lu et al., 2010). Stress or exercise before slaughter resulted in faster pH decline. The pH value of LD muscle in finishing pigs decreased gradually with storage (45 min, 24 h, and 48 h); this result could be attributed to the accumulation of lactate post-mortem. H30 and PF pigs had higher LDH activities and lactate level in LD muscle, similar to the chronically heat-stressed broilers (Zhang et al., 2012). Therefore, LD muscle of constantly heat-exposed or pair-fed pigs may have more pyruvate converted to lactate, leading to lower pH values.
Chronic heat stress resulted in poor WHC; similar results have been reported in growing pigs (Lu et al., 2010). The water content of meat is one of the essential quality parameters as it relates to the final yield of end product and is also important in terms of eating (Cheng et al., 2008). The excessive loss of water in H30 pigs had an adverse effect on product appearance and shelf life, which also implied financial loss. The results of this study revealed higher shear force values in H30 pigs than in PF or CON pigs, similar to the findings of Lu et al. (2010), who reported that chronic heat stress decreases meat tenderness in growing swine. Longissimus dorsi muscle of H30 pigs had higher drip loss and shear force than PF, indicating that direct effects of high ambient temperature stress caused the changes instead of relying on the decreased feed-intake.
Muscle a* and b* values at 24 h post-mortem were positively correlated with pH24 h values (r = 0.66, p<0.01 and r = 0.63, p<0.01, respectively), while muscle L* values at 45 min post-mortem were negatively correlated with pH48 h values (r = −0.40, p<0.05). Color change was visible to the unaided eye when muscle pH was less than 6.1 (Abril et al., 2001), which was in agreement with the lower muscle pH and paler meat color characteristics in the study. Warriss et al. (1987) reported that pH determines drip loss in porcine muscle. Muscle water is driven towards the extracellular spaces at lower pH values, which leads to lower pork WHC. In this study, pH24 h was negatively correlated with drip loss at 24 h post-mortem (r = −0.52, p<0.01), which indicated that the decline pH values in LD muscle of H30 pigs were in accordance with the poor WHC in the study.
Effects of constant heat stress on carnosine synthetase mRNA expression and carnosine content of longissimus dorsi muscle in finishing pigs
Carnosine is a naturally-occurring dipeptide that is synthesized by the CARNS1 from β-alanine and L-histidine. It is widely accepted that carnosine acts as a potent endogenous antioxidant in its function of metal chelation, hydrogen donorship and oxygen free-radical scavenging. Supplementation of carnosine (100 mg/kg diet) results in reduction of muscle MDA concentration in finishing pigs (Ma et al., 2010). It was reported that carnosine is an effective agent to decrease prooxidant status in brain of oxidative stress rats (Kalaz et al., 2012).
Carnosine synthetase has been isolated from chicken sternal muscle and human skeletal and heart muscles (Bulygina et al., 1995). In this study, the down-regulation of CARNS1 mRNA expression was in accordance with the decreased muscle carnosine content in H30 pigs. Higher muscle carnosine level and CARNS1 mRNA expression were observed in H30 pigs than PF, revealing that these remarkable influences were caused by direct effects of high ambient temperature stress.
Carnosine content of H30 pigs (4.98 mg/g) was significantly lower than that of CON (5.62 mg/g) and PF (5.58 mg/g). On the other hand, Manhiani et al. (2011) reported that carnosine content in breast of short-term (15 min) stressed birds (17.39 mg/g) was ten times higher than that of non-stressed birds (1.85 mg/g). Additionally, carnosine content of thigh tissue of stressed birds (21.25 mg/g) was approximately 2-fold higher than that of non-stressed birds (11.10 mg/g). In horses, Dunnett et al. (2002) found higher plasma carnosine concentration at 5 to 30 min and lower carnosine concentrations at 120 min post-exercise; however, carnosine values returned to normal after 1 d. Differences in muscle carnosine content among animals exposed to stress may be accounted for by the difference in species, stress duration time and intensity. It was possible that the content of carnosine in skeletal muscle of birds and plasma of horses increased under short-time stress and then may have decreased over time, similar to the situation in LD muscle of finishing pigs suffering from constant heat stress.
Effects of constant heat stress on antioxidant capacity of longissimus dorsi muscle in finishing pigs
According to previous reports, heat stress accelerates the oxidation of muscle tissue (Wang et al., 2009), resulting in a shift in the pro-oxidant/antioxidant balance (Lin et al., 2006). The MDA is a marker of mitochondrial and muscle lipid peroxidation, CAT and SOD are antioxidant parameters.
