Abstract
Hemocompatibility of tissue-engineered vascular grafts remains a major hurdle to clinical utility for small-diameter grafts. Here we assessed the feasibility of using autologous blood outgrowth endothelial cells to create an endothelium via lumenal seeding on completely biological, decellularized engineered allografts prior to implantation in the sheep femoral artery. The 4-mm-diameter, 2- to 3-cm-long grafts were fabricated from fibrin gel remodeled into an aligned tissue tube in vitro by ovine dermal fibroblasts prior to decellularization. Decellularized grafts pre-seeded with blood outgrowth endothelial cells (n=3) retained unprecedented (>95 %) monolayer coverage 1 h post-implantation and had greater endothelial coverage, smaller wall thickness, and more basement membrane after 9-week implantation, including a final week without anti-coagulation therapy, compared with contralateral non-seeded controls. These results support the use of autologous blood outgrowth endothelial cells as a viable source of endothelial cells for creating an endothelium with biological function on decellularized engineered allografts made from fibroblast-remodeled fibrin.
Keywords: Endothelial cells, Vascular graft, Tissue engineering
Introduction
A primary limitation of current tissue-engineered small-diameter arteries (tissue-engineered vascular grafts, TEVG) is propensity for thrombosis following implantation. The use of anti-coagulation therapies for the duration of study is thus common. To eliminate the need for sustained anti-coagulation, a number of strategies are being investigated for imparting anti-thrombogenic functionality to the lumenal surface prior to implantation [1–5]. A recent study performed in the porcine model demonstrates clear benefit of pre-seeding endothelial cells (EC) [1]: patency was substantially improved and neo-intima formation was attenuated with both autologous vessel EC and circulating endothelial progenitor cells (EPC). Use of autologous blood outgrowth endothelial cells (BOEC, also referred to as late-outgrowth EPC), presents a uniquely advantageous strategy for fabricating TEVG with anti-thrombogenic functionality [6, 7]. These cells can be isolated from a small amount of blood obtained via routine venipuncture and expanded extensively (to 1019 cells) [6], including some patients with coronary artery disease [8]. BOEC also tolerate cryopreservation [9] and retain phenotypic and gene expression stability at high-fold expansion [10].
In this study, we evaluated decellularized TEVG fabricated from allogeneic fibroblasts (a completely biological cell-produced extracellular matrix) that were seeded with autologous BOEC prior to implantation in the ovine femoral artery. A pulsatile flow loop was used to determine a shear pre-conditioning regimen that yielded monolayer retention under simulated femoral artery blood flow. Some grafts were removed after 1 h of implantation to assess any acute loss of BOEC. In order to assess hemocompatibility and anti-thrombogenic functionality of the seeded BOEC in the grafts implanted for 9 weeks, all anti-coagulation therapy was terminated at 8 weeks, preceding the final week of implantation. The grafts were monitored with ultrasonography and characterized with cellular, biochemical, and biomechanical analyses at implantation and explantation.
Methods
BOEC Isolation and Characterization
Autologous BOEC were isolated using a modified method described by Lin et al., 2000. Briefly, 100 ml of heparinized (10 U/ml) blood was acquired via venipuncture from juvenile sheep destined for BOEC-seeded graft implantation. The heparinized blood was diluted 1:1 with 37 °C Hanks Balanced Salt Solution (HBSS) and layered over Ficoll-Paque Premium (d=1.077 g/ml, GE Healthcare 17-5442-02). Following centrifugation at 400×g for 30 min, mononuclear cell fractions were isolated and washed three times with warm HBSS. The remaining pellet was resuspended in EGM-2 (Lonza cc-3156) supplemented with an extra 8 % fetal bovine serum (FBS), and 100 U/ml penicillin, 100 μg/ml streptomycin and plated into 6-well plates coated with 50 ug/ml collagen I. This medium was changed daily for 1 week and every other day thereafter until colonies appeared, approximately 2–3 weeks, at which point colonies were subcultured and replated at 104/cm2. BOEC were passaged at 90 % confluence and used for seeding at passage 7. An endothelial phenotype was verified via low-density lipoprotein (LDL) uptake (Biomed Tech Inc BT-902), expression of VE-Cadherin (Santa Cruz SC-6458) and vWF (Abcam ab6994), and negative CD45 expression (US Biological C2399-07B). Antithrombogenicity of each BOEC isolation in monolayer culture was confirmed using a washed-platelet adhesion assay. Briefly, platelet rich plasma (PRP) was isolated from citrated ovine whole blood via centrifugation. Platelets were pelleted from the PRP via centrifugation, resuspended in Tyrode's solution, and incubated for 2 h at 37 °C with BOEC from each isolation, with a segment of graft serving as a positive control. Following this, each sample was washed to remove unbound platelets, fixed for 30 min in 1 % paraformaldehyde, and immunostained for GpIIb/IIIa (VMRD CAPP2A). Platelet coverage was quantified using ImageJ (NIH).
