Abstract
Present study analyzed the changes in peripheral blood testosterone concentrations and testicular cytogram in relation to age and semen quality in crossbred males. Three different age groups of crossbred males viz. bull calves (6 months, n = 5), young bulls (15 months, n = 5) and adult bulls (4 to 6 years, n = 8) were utilized for the study. Testicular fine needle aspiration cytology technique was used to quantify testicular cytology and their indices. Peripheral blood testosterone concentrations were measured using enzyme-linked immunosorbent assay method. Semen samples collected from adult bulls were microscopically evaluated for quality parameters. Mean peripheral blood testosterone concentrations in bull calves, young bulls and adult bulls were 2.28±0.09 ng/mL, 1.42±0.22 ng/mL and 5.66±1.08 ng/mL respectively, and that in adult bulls were significantly different (p<0.01) from young bulls and bull calves. There was no significant difference between the proportion of different testicular cells in bull calves and young bulls. Between young and adult bulls, significant differences (p<0.01) were observed in the proportion of spermatocytes, spermatozoa, and sperm: Sertoli cell ratio. The proportions of Sertoli cells showed a significant difference (p<0.01) between the three age groups. The number of primary spermatocytes had a positive correlation with peripheral blood testosterone concentrations in bull calves (r = 0.719, p<0.01). Number of Sertoli cells per 100 germ cells was negatively correlated with blood testosterone concentration in young bulls (r = −0.713, p<0.01). Among different semen parameters in adult bulls, ejaculate volume (r = 0.790, p<0.05) had positive relationship, and sperm motility had significant negative correlation (r = −0.711, p<0.05) with testosterone concentrations. The number of Sertoli cells and Sertoli cell index had a positive correlation with various semen quality parameters (p<0.001). Results of the present study conclude that number of Sertoli cells and Sertoli cell index are good indicators of semen quality, but peripheral blood testosterone concentrations may not have a direct relationship with various seminal attributes in crossbred bulls.
Keywords: Fine Needle Aspiration Cytology, Testicular Cytogram, Testicular Cell Indices, Crossbred Bulls
INTRODUCTION
Crossbreeding of low producing zebu (Bos indicus) cattle with high profile exotic breeds (Bos taurus) is widely adopted to improve the milk productivity in developing countries. Several crossbreds have been developed during the last few decades with substantially greater productivity than their purebred counterparts (Singh, 2005). Although, reported fertility problems in crossbred females are low, the incidence of infertility is very high in crossbred males (Mukhopadhyay et al., 2010; Khatun et al., 2013). Infertility or sub-fertility in crossbred males due to poor semen quality, poor libido and poor freezability are major problems to reject several bulls at semen stations (Vijetha et al., 2014). In several studies conducted in India, it was found that nearly 50% crossbred males were removed from breeding stocks due to low quality semen production (Sudheer and Xavier, 2000; Mathur et al., 2002; Mukhopadhyay et al., 2010; Khatun et al., 2013; Rajak et al., 2013). This slows the genetic improvement programmes, which can cause heavy economic losses in terms of time, labour and money in rearing infertile bulls up to semen collection age.
Poor semen quality may be attributed to impaired spermatogenesis, endocrine disturbances, alterations in microenvironment of seminiferous tubules, aberrations during epididymal transit and accessory gland infections (Robaire and Viger, 1995; Hinton et al., 1996). The latter two factors can be diagnosed readily by observing the spermatozoa for maturation associated changes (e.g. protoplasmic droplets) and deviations in the quality of seminal plasma (Hinton et al., 1996); however, the other factors also need to be investigated. Testosterone in males is responsible for libido, normal spermatogenesis and normal function of the reproductive tract (McLachlau et al., 1996; Goeritz et al., 2003). Insufficient amount of androgen leads to an immediate arrest in meiotic transformation of primary spermatocytes to spermatids which causes an effective block in sperm production (Suresh et al., 1995). Several studies have analyzed the relationship between serum testosterone concentration, sperm quality and fertility in bulls with few reporting a strong relationship (Wolfe, 1996; Tuli et al., 1991; Javed et al., 2000) while others did not find any relationship (Schallenberger et al., 1991; Sekasiddhi and Buban, 1997; Santos et al., 2004; Rajak et al., 2014). Substantial differences were reported in the plasma testosterone concentration between various breeds of bulls (Post and Bindon, 1983; Chase et al., 1997), with temperate breeds (Bos taurus) having higher testosterone concentration than tropical cattle (Chase et al., 1997), however studies in this aspect are limited in crossbred bulls. Similarly, the relationship among testicular cytology, their indices and testosterone concentrations of bulls have not been studied in detail.