Higher MDA level and lower enzyme activity of SOD, CAT in LD muscle of H30 pigs were obtained than CON in this study. The MDA levels of LD muscle in H30 pigs were higher than PF, while CAT activities in H30 muscle were lower than PF, showing the direct effects of constant high ambient temperature lead to oxidative stress in LD muscle of finishing pigs. It has been reported that weaning stress enhances MDA levels, decreases SOD activity in serum of piglets (Zhu et al., 2012). Increased MDA concentration and decreased SOD activity were observed after 48 hours oxidative stress in porcine kidney cells (Chen et al., 2013). Azad et al. (2010) and Mahmoud et al. (2003) demonstrated that constant heat stress results in higher MDA levels in skeletal muscle of broiler chickens. Addition of hexachlorocyclohexane to feedstuff reduced liver SOD and serum CAT activity, increased serum MDA in growing/finishing pigs (Wang et al., 2006). Hepatic and brain CAT activities decrease in rats exposed to chronic cold stress (Kalaz et al., 2012).
The MDA is generally used as a biomarker for radical-induced damage and endogenous lipid peroxidation, it is implied that the pro-oxidant/antioxidant balance is disrupted with higher MDA levels of LD muscle in H30 pigs. The SOD catalyzes the conversion of superoxide to hydrogen peroxide and functions in conjunction with CAT to convert hydrogen peroxide into water and molecular oxygen (Finkel and Holbrook, 2000). The decreased antioxidant capacity (SOD and CAT) and enhanced lipid peroxidation (MDA) in LD muscle of H30 pigs indicated an oxidative stress under constant high temperature condition.
Carnosine may affect meat quality of longissimus dorsi muscle in finishing pigs through antioxidant capacity under constant heat stress
Carnosine is a potent ROS scavenger, which inhibits lipid peroxidation and protein damage (Namgung et al., 2010). Carnosine supplementation decreases MDA levels in both liver and brain tissues of constant cold-stressed rats (Kalaz et al., 2012). In this study, muscle MDA content was negatively correlated with carnosine content (r = −0.52, p<0.01), while muscle CAT activities were positively correlated with carnosine content (r = 0.43, p<0.05). These results revealed that reduced muscle carnosine content was positively correlated with the oxidant status of muscle tissue.
In this study, low antioxidant capacity of muscle tissue was associated with poor meat quality. There was a significant positive correlation between muscle MDA content and shear force (r = 0.73, p<0.01), while a significant negative correlation was obtained between CAT activity and shear force (r = −0.62, p<0.05), indicating that less tender meat has higher oxidant status. Zhang et al. (2012) reported that lower a* values in heat-exposed birds were due to oxidized myoglobin pigments, which is consistent with the positive correlation (r = 0.41, p<0.1) between a* values at 24 h and CAT activity obtained in this study. The MDA content was positively correlated with L* values at 24 h post-mortem (r = 0.69, p<0.01); CAT activity was negatively correlated with L* values at 24 h post-mortem (r = −0.64, p<0.01), which suggests that paler meat color characteristics were accompanied with lipid peroxidation in heat-stressed pigs.
The effects of carnosine on meat quality have been reported. Ma et al. (2010) reported that the addition of 100 mg carnosine per kg diet increases pH values of muscle at 45 min, 24 h, and 48 h post-mortem in LD muscle of finishing pigs. Cheng et al. (2013) and Das et al. (2006) reported that carnosine increases pH values of pre-cooked pork patties and ground buffalo meat. Carnosine supplementation increases the redness of meat in finishing pigs (Ma et al., 2010) and ground beef (Sanchez-Escalante et al., 2003). Decker et al. (1990) reported that carnosine is effective in inhibiting myoglobin oxidation in salted, frozen, ground pork. Even though there were no significant correlations between muscle carnosine content and a* values in this study, there were significant negative correlations between L* values (at 45 min and 24 h post-mortem) and carnosine content (r = −0.45, p<0.05 and r = −0.50, p<0.05, respectively). A significant negative correlation was obtained between muscle carnosine content and drip loss at 48 h post-mortem (r = −0.58, p<0.01); pigs exposed to constant heat stress have lower carnosine content and higher drip loss in LD muscle, similar to the findings of Ma et al. (2010), who observed decreased drip loss at 48 h post-mortem in pigs of carnosine addition.
A lot of researches above verified that carnosine supplementation improves meat quality. The correlation among lower carnosine content and decreased antioxidant capacity, and the relationship among decreased antioxidant capacity and poor meat quality were observed in the study. Therefore, peroxidation and poor meat quality in heat-stressed pigs may be attributed to lower carnosine content.
In conclusion, the results confirmed that constant heat stress results in lower muscle pH, a* values, and tenderness, and higher drip loss and L* values. The meat quality is associated with increased MDA content and decreased antioxidant capacity (CAT and SOD) in LD muscle of chronically heat-stressed pigs, which might be attributed to the reduction in meat carnosine content.