BOEC Seeding of TEVG
Grafts were made from fibrin gel tubes remodeled into collagenous tissue by allogeneic dermal fibroblasts as previously described [11] and then decellularized via sequential treatment with 1 % sodium dodecyl sulfate (SDS, Sigma) in distilled water for 6 h, 1 % Triton X-100 (Sigma) in distilled water for 30 min, and deoxyribonuclease enzyme (Worthington Biochemical) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % FBS for 4 h [12]. They were stored at 4 °C in sterile phosphate buffered saline (PBS) for 5 months prior to use, during which time there was no change in burst pressure (4200±180 mmHg and 4370±70 mm Hg before and after storage, respectively). The decellularized grafts were mounted in a custom cell-seeding apparatus and incubated overnight in EGM-2 medium. Graft lumens were treated for 4 h with 50 μg/ml bovine fibronectin (Sigma F1141). A BOEC suspension of 107/ml was injected into graft lumens (a total of ∼5×106 cells) and rotated at 0.3 rpm for 2 h. Following rotation, grafts were incubated statically for 60 h prior to implantation with medium changes every 24 h. A flow-conditioning period was employed 1.5 h before surgery during which the seeded grafts were mounted in a system designed for subjecting grafts to pulsatile fluid flow via a reciprocating syringe pump since this maximized BOEC retention in vitro. Physiological shear stresses (25 dyne/cm2 peak) were attained by steadily increasing the pulsation frequency over 30 min to 1.5 Hz while maintaining a constant stroke volume. Following flow-conditioning, the BOEC-seeded grafts were transported to the surgical suite for implantation.
TEVG Mechanical Properties Measurement
TEVG were mounted in a system designed for pressurizing individual grafts to failure. Each graft was cannulated and tested for compliance and burst strength as described previously [11]. Additionally, TEVG samples were tested for tensile properties as described previously [11].
Implant Procedures
All protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). Surgeries were performed by the University of Minnesota Experimental Surgical Services. Both acellular grafts and autologous BOEC-seeded grafts were evaluated in a total of three animals utilizing both femoral arteries. Animals were sedated with 0.04 mg/kg intramuscular atropine and 10 mg/kg intramuscular ketamine. Following sedation, a jugular vein IV was placed and animals were anesthetized with 10–12 mg/kg intravenous propofol and maintained on isoflurane during surgery. Interpositional femoral artery implants were performed in all cases. Grafts were secured using running 7–0 prolene sutures. To evaluate the utility of seeding autologous BOEC on the graft lumenal surface, a 9-week study duration was employed with a subset of seeded grafts harvested at 1 h post-implantation to evaluate the impact of surgical manipulation and physiological blood flow on BOEC retention. A total of six BOEC-seeded grafts were implanted into three sheep: n=3 grafts harvested at 1 h, replaced with n=3 grafts harvested at 9 weeks. In each case of a 9-week implant, the contralateral femoral artery contained a non-seeded graft (n=3) for comparison. Endothelial retention of 1 h explants was quantified with en face staining for F-actin and nuclei. Endothelial coverage was averaged along the length of each graft explanted at 1 h. Animals were maintained on daily subcutaneous injections of enoxaparin (1 mg/kg) beginning immediately post-operatively, as well as aspirin (80 mg/day) and clopidogrel (75 mg/day) beginning 3 days prior to surgery. Starting at day 8, enoxaparin was removed and animals were maintained on only aspirin and clopidogrel until the end of the eighth week at which point anti-coagulation therapy was ceased. Animals were sacrificed at 9 weeks. Ultrasound analysis was performed at 1 and 8 weeks.