Production of fertile sperm is a function of normal mitosis and meiosis of germ cell and proper function of germ and Sertoli cells would give idea about the semen quality (Johnson, 1991). Since the quality of spermatozoa reflects the health of seminiferous tubules, and proportion of different cells of seminiferous tubules, especially the germ cells and Sertoli cells, it could be used to assess the status of spermatogenesis. Many researchers reported that number of Sertoli cells is highly correlated to sperm production ability of animals (Russell and Peterson, 1984; França and Russell, 1998; Leal et al., 2004). Interestingly reports are also available that testosterone plays an important role in the proliferation of Sertoli cells, even within the developing testis itself (Johnston et al., 2004; Scott et al., 2007; 2008; Sharpe, 2010). But information on the relationship among these three parameters, i.e. testosterone concentration, number of testicular cells and various semen quality parameters, are very limited in bulls (Chapwanya et al., 2008). Therefore, the present study was conducted to determine the relationship among peripheral blood testosterone concentrations, different testicular cells and their indices and semen quality in crossbred bulls of different age groups.
MATERIAL AND METHODS
Experimental animals and their management
The present investigation was conducted on Karan Fries crossbred bulls (Holstein Friesian×Tharparkar) maintained at Artificial Breeding Research Centre and Livestock Research Centre, National Dairy Research Institute (NDRI), Karnal, India. Three subject groups viz. bull calves (6 months age, n = 5), young bulls (15 months age, n = 5) and adult bulls (4 to 6 years age, n = 8) were selected randomly to study the parameters at pre-pubertal, pubertal and adult stages of life.
Adult and young bulls were kept in individual pens (30′×10′) on concrete floor and the bull calves were kept under group system of housing. The adult and young bulls were fed with ad libitum green fodder and measured quantities of concentrate (2.5 kg/d for adult bulls and 1.5 kg/d for young bulls) containing 21% crude protein and 70% total digestible nutrients. Vaccinations, de-worming, regular check-up for communicable diseases and other herd-health programmes were followed as per standard farm schedule, to protect the animals from diseases. All the experimental procedures and animal experimentation methods were approved by the Institutional Animal Ethical Committee of NDRI (No. IAEC/5/11).
Blood sample collection and testosterone estimation
Three blood samples were collected on three consecutive days at 8:00 am from all the experimental animals from jugular vein using 9 mL vacutainer tubes (BD vacutainer, Franklin Lakes, NJ, USA) without anticoagulant for testosterone estimation. The blood samples were allowed to clot for 4 to 5 hours at 4°C to 5°C, serum was separated by centrifugation (1,500 g; 15 min) and the testosterone concentrations were estimated using bovine testosterone enzyme-linked immunosorbent assay Kit (Endocrine Technologies Inc, Newark, CA, USA). The sensitivity of the test was 20 pg/mL and the reliability was checked using reference positive control.
Testicular cytology and estimation of cell indices
For testicular fine needle aspiration cytology, animals were restrained properly and epidural anaesthesia (2% Lignocaine hydrochloride; Dosages, 2 to 3 mL in bull calves, 4 to 5 mL in young bulls and 7 to 10 mL in adult bulls) was administered at sacro-coccygeal junction. A 22 gauge needle was inserted through the scrotum into the posterior part of the testis at right angle. When plunger was pulled back, the needle was moved little forward and backward within the testis two to three times for approximately 4 seconds for dislodging of cells and easy suction into the needle. Two aspirations per each testis were taken from all the animals. The aspirate was expelled on a clean glass slide and a thin smear was prepared using another slide. Four slides per animal were prepared for analysis.
The smears were stained with May-Grunwald Giemsa (Wild-Leitz, Wetzlar, Germany) stain. After air drying, the smear was immersed in methanol for 15 minutes; washed in running tap water for one minute, and smear was air dried and then stained with diluted May-Grunwald stain (1:1 with phosphate-buffered saline [PBS]) for 15 minutes. The smear was washed with tap water and air dried. Diluted Giemsa stain (1:3 with PBS) was then poured on the smear and allowed to stain for 45 minutes before washing and air drying. The smear was examined under phase contrast microscope at 400× and 1,000× magnifications (Nikon Eclipse E600, Tokyo, Japan). A total 200 clearly identifiable cells were counted from each slide for analysing the number of various testicular cells and their indices.
Different spermatogenic cells (spermatogonia, primary spermatocytes, early spermatids, late spermatids and spermatozoa) and Sertoli cells were identified according to their distinct morphological characteristics as described by Leme and Papa (2010) and Santos et al. (2010). Different testicular cell indices viz. spermatic index (number of spermatozoa per 100 spermatogenic cells), Sertoli cell index (number of Sertoli cell per 100 spermatogenic cells), sperm: Sertoli cell ratio (ratio of number of spermatozoa to the number of Sertoli cells), spermatogenic:Sertoli cell ratio (ratio of total no. of spermatogenic cell to total number of Sertoli cells) were also estimated.
Semen quality assessment in adult bulls
Sixteen ejaculates (weekly once, for four months) were collected from each adult bull (n = 8) at 8:00 am, using artificial vagina method and microscopically examined for mass activity, individual motility, sperm concentration, percentages of live, acrosome intact and membrane intact spermatozoa.