ACKNOWLEDGMENTS
This study was supported by grants from the National Key Basic Research Program of China (National 973 Project) (2012CB124706), Key Project in the National Science & Technology Pillar Program (2012BAD39B0202) and The Agricultural Science and Technology Innovation Program (ASTIP-IAS07). The authors greatly appreciate Dr Yanjun Cui, Mr Zhenghui Cao and Chunhe Yang for their skilled help in meat sampling and in several laboratory analyses. The authors would like to thank the reviewers for their valuable comments and suggestions.
REFERENCES
- Abril M, Campo MM, Önenç A, Sanudo C, Albertí P, Negueruela AI. Beef colour evolution as a function of ultimate pH. Meat Sci. 2001;58:69–78. doi: 10.1016/s0309-1740(00)00133-9. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Antonopoulou E, Kentepozidou E, Feidantsis K, Roufidou C, Despoti S, Chatzifotis S. Starvation and re-feeding affect Hsp expression, MAPK activation and antioxidant enzymes activity of European Sea Bass (Dicentrarchus labrax) Comp Biochem Physiol A Mol Integr Physiol. 2013;165:79–88. doi: 10.1016/j.cbpa.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Azad MAK, Kikusato M, Maekawa T, Shirakawa H, Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp Biochem Physiol A Mol Integr Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Bulygina ER, Kramarenko GG. Isolation of carnosine synthetase from animal and human muscles. Vopr Med Khim. 1995;41:27–30. [PubMed] [Google Scholar]
- Chen X, Ren F, Hesketh J, Shi X, Li J, Gan F, Huang K. Interaction of porcine circovirus type 2 replication with intracellular redox status in vitro. Redox Rep. 2013;18:186–192. doi: 10.1179/1351000213Y.0000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JH, Wang ST, Ockerman HW. Quality preservation of reduced sodium pork patties: Effects of antioxidants on color and lipid stability. J Sci Food Agric. 2013;93:2959–2962. doi: 10.1002/jsfa.6124. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Sun DW. Factors affecting the water holding capacity of red meat products: A review of recent research advances. Crit Rev Food Sci Nutr. 2008;48:137–159. doi: 10.1080/10408390601177647. [DOI] [PubMed] [Google Scholar]
- Chulayo AY, Muchenje V. The Effects of pre-slaughter stress and season on the activity of plasma creatine kinase and mutton quality from different sheep breeds slaughtered at a smallholder Abattoir. Asian Australas J Anim Sci. 2013;26:1762–1772. doi: 10.5713/ajas.2013.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AK, Anjaneyulu ASR, Biswas S. Effect of carnosine preblending on the quality of ground buffalo meat. Food Chem. 2006;97:531–538. [Google Scholar]
- Decker EA, Faraji H. Inhibition of lipid oxidation by carnosine. J Am Oil Chem Soc. 1990;67:650–652. [Google Scholar]
- Dunnett M, Harris RC, Dunnett CE, Harris PA. Plasma carnosine concentration: Diurnal variation and effects of age, exercise and muscle damage. Equine. Vet. J. 2002;(Suppl 34):283–287. doi: 10.1111/j.2042-3306.2002.tb05434.x. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Imik H, Ozlu H, Gumus R, Atasever MA, Urcarand S, Atasever M. Effects of ascorbic acid and a-lipoic acid on performance and meat quality of broilers subjected to heat stress. Br Poult Sci. 2012;53:800–808. doi: 10.1080/00071668.2012.740615. [DOI] [PubMed] [Google Scholar]
- Jurie C, Ortigues-Marty I, Picard B, Micol D, Hoc-quette JF. The separate effects of the nature of diet and grazing mobility on metabolic potential of muscles from Charo-lais steers. Livest Sci. 2006;104:182–192. [Google Scholar]
- Kalaz EB, Evran B, Develi-İş S, Vural P, Doğru-Abbasoğlu S, Uysal M. Effect of carnosine on prooxidant-antioxidant balance in several tissues of rats exposed to chronic cold plus immobilization stress. J Pharmacol Sci. 2012;120:98–104. doi: 10.1254/jphs.12107fp. [DOI] [PubMed] [Google Scholar]
- Kauffman RG, Eikelenboom G, van der Wal PG, Merkus G, Zaar M. The use of filter paper to estimate drip loss of porcine musculature. Meat Sci. 1986;18:191–200. doi: 10.1016/0309-1740(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Küchenmeister U, Kuhn G, Ender K. Seasonal effects on Ca2+ transport of sarcoplasmic reticulum and on meat quality of pigs with different malignant hyperthermia status. Meat Sci. 2000;55:239–245. doi: 10.1016/s0309-1740(99)00149-7. [DOI] [PubMed] [Google Scholar]
- Lin H, Buyse J, Decuypere E. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Lu QP, Wen J, Zhang HF. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci. 2007;86:1059–1064. doi: 10.1093/ps/86.6.1059. [DOI] [PubMed] [Google Scholar]