Explant Analysis
Grafts were cut into ring sections and were imaged. The two end rings and mid-graft ring were fixed for histological analyses, and the remainder of the rings were distributed for SEM, mechanical, and biochemical analyses.
Histology
Grafts pre-implant and post-explant were fixed in 4 % paraformaldehyde, embedded in OCT (Tissue-Tek), and frozen in liquid N2. Sections of 9-μm thickness were stained with Lillie's trichrome. Samples for SEM imaging were fixed as described previously [13] and were imaged using a Hitachi S-4700 Scanning Electron Microscope at 3.0 kV.
Immunostaining
Histological sections of grafts were stained for αSMA (Sigma, A5228), vWF (Abcam ab6994), VE-cadherin (Santa Cruz sc-6458), CD45 (US Biological C2399-07B), GpIIb/IIIa (VMRD CAPP2A), laminin (Abcam ab11575), fibronectin (Abcam ab6584), and collagen IV (Abcam ab6586).
All samples were blocked with 5 % normal donkey serum, incubated in primary antibody at 5 ug/ml and stained with a Cy2-, 3-, or 5-conjugated, species-matched secondary antibody (Jackson Immunoresearch) [11]. Nuclei were counter-stained with Hoechst 33342 (Invitrogen H3570). Platelet-endothelial co-localization was calculated as the area fraction of vWF staining on the lumenal surface that co-stained for GpIIb/IIIa for mid-graft sections.
Statistical Analyses
For all experiments, statistical significance of differences between groups was determined using Student's t-test for two treatments and one-way ANOVA for more than two treatments with the Tukey post hoc test in GraphPad Prism® software for Windows. Any reference to a difference in the Results and Discussion sections implies statistical significance at the level p<0.05. In all cases, where the difference was significant, symbols are used to indicate the difference and explained in the figure captions.
Results
BOEC and Non-Seeded TEVG
BOEC isolated from multiple sheep were positive for expression of VE-cadherin (Fig. 1a), von Willebrand Factor (vWF, Fig. 1b), and LDL uptake (Fig. 1c). Endothelialized grafts were evaluated in vitro for tensile properties in comparison to acellular grafts. Tensile properties were unchanged from the BOEC seeding (UTS: 656±77kPa vs. 707±46 kPa; modulus: 1,333±65 kPa vs. 1,320±138 kPa; n=3). Control grafts displayed 30 % platelet coverage whereas a BOEC monolayer displayed platelet coverage <1 % (Fig. 1e–g).
Fig. 1.
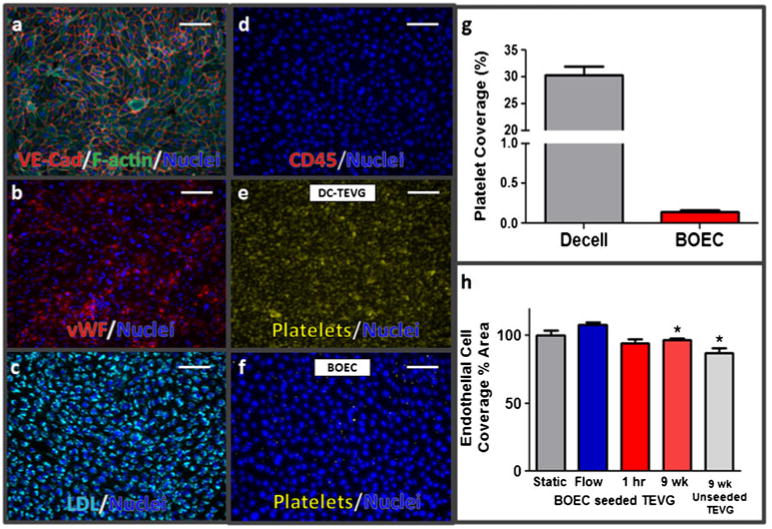
BOEC and BOEC-seeded TEVG characterization. BOEC were positive for a VE-cadherin, b von Willebrand factor, c LDL uptake, and negative for d. CD45 (scale bar=200 μm). An in vitro platelet adhesion assay comparing e non-seeded grafts to f BOEC in monolayer culture and g area percentage with platelet coverage. Endothelial retention under flow (h) was quantified using en face staining of 1-h implants (1 h) and compared with statically incubated (Static) and flow-conditioned (Flow) pre-implantation samples, as well as explanted endothelial coverage quantified from vWF staining of histological cross-sections (‘9 week’, and ‘9 week unseeded TEVG’ in ‘h.’)