The mass activity and individual motility was estimated using microscope. The sperm concentration was estimated using the Haemocytometer (improved Neubauer’s chamber) as described by Salisbury et al. (1985). Percentage of live spermatozoa was determined by Eosin-Nigrosin staining (Campbell et al., 1953). Total or partly stained spermatozoa were considered as dead. Percentage of spermatozoa with intact acrosome was evaluated on 1,000× magnification by Giemsa staining method as per procedure given by Watson (1975). The percentage of membrane intact spermatozoa was evaluated using hypo-osmotic swelling test method as described by Jeyandran et al. (1984) using hypo osmotic solution containing sodium citrate and fructose (150 mOsm/L). Sperms with coiled tail were considered as membrane intact sperms and those with straight tail as membrane damaged sperms. For analysing live, acrosome intact and membrane intact spermatozoa, at least 200 sperms per smear were counted using tally counter. All semen evaluations were performed by same person throughout the study.
Statistical analysis
Descriptive statistics were calculated for testosterone concentrations, testicular cells and their indices in different experimental groups and the results are represented as mean ±standard error (SE). One way analysis of variance was performed to compare the testosterone concentrations, testicular cells and their indices in different categories of bulls. Pearson product moment correlation was used for the analysis of correlation among testosterone concentrations, seminal parameters and number of different testicular cells and indices in different categories. When the p value was <0.05, the means were considered to be significantly different between the groups. The analyses were performed using Sigma Plot 11 software package (Systat software Inc., San Jose, CA, USA).
RESULTS
Peripheral blood testosterone concentrations
The peripheral blood testosterone concentrations in animals of different experimental groups are shown in Figure 1. Differences in testosterone concentrations were observed between adult bulls and bull calves (p<0.001) and young and adult bulls (p<0.001). But, there was no difference in testosterone concentrations between bull calves and young bulls.
Figure 1.
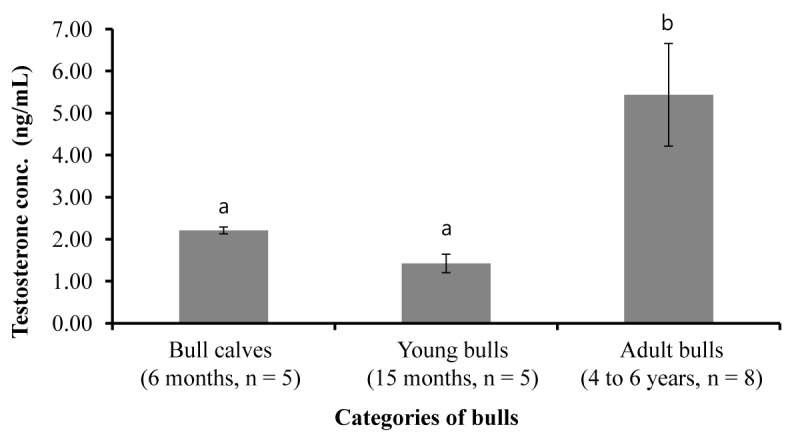
Peripheral blood serum testosterone concentrations (mean±standard error) in crossbred males of different age groups (mean with different superscript differ significantly at p<0.001).
Testicular cells and their indices
Different cells in the testicular aspirate are shown in Figure 2. Different cell counts in testicular fine needle aspiration cytology of bull calves, young bulls and adult bulls are presented in Table 1. The proportion of testicular cells and their indices did not differ between bull calves and young bulls, except for spermatozoa, which were absent in bull calves. But between young and adult bulls, the numbers of spermatocytes and spermatozoa, spermatic index, and sperm-Sertoli cell ratio were different (p<0.01). The proportion of Sertoli cells in bull calves, young bulls and adult bulls did not have significant difference between the groups.
Figure 2.
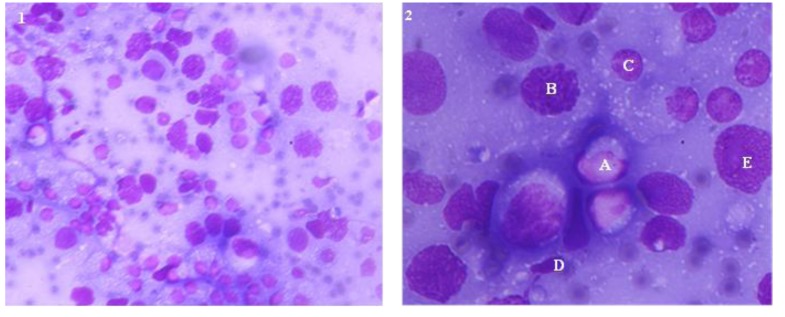
Testicular cytogram at 1) 400× and 2) 1,000× magnifications. Testicular cells were extracted from live bulls using fine needle aspiration and smear was stained withMay-Grunwald Giemsa method. A, Spermatogonia; B, Spermatocyte; C, Round spermatid; D, Elongated spermatid; E, Sertoli cell.
Table 1.