- Lu QP, Zhang HF, Liu QW, Liu SJ. The effect of high ambient temperature on muscle fiber characteristics and meat quality in growing pig. Proceedings of the Sixth National Conference on Feed Nutrition; Yangling, China. 2010. pp. 542–543. [Google Scholar]
- Ma XY, Jiang ZY, Lin YC, Zheng CT, Zhou GL. Dietary supplementation with carnosine improves antioxidant capacity and meat quality of finishing pigs. J. Anim. Physiol. Anim. Nutr (Berl). 2010;94:e286–e295. doi: 10.1111/j.1439-0396.2010.01009.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud KZ, Edens FW. Influence of selenium sources on age-related and mild heat stress-related changes of blood and liver glutathione redox cycle in broiler chickens (Gallus domesticus) Comp Biochem Physiol B Biochem Mol Biol. 2003;136:921–934. doi: 10.1016/s1096-4959(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Mancini RA, Hunt MC. Current research in meat color. Meat Sci. 2005;71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Manhiani PS, Northcutt JK, Han I, Bridges WC, Scott TR, Dawson PL. Effect of stress on carnosine levels in brain, breast, and thigh of broilers. Poult Sci. 2011;90:2348–2354. doi: 10.3382/ps.2011-01426. [DOI] [PubMed] [Google Scholar]
- McKee SR, Sams AR. The effect of seasonal heat stress on rigor development and the incidence of pale, exudative turkey meat. Poult Sci. 1997;76:1616–1620. doi: 10.1093/ps/76.11.1616. [DOI] [PubMed] [Google Scholar]
- Morales A, Grageola F, García H, Arce N, Araiza B, Yáñez J, Cervantes M. Performance, serum amino acid concentrations and expression of selected genes in pair-fed growing pigs exposed to high ambient temperatures. J. Anim. Physiol. Anim. Nutr. (Berl) 2014 doi: 10.1111/jpn.12161. [DOI] [PubMed] [Google Scholar]
- Namgung N, Shin DH, Park SW, Paik IK. Effects of supplementary blood meal on carnosine content in the breast meat and laying performance of old hens. Asian Australas J Anim Sci. 2010;23:946–951. [Google Scholar]
- Ōyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984;142:290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- Pérez MP, Palacio J, Santolaria MP, Aceña MC, Chacón G, Gascón M, Calvo JH, Zaragoza P, Beltran JA, García-Belenguerd S. Effect of transport time on welfare and meat quality in pigs. Meat Sci. 2002;61:425–433. doi: 10.1016/s0309-1740(01)00216-9. [DOI] [PubMed] [Google Scholar]
- Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyldialde-hyde) in biochemical systems. Anal Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Rosenvold K, Andersen HJ. The significance of pre-slaughter stress and diet on color and colour stability of pork. Meat Sci. 2003;63:199–209. doi: 10.1016/s0309-1740(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Salmi B, Trefan L, Bünger L, Doeschl-Wilson A, Bidanel JP, Terlouw C, Larzul C. Bayesian meta-analysis of the effect of fasting, transport and lairage times on four attributes of pork meat quality. Meat sci. 2012;90:584–598. doi: 10.1016/j.meatsci.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Sanchez-Escalante A, Djenane D, Torrescano G, Giménez B, Beltrán JA, Roncalés P. Evaluation of the antioxidant ability of hydrazine-purified and untreated commercial carnosine in beef patties. Meat Sci. 2003;64:59–67. doi: 10.1016/s0309-1740(02)00162-6. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Science and Technology Ministry of China. The guiding suggestion about treating experimental animals amicablely. 2006. No. 398 Document. [Google Scholar]
- Velasco V, Williams P. Improving meat quality through natural antioxidants. Chil J Agric Res. 2011;71:313–322. [Google Scholar]
- Wang F, Xu ZR, Su JH. Effect of HCH contamination of diet on the growth performance and immune and antioxidant ability in growing/finishing pigs. Vet Res Commun. 2006;30:645–654. doi: 10.1007/s11259-006-3327-z. [DOI] [PubMed] [Google Scholar]
- Wang RR, Pan XJ, Peng ZQ. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult Sci. 2009;88:1078–1084. doi: 10.3382/ps.2008-00094. [DOI] [PubMed] [Google Scholar]
- Warriss PD, Brown SN. The relationship between initial pH, reflectance and exudation in pig muscle. Meat Sci. 1987;20:65–72. doi: 10.1016/0309-1740(87)90051-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Jia GQ, Zuo JJ, Zhang Y, Lei J, Ren L, Feng DY. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhu LH, Zhao KL, Chen XL, Xu JX. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2012;90:2581–2589. doi: 10.2527/jas.2012-4444. [DOI] [PubMed] [Google Scholar]