In Vitro Shear Flow Behavior of BOEC-seeded TEVG
BOEC shear resistance was evaluated by quantifying graft surface coverage before and after subjecting seeded grafts to a supraphysiological pulsatile shear stress (40 dyn/cm2 peak shear stress at 1.5 Hz) for 1 h. Coverage was also evaluated following surgical manipulation, implantation, and femoral blood flow for 1 h. BOEC surface coverage normalized to unsheared controls remained unchanged between statically incubated (100±4 %), in vitro sheared (108±2 %), and 1 h in vivo (95±3 %) (Fig. 1h). When BOEC coverage was quantified and averaged along the length of the 1 h implanted grafts and compared with pre-implantation coverage values, no difference was found.
BOEC-seeded TEVG Remodeling in vivo
Basement membrane deposition was evaluated on BOEC-seeded grafts and non-seeded contralateral control grafts at the time of implantation and at the 9-week explantation. BOEC seeding led to a qualitatively increased presence of basement membrane components at implantation compared with control grafts, and at the 9-week explantation, basement membrane deposition was markedly elevated in BOEC-seeded grafts as indicated through substantial elevation in the observed deposition of the basement membrane components fibronectin, collagen IV, and laminin (Fig. 2a–c). Grafts were evaluated by ultrasonography at 1 and 8 weeks with no indication of aneurysm. At 8 weeks, there was a reduction in diameter at the anastomoses of the control grafts relative to the mid-graft region (2.3±0.2 vs. 3.4±0.3 mm); however, the BOEC-seeded grafts did not exhibit a decrease in diameter (2.7±0.5 vs. 3.8±0.6 mm).
Fig. 2.
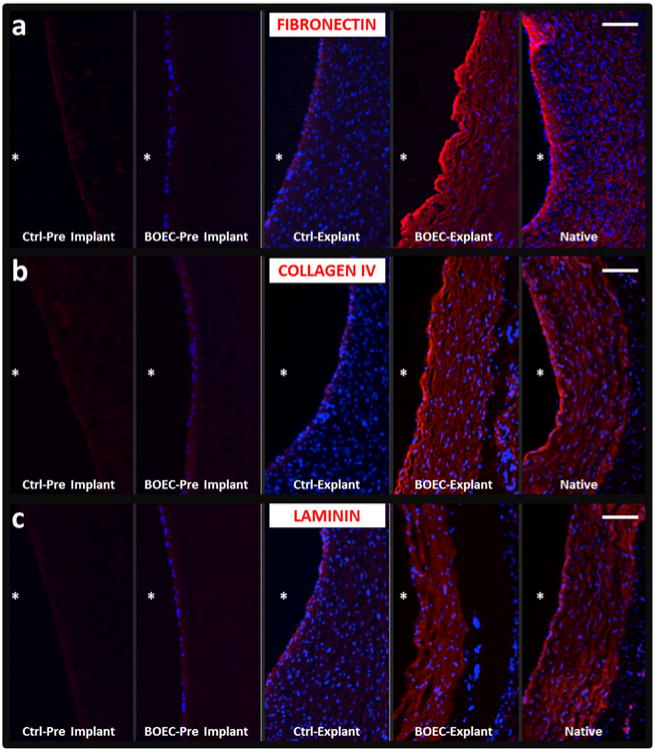
Basement membrane staining of BOEC-seeded vs. control grafts with comparison to native vessels. Cryosections were evaluated for evidence of deposition of the basement membrane proteins a fibronectin, b type-IV collagen, and c laminin. Immunostaining of acellular grafts is shown in the first column (Ctrl pre-implant), BOEC-seeded grafts at the time of implant are shown in the second column (BOEC pre-implant), 9-week explanted acellular control TEVG (Ctrl-Explant) and BOEC-seeded explanted TEVG (BOEC-Explant) are shown in the third and fourth columns, respectively, and immunostained sections of native femoral artery are shown in the final column. Scale bars= 100 μm and Asterisk indicates the lumenal surface