Different testicular cells (%) and their indices in crossbred males of different age groups
| Parameters | Bull calves (6 months; n = 5) | Young bulls (15 months; n = 5) | Adult bulls (4 to 6 years; n = 8) |
|---|---|---|---|
| Testicular cells (%) | |||
| Spermatogonia | 16.6±1.99 | 13.0 ±3.39 | 10.0±0.59 |
| Primary Spermatocytes | 28.4ab±2.46 | 35.8a±2.17 | 23.87b±1.67 |
| Early spermatids | 19.4±2.82 | 19.8±3.15 | 21.50±3.71 |
| Late spermatids | 21.4±1.72 | 13.2±3.72 | 12.50±2.17 |
| Spermatozoa | - | 4.0a±0.77 | 14.0b±2.08 |
| Sertoli cells | 14.2±1.93 | 14.2±2.75 | 18.12±2.82 |
| Testicular cell indices | |||
| Spermatic index | - | 4.99a ±1.10 | 23.02b±4.93 |
| Sertoli cell index | 16.78±2.65 | 17.94±3.98 | 29.91±6.55 |
| Sperm:Sertoli cell ratio | - | 0.31a ±0.05 | 0.78b±0.03 |
| Spermatogenic:Sertoli cell ratio | 6.62±1.09 | 7.10±1.81 | 4.85±1.04 |
| Sertoli cells/100 germ cells | 16.78±2.65 | 17.03±3.75 | 23.18±4.10 |
Means with different superscripts within a row differ significantly between different age groups (p<0.01).
Relationship of testicular cells and indices with testosterone concentration
The correlation between different testicular cell and their indices with peripheral testosterone concentration in different age groups are depicted in Table 2. In bull calves, the proportions of spermatocytes were positively correlated and the spermatogenic:Sertoli cell ratio was negatively correlated with testosterone concentrations (P<0.01). In young bulls, the number of Sertoli cells per 100 germ cells was negatively correlated with testosterone concentration (p<0.01). However, there was no significant relationship between any of the testicular cells and their indices with testosterone concentrations in adult bulls.
Table 2.
Correlation coefficients for association of testosterone concentrations with testicular cell number and indices in crossbred bulls of different age groups
| Testicular cells and indices | Testosterone concentration (ng/mL) | ||
|---|---|---|---|
|
| |||
| Bull calves (6 months; n = 5) | Young bulls (15 months; n = 5) | Adult bulls (4 to 6 years; n = 8) | |
| Spermatogonia | −0.146 | 0.028 | −0.182 |
| Primary spermatocytes | 0.719* | 0.006 | 0.193 |
| Early spermatids | −0.679 | −0.014 | 0.110 |
| Late spermatids | −0.062 | −0.018 | 0.108 |
| Spermatozoa | - | −0.069 | 0.135 |
| Sertoli cells | 0.269 | 0.019 | −0.072 |
| Spermatic index | - | −0.070 | −0.071 |
| Sertoli cell index | 0.258 | 0.016 | −0.053 |
| Sperm:Sertoli cell ratio | - | −0.064 | 0.008 |
| Spermatogenic:Sertoli cell ratio | −0.371* | −0.006 | 0.134 |
| Sertoli cells/100 germ cells | 0.258 | −0.713* | −0.056 |
Indicates significant relationship between a pair of variables (p<0.001).
Relationship between testosterone concentration and seminal characteristics
The mean (±SE) of mass activity, individual motility (%), viability (%), acrosomal integrity (%), and membrane intact spermatozoa (%) were 2.38±0.31, 55.61±4.16, 68.79±4.95, 97.44±0.19, and 41.69±2.93, respectively. The relationship between blood testosterone concentrations and seminal attributes of bulls are given in Table 3. The peripheral blood testosterone concentrations were positively correlated with ejaculate volume and negatively correlated with individual motility (p<0.05). The other semen parameters studied did not have any sensible correlation with blood testosterone concentrations.
Table 3.
Correlation coefficients for association of testosterone concentrations and seminal characteristics in adult bulls (n = 8)
| Parameters | Serum testosterone concentration |
|---|---|
| Volume (mL) | 0.790* |
| Mass activity (0 to 5 scale) | −0.478 |
| Individual motility (%) | −0.711* |
| Live spermatozoa (%) | −0.459 |
| Sperm concentration (106/mL) | −0.443 |
| Membrane intact spermatozoa (%) | −0.528 |
| Acrosome integrity (%) | −0.401 |
Indicates significant relationship between a pair of variables (p<0.05).