Post-explantation, the grafts were evaluated grossly, with BOEC-seeded grafts exhibiting a markedly thinner graft wall (Fig. 3a). Wall thicknesses for BOEC-seeded grafts (0.55±0.07 mm) were substantially less than the control grafts (0.92±0.08 mm) (Fig. 3b). Both the UTS and modulus of BOEC-seeded grafts were higher than control grafts (Fig. 3c, d). Trichrome staining indicated a substantially thinner cellular region towards the lumenal surface of BOEC-seeded grafts in comparison to control grafts (Fig. 4a, b). The infiltrating cells were predominantly αSMA+ (Fig. 4c, d). There were no remarkable differences in the inflammatory response between the BOEC-seeded and non-seeded control grafts based on CD45 immunostaining (Fig. 4e, f). Gross clotting was not observed in any of the implanted grafts. Immunostaining for vWF at multiple regions along the length of each graft (Fig. 4g, h) indicated higher endothelial coverage on BOEC-seeded grafts (96.7±1.5 %) compared with control grafts (86.9±3.6 %). When analyzed for platelet-endothelial co-localization, histological sections of explanted grafts revealed no difference in platelet binding between BOEC-seeded and control grafts (0.014 %+/−0.01 % and 0.009 % +/−0.01 %, respectively). SEM revealed regions of complete endothelial coverage in BOEC-seeded grafts (Fig. 5a) exhibiting tight junctions (Fig. 5b). There were also sparse regions with exposed graft matrix where platelets had bound (Fig. 5c), which was also true for the control grafts.
Fig. 3.
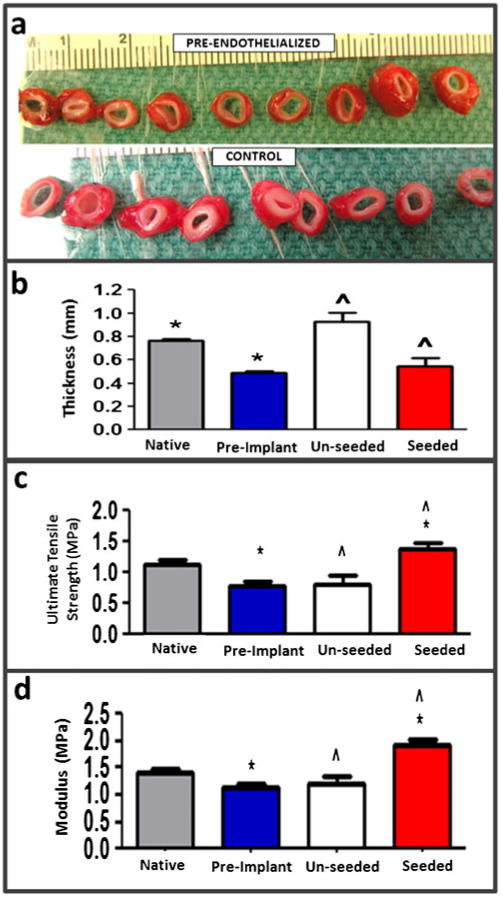
After 9-week implantation, pre-endothelialized and control grafts were harvested and cut into ring segments for gross visual analysis (a). Measurements of the explanted graft properties were conducted. Thickness (b), ultimate tensile strength (c), and modulus (d) of pre-endothelialized (seeded, n=3) and control (unseeded, n=3) TEVG in comparison to femoral artery and pre-implantation TEVG (n=3) are shown
Fig. 4.