Relationship of testicular cells and indices with seminal parameters
The relationship among testicular cells, their indices and seminal parameters in adult bulls are presented in Table 4. The proportion of spermatogonia in testicular cytology had positive correlation with ejaculate volume (p<0.01), mass activity (p<0.05) and individual motility (p<0.05) of spermatozoa. The proportion of spermatocytes in testicular cytology had negative correlation with mass activity (p<0.01) and individual motility, but the proportion of early spermatids had significant negative correlation (p<0.01) with volume, mass activity, individual motility and viability of spermatozoa. Both, the proportion of spermatozoa and Sertoli cells were positively correlated with mass activity (p<0.001), individual motility (p<0.001), sperm concentration (p<0.05) and membrane integrity (p<0.001). However, the viability of spermatozoa had positive correlation with the number of Sertoli cells (p<0.001). Among the testicular cell indices, spermatic and Sertoli cell index had positive correlation with mass activity (p<0.001), individual motility (p<0.001), sperm concentration (p<0.05) and membrane integrity (p<0.001). The spermatic index also had positive correlation with viability of spermatozoa. Sperm:Sertoli cell ratio and spermatogenic to Sertoli cell ratio had negative correlation with mass activity (p<0.001), individual motility (p<0.001), viability (p<0.001) and membrane integrity (p<0.005) of spermatozoa, but spermatogenic to Sertoli cell ratio had negative correlation with sperm concentration (p<0.05).
Table 4.
Correlation coefficients for association of testicular cell number and indices with seminal parameters in adult bulls
| Testicular cell and indices | Seminal parameters | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Volume | Mass activity | Individual motility | Viability | Sperm concentration | Acrosome integrity | Membrane integrity | |
| Spermatogonia | 0.327* | 0.219* | 0.221* | −0.010 | 0.094 | 0.066 | 0.047 |
| Spermatocytes | 0.014 | −0.420* | −0.348* | −0.075 | −0.092 | −0.057 | −0.162 |
| Early spermatid | −0.238* | −0.347* | −0.318* | −0.123* | 0.052 | 0.028 | −0.237* |
| Late spermatid | −0.030 | −0.076 | −0.168 | −0.042 | −0.069 | 0.076 | −0.145 |
| Spermatozoa | −0.025 | 0.730* | 0.706* | −0.042 | 0.234* | 0.140 | 0.379* |
| Sertoli cells | −0.003 | 0.772* | 0.738* | 0.292* | 0.206* | 0.142 | 0.428 * |
| Spermatic index | −0.028 | 0.728* | 0.704* | 0.228* | 0.255* | 0.124 | 0.381 * |
| Sertoli cell index | −0.018 | 0.752* | 0.725* | 0.269 | 0.213* | 0.122 | 0.417* |
| Sperm:Sertoli cell ratio | −0.081 | −0.314* | −0.253* | −0.212* | 0.016 | −0.080 | −0.210* |
| Spermatogenic:Sertoli cell ratio | −0.041 | −0.770* | −0.722* | −0.275 * | −0.220 * | −0.181* | −0.387* |
| Sertoli cell/100 germ cell | −0.013 | 0.760* | 0.731* | 0.287* | −0.200* | 0.132 | 0.427* |
Indicates significant relationship between a pair of variables (p<0.001).
DISCUSSION
Subfertility in breeding bulls is a serious problem limiting the success of artificial breeding programme in cattle. Specifically, the magnitude of fertility problems is very high in crossbred bulls. Several workers reported that a considerable proportion of crossbred bulls produced inferior quality semen and were culled from the semen station (Sudheer and Xavier, 2000; Mathur et al., 2002; Mukhopadhyay et al., 2010; Khatun et al., 2013). The reasons for high incidence of infertility in crossbred bulls are yet to be identified. Since the quality of spermatozoa is determined by the environment at the seminiferous tubules, we hypothesized that the proportion of spermatogenic cells and Sertoli cells would reflect the quality of semen produced. The present study assessed the testicular cytology and peripheral testosterone concentrations in crossbred bull calves, young and adult bulls and studied the relationship among these parameters.
Among the different spermatogenic cells studied, the proportion of spermatocytes, spermatozoa, spermatic index and sperm:Sertoli cell ratio differed between young and adult bulls. However, the proportion of Sertoli cells did not differ significantly between various age groups. It has been reported that proliferation of Sertoli cells seize to divide after puberty (Yang and Han, 2010) and our finding confirm these observations. Spermatozoa were not observed in the testicular cytology of bull calves indicating that sperm formation did not start at six months age in crossbred bulls. However, the numbers of early and late spermatids in the testicular cytology of bull calves were almost similar to that of young and adult bulls. This indicates that the spermatogenesis had already started in the bull calves but transformation of spermatids to spermatozoa had not started. Among different testicular cytology indices, the spermatic index corresponds to the end products of final maturation step and spermatogenesis. The spermatic index observed in present study was lower than reported earlier in other species viz. stallion (31.5±8.5; Leme and Papa, 2000), dog (26±3.8; Santos et al., 2010) and men (34±13.3; Foresta and Varatto, 1992).
Thereafter we analysed the relationship of testicular cells and their indices with semen quality in adult crossbred bulls. To the best of our knowledge, this study was first of its kind in crossbred bulls to report the relationship between testicular cells and their indices with seminal parameters. Among different testicular cells, the proportion of Sertoli cells in the testicular cytology was found to be significantly related to several semen quality parameters. Similarly, the indices related to the Sertoli cells also had strong relationship with semen quality parameters indicating the significance of Sertoli cells in maintaining semen quality. Several researchers reported that number of Sertoli cells is highly correlated to sperm production ability of animals (Russell and Peterson, 1984; França and Russell, 1998; Leal et al., 2004) and specifically in humans (Hentrich et al., 2011) and horses (Sharpe et al., 2003). The functional efficiency of Sertoli cells can be recognized from the number of germ cells nourished by single Sertoli cell, which is also highly correlated to indicator of sperm production efficiency (Russell and Peterson, 1984; França and Russell, 1998). We also found that Sertoli cells per 100 germ cells had a positive relationship with mass activity, individual motility; viability and membrane integrity of spermatozoa.
Testosterone is primarily responsible for the maintenance of secondary sexual characteristics and libido, which promotes quality sperm production in bulls (Hafez, 2000). Several studies revealed that poor semen quality has been associated with deficiency in circulating testosterone concentrations (Schallenberger et al., 1991; Gulia et al., 2010). The concentrations of testosterone observed in the study were higher than those reported earlier in Holstein bull calves (0.49±0.66 ng/mL; Gholami et al., 2010), but comparable with other reports in crossbred young bulls (1.96 to 4.08 ng/mL; Santra et al., 1999) and crossbred adult bulls (0.44 to 5.60 ng/mL; Kumar et al., 2006). Few studies reported that testosterone concentration in males increase with advancing age (Holstein bulls, Gholami et al., 2010; crossbred bulls, Gulia et al., 2010). Even though the testosterone concentrations observed in the present study falls within the range of previous observations, we did not find any proportionate change in testosterone concentrations in different age groups of bulls. This could likely be attributed to variation between bulls or breeds. In consensus to our observations, Rawlings et al. (1972) also reported large variation in the androgen secretion among bulls within ages and reported that testosterone concentration dropped after 11 months of age. Since the role of testosterone on the spermatogenic process is well established, it is expected that the testosterone concentrations are associated with semen quality (Javed et al., 2000). However, results of the present study did not support their findings. Earlier studies also reported that the circulating blood testosterone concentrations are not related to libido (Schallenberger et al., 1991; Sekasiddhi and Buban, 1997) and semen quality (Souza et al., 2011) or have only low correlation with mass activity and motility (Santos et al., 2004; Souza et al., 2011). Since testosterone concentration varies with many factors like species, breed, individual, age, season, environmental conditions and time, frequent estimation of testosterone in short intervals taken with objective measures of other traits may be necessary to accurately assess relationships of testosterone concentrations with seminal characteristics.
CONCLUSION
Since the number of Sertoli cells and Sertoli cell index showed a strong relationship with semen quality, these parameters can be used to assess the quality semen production potential of the adult crossbred bulls. Since there were no significant changes in these indices among bulls of different age groups, they can be used in combination with other routine parameters to select high performance breeding bulls at younger ages, but validation of this assertion with a larger sample size will be needed before final proposition. The relationship of testosterone concentrations with semen quality was not significant indicating that determination of testosterone profile alone may not predict the quality semen production ability in crossbred bulls.
ACKNOWLEDGMENTS
The authors are thankful to the Director, NDRI, Karnal for providing the facilities to conduct the research. The first author was a recipient of Junior Research Fellowship from ICAR, New Delhi. The project was funded by World bank supported NFBSFARA project (NFBSFARA/BS-3009) and National Dairy Research Institute, ICAR.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Campbell RG, Hancock JC, Rothschild L. Counting live and dead bull spermatozoa. J Exp Biol. 1953;30:44–49. [Google Scholar]