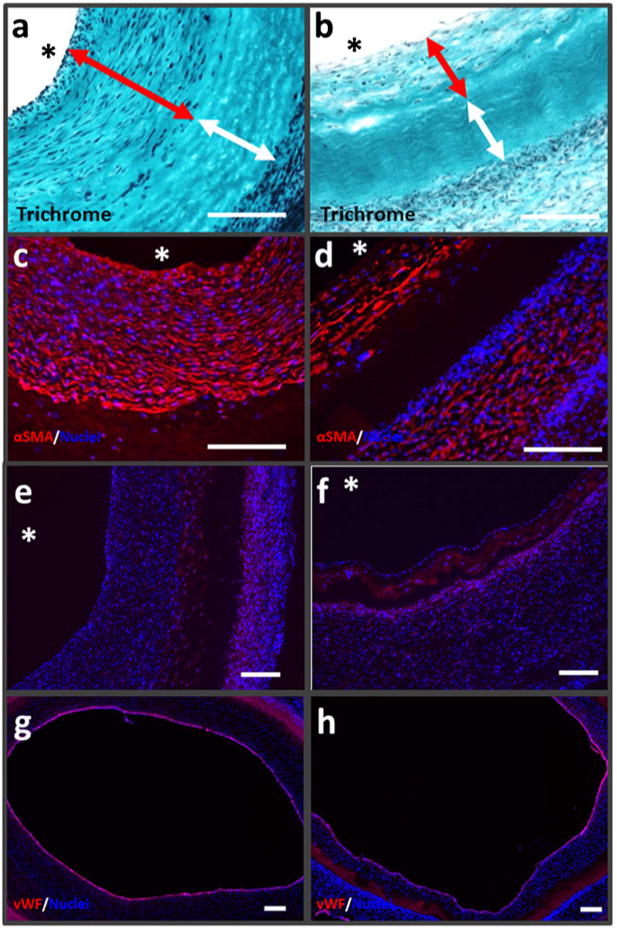
BOEC-seeded TEVG characterization at explantation. Trichrome images of 9-week explanted grafts with red arrows showing cellular regions adjoining the lumenal surface and white arrows the internal acellular regions for a unseeded TEVG and b BOEC-seeded TEVG. αSMA, CD45, and vWF staining on unseeded TEVG (c, e, g) and BOEC-seeded TEVG (d, f, h) near mid-graft (white scale bar=200 μm)
Fig. 5.
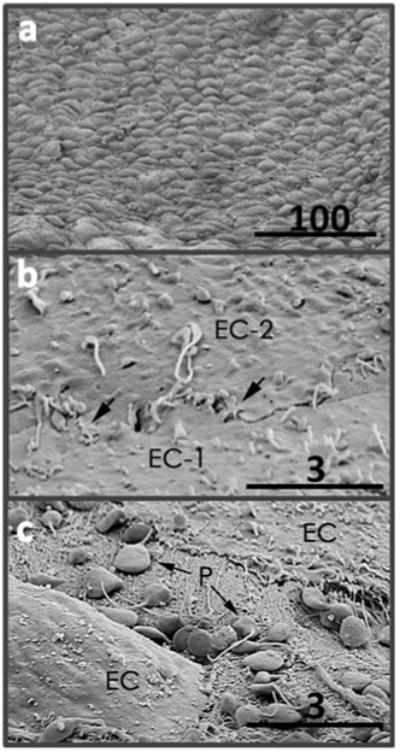
SEM images of BOEC-seeded TEVG lumenal surface. Regions with a flow-aligned confluent monolayer (a), tight junctions between cells (b), and rare location with exposed matrix and bound platelets (c) (scale bars with values in μm are shown)
Discussion
In this study, we utilized the ovine femoral artery model to evaluate a completely biological, acellular TEVG made from fibrin remodeling by allogeneic fibroblasts in vitro subsequently seeded with autologous BOEC as a potential solution for the clinical need of small-diameter arterial grafts possessing a functional endothelium. We report elsewhere the favorable remodeling of these grafts without seeded BOEC (under sustained anti-coagulation therapy) in the same ovine femoral artery model after 8- and 24-week implantation [12].