- Chapwanya A, Callanan J, Larkin H, Keenan L, Vaughan L. Breeding soundness evaluation by semen analysis, testicular fine needle aspiration cytology and trans-scrotal ultrasonography. Irish Vet J. 2008;61:315–318. doi: 10.1186/2046-0481-61-5-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase CC, Chenoweth PJ, Larsen RE, Olson TA, Hammond AC, Menchaca MA, Randel RD. Growth and reproductive development from weaning through 20 months of age among breeds of bulls in subtropical Florida. Theriogenology. 1997;47:723–745. doi: 10.1016/s0093-691x(97)00030-7. [DOI] [PubMed] [Google Scholar]
- Foresta C, Varotto A. Assessment of testicular cytology by fine needle aspiration as a diagnostic parameter in the evaluation of the oligospermic subject. Fertil Steril. 1992;58:1028–1033. doi: 10.1016/s0015-0282(16)55455-7. [DOI] [PubMed] [Google Scholar]
- Franca LR, Russell LD. The testis of domestic animals. In: Martínez-Garcia F, Regadera J, editors. Male Reproduction: A Multidisciplinary Overview. Churchill Livingstone; Madrid, Spain: 1998. pp. 197–219. [Google Scholar]
- Gholami H, Towhidi A, Shahneh Z, Dirandeh E. The relationship of plasma leptin concentration and puberty in Holstein bull calves (Bos taurus) J. Anim. Physiol. Anim. Nutr. (Berl.) 2010;94:797–802. doi: 10.1111/j.1439-0396.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- Goeritz F, Quest M, Wagener A, Fassbender M, Broich A, Hildebrandt, Hildebrandt TB, Hofmann RR, Blottner S. Seasonal timing of sperm production in roe deer: interrelationship among changes in ejaculate parameters, morphology and function of testis and accessory glands. Theriogenology. 2003;59:1487–1502. doi: 10.1016/s0093-691x(02)01201-3. [DOI] [PubMed] [Google Scholar]
- Gulia S, Sarkar M, Kumar V, Meyer HH, Prakash BS. Divergent development of testosterone secretion in male zebu (Bos indicus) and crossbred cattle (Bos indicus×Bos taurus) and buffaloes (Bubalus bubalis) during growth. Trop Anim Health Prod. 2010;42:1143–1148. doi: 10.1007/s11250-010-9538-x. [DOI] [PubMed] [Google Scholar]
- Hafez ESE. Reproduction in Farm Animals. 7th Edition. Lippincott Williams and Wilkins; 2000. pp. 293–397. [Google Scholar]
- Hentrich A, Wolter M, Szardening-Kirchner C, Lüers GH, Bergmann M, Kliesch S, Konrad L. Reduced numbers of Sertoli, germ, and spermatogonial stem cells in impaired spermatogenesis. Mod Pathol. 2011;24:1380–1389. doi: 10.1038/modpathol.2011.97. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Palladino MA, Rudolph D, Lan ZJ, Labus JC. The role of epididymis in the protection of spermatozoa. Curr Top Dev. 1996;33:61–102. doi: 10.1016/s0070-2153(08)60337-3. [DOI] [PubMed] [Google Scholar]
- Javed MT, Khan A, Ali M. Influnce of season on seminal plasma testosterone and oesterogen in healthy and abnormal buffalo bulls and their relationship with other semen parameters. Vet Arhiv. 2000;70:141–149. [Google Scholar]
- Jeyendran RS, Vander-Ven HH, Perez PM, Crabo BG, Zaneveld LJD. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–228. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- Johnson L. Spermatogenesis. In: Cupps PT, editor. Reproduction in Domestic Animals. Fourth ed. Academic Press Inc; San Diego, CA, USA: 1991. pp. 173–219. [Google Scholar]
- Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O’Shaughnessy PJ. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- Khatun M, Kaur S, Kanchan, Mukhopadhyay CS. Subfertility problems leading to disposal of breeding bulls. Asian Australas J Anim Sci. 2013;26:303–308. doi: 10.5713/ajas.2012.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Verma RP, Singh LP, Varshney VP, Dass RS. Effect of different concentrations and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone concentration in crossbred cattle (Bos indicus×Bos taurus) bulls. Reprod Nutr Dev. 2006;46:663–675. doi: 10.1051/rnd:2006041. [DOI] [PubMed] [Google Scholar]
- Leal MC, Becker-Silva SC, Chiarini-Garcia H, França LR. Sertoli cell efficiency and daily sperm production in goats (Capra hircus) Anim Reprod. 2004;1:122–128. [Google Scholar]
- Leme DP, Papa FO. Cytologic identification and quantification of testicular cell types using fine needle aspiration in horses. Equine Vet J. 2000;32:444–446. doi: 10.2746/042516400777591156. [DOI] [PubMed] [Google Scholar]
- Leme DP, Papa FO. How to perform and interpret testicular fine needle aspiration in stallion. J Equine Vet Sci. 2010;30:590–596. [Google Scholar]
- Mathur AK, Tyagi S, Singh SP. Frieswal bull - an experience of HF with Sahiwal. J Livest Poult Prod. 2002;18:21–23. [Google Scholar]
- McLachlau RI, Wreford NG, O’Donnell L, de Kretser DM, Robertson DM. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol. 1996;148:1–9. doi: 10.1677/joe.0.1480001. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay CS, Gupta AK, Yadav BR, Khate K, Raina VS, Mohanty TK, Dey PP. Subfertility in males: an important cause of bull disposal in bovines. Asian Australas J Anim Sci. 2010;23:450–455. [Google Scholar]
- Post TB, Bindon BM. Plasma luteinizing hormone and testosterone concentrations in different breeds of young beef bulls in the tropics. Aust J Biol Sci. 1983;36:545–550. doi: 10.1071/bi9830545. [DOI] [PubMed] [Google Scholar]
- Rajak SK, Tripathi UK, Attupuram NM, Boro P, Layek SS, Aslam MKM, Kumaresan A, Mohanty TK, Sreela L, Prakash MA. Relationship of blood and seminal plasma testosterone concentrations with semen quality in crossbred bulls. Indian J Dairy Sci. 2014;67:162–167. [Google Scholar]
- Rajak SK, Kumaresan A, Gaurav MK, Aslam MKM, Mohanty TK, Prasad S, Chakravarty AK, Venkatasubramanian V. Testicular biometry and semen quality is not altered by the process of fine needle aspiration in crossbred bulls. Indian J Anim Sci. 2013;83:732–735. [Google Scholar]
- Rawlings NC, Hafs HD, Swanson LV. Testicular and blood plasma androgens in Holstein bulls from birth through puberty. J Anim Sci. 1972;34:435–440. doi: 10.2527/jas1972.343435x. [DOI] [PubMed] [Google Scholar]
- Robaire B, Viger RS. Regulation of epdidymal epithelial cell function. Biol Reprod. 1995;52:226–236. doi: 10.1095/biolreprod52.2.226. [DOI] [PubMed] [Google Scholar]
- Russell LD, Peterson RN. Determination of the elongate spermatid-Sertoli cell ratio in various mammals. J Reprod Fertil. 1984;70:635–641. doi: 10.1530/jrf.0.0700635. [DOI] [PubMed] [Google Scholar]
- Salisbury GW, VanDenmark NL, Lodge JR. Physiology of Reproduction and Artificial Insemination of cattle. 2nd edition. CBS Publ. Dist; New Delhi, India: 1985. [Google Scholar]
- Santos M, Marcos R, Canictti M. Cytologic study of normal canine testis. Theriogenology. 2010;73:208–214. doi: 10.1016/j.theriogenology.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Santos MD, Torres CAA, Ruas JRM, Guimaraes JD, Filho JMS. Reproductive potential of Nelore bulls submitted to different bull:cow proportion. Arq Bras Med Vet Zootec. 2004;56:497–503. [Google Scholar]
- Santra A, Agrwal N, Kamara DN, Pathak NN. Effect of level of concentrate supplement on blood biochemical changes and testosterone level in crossbred (Bos indicus×Bos Taurus) calves. Asian Australas J Anim Sci. 1999;12:881–885. [Google Scholar]
- Schallenberger E, Hartl P, Schams D, Lorrmann W, Hahn R. Hormone profiles and fertility in bulls. Tierzuchter. 1991;43:402–403. [Google Scholar]
- Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, DeGendt K, Verhoeven G, O’Shaughnessy PJ, Sharpe RM. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148:2027–2036. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Jobling MS, McKinnell C, Drake AJ, Sharpe RM. Relationship between androgen action in the ‘male programming window’, fetal Sertoli cell number and adult testis size in the rat. Endocrinology. 2008;149:5280–5287. doi: 10.1210/en.2008-0413. [DOI] [PubMed] [Google Scholar]
- Sekasiddhi P, Buban S. Hormone profiles in low libido bulls. J Thai Vet Med Asso. 1997;48:43–49. [Google Scholar]
- Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Phil. Trans. R. Soc. B. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Singh A. Crossbreeding of cattle for increasing milk poduction in India: A Review. Indian J Anim Sci. 2005;75:383–390. [Google Scholar]
- Souza LWO, Andrade AFC, Celeghini ECC, Negrão JA, de-Arruda RP. Correlation between sperm characteristics and testosterone in bovine seminal plasma by direct radio immunoassay. R Bras Zootec. 2011;40:2721–2724. [Google Scholar]
- Sudheer S, Xavier CJ. Disposal pattern of breeding bulls in Kerala. Ind J Anim Reprod. 2000;21:72–73. [Google Scholar]
- Suresh R, Medhamurthy R, Moudgal NR. Comparative studies on the effects of specific immunoneutralisation of endogenous FSH or LH on testicular germ cell transformation in the adult bonnet monkey (Macaca radiata) Am J Reprod Immunol. 1995;34:35–43. doi: 10.1111/j.1600-0897.1995.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Tuli RH, Lohan IS, Goyal RL, Singal SP. Testosterone and progesterone hormones in buffalo bull seminal plasma and their correlations with seminal characteristics. Indian J Dairy Sci. 1991;44:587–589. [Google Scholar]
- Vijetha BT, Layek SS, Kumaresan A, Mohanty TK, Gupta AK, Chakravarty AK, Manimaran A, Prasad Shiv. Effects of pedigree and exotic genetic inheritance on semen production traits of dairy bulls. Asian Pac J Reprod. 2014;3:13–17. [Google Scholar]
- Watson PF. Use of Giemsa stain to detect changes in acrosome of frozen ram spermatozoa. Vet Rec. 1975;97:12–15. doi: 10.1136/vr.97.1.12. [DOI] [PubMed] [Google Scholar]
- Wolfe F. Thesis. Tierarztliche Hochschule; Hannover: 1996. Biochemical and endocrinological parameters as indicators of the freezability of the semen of bulls of different ages. [Google Scholar]
- Yang Y, Han C. GDNF stimulates the proliferation of cultured mouse immature Sertoli cells via its receptor subunit NCAM and ERK1/2 signalling pathway. BMC Cell Biol. 2010;11:78. doi: 10.1186/1471-2121-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]