The seeded BOEC were almost totally retained (>95 %) after 1-h implantation, indicating excellent short-term shear resistance. This compares favorably to the 64 % retention of autologous porcine BOECs prior to implantation in the sole study reporting implantation of a BOEC-seeded TEVG (where outgrowth cells on fibronectin-coated flasks, not collagen-coated flasks as used in this study, were seeded on a decellularized TEVG made from smooth muscle cells cultured on a polyglycolic acid fiber scaffold) [1]. While there was a reduction in diameter at the anastomoses of the non-seeded contralateral control grafts relative to the mid-graft region at 8 weeks, a diameter reduction did not occur in the BOEC-seeded grafts. In addition, a substantial reduction in graft thickness was observed in BOEC-seeded grafts after 9-week implantation compared with control grafts, indicating a clear effect of the seeded BOEC monolayer at implantation. BOEC-seeded grafts also showed a more advanced basement membrane development at the lumenal surface, at both the time of implantation and at explantation. The control grafts did not clot in the final week of implantation when anti-coagulation therapy was removed, which was unexpected since prior 8-week studies of control grafts showed that spontaneous endothelialization was incomplete mid-graft [12]. This precludes any conclusion regarding an anti-thrombogenic benefit of the seeded BOECs. Nonetheless, there was a greater overall endothelial coverage of the BOEC-seeded grafts. The lack of thrombus formation on the control grafts could be due to the spontaneous endothelialization that occurred, passivation of the graft lumen throughout the implant duration, and/or a persistent effect of the clopidogrel, which irreversibly inhibits platelet binding for their entire 7- to 10-day lifespan [14]. When analyzed for platelet-endothelial co-localization, no difference existed in platelet binding between BOEC-seeded and control grafts, indicating that, if still present, the BOEC were exhibiting platelet interactions indistinguishable from EC that spontaneously repopulated the control grafts.
Both the mitigation of anastomotic narrowing and advanced basement membrane deposition in the BOEC-seeded TEVG are consistent with published results showing that the existence of a robust basement membrane provides a physical barrier for transmigration of smooth muscle cells (SMC) to the vessel lumen, an early event in the progression of intimal hyperplasia [15]. While the enhanced basement membrane observed at explantation of BOEC-seeded grafts compared with control grafts provides an explanation for the decrease in wall thickening observed, at least one alternative explanation exists. Recent studies have shown that mobilization of bone marrow-derived mesenchymal stem cells following vascular injury contributes to neointima formation resulting from induction of a SMC phenotype following injury-site adhesion. Through secretion of paracrine signaling molecules, BOEC might have attenuated this phenomenon by directing differentiation of the injury-localized mesenchymal stem cells towards an endothelial phenotype and thereby attenuating neointimal progression and promoting vascular repair [16]. While further studies must be done to elucidate the mechanisms by which wall thickening was decreased in the BOEC-seeded grafts, it is encouraging that published results are consistent with our observations. Unlike the contralateral non-seeded control grafts, which exhibited anastomotic narrowing as found in our prior study 8 weeks post-implantation [12], ultrasonography of the BOEC-seeded grafts taken at both 1 and 8 weeks post-implantation indicated no evidence of stenosis or altered hemodynamics, with only minor neointima formation. This could be due to paracrine factors released by the BOECs that inhibit proliferation and extacellular matrix deposition of the invaded cells. For example, nitric oxide released from the endothelium is well known to suppress intimal hyperplasia following arterial injury by inhibiting proliferation of vascular SMC [17]. It would be desirable to unambiguously establish the presence of BOEC on the lumenal surface of the graft at the time of the 9 weeks explantation by pre-labeling the BOEC.
Employing the use of autologous endothelialization pre-implantation delays the clinical availability and complicates the commercialization of the TEVG described herein and any TEVG using this strategy to confer a non-thrombogenic property. However, there is no proven alternative strategy currently. It should be noted that the BOECs in these studies were used at passage 7 in order to attain sufficient cell stocks necessary to develop a repeatable method for fabricating a robust, shear-resistant endothelium. To achieve this required approximately 6–7 weeks from the time of the initial blood draw. Given the BOEC growth characteristics we have observed, this delay could be reduced to as little as 3 weeks to achieve the necessary cell number to fully endothelialize a 15-cm graft for utilization in coronary artery bypass. In addition, studies have described the ability to enhance endothelial progenitor mobilization through systemic delivery of cytokines [18, 19], which could substantially diminish this delay even further. Short TEVG (2–3 cm) implanted interpositionally into the ovine femoral artery were used in these studies to evaluate the effects of BOEC seeding on graft remodeling and for comparison to our prior studies implanting unseeded TEVG in this pre-clinical model. Substantially, longer TEVG made from human dermal fibroblasts are needed to be clinically relevant as coronary artery bypass grafts and they must be evaluated as bypass grafts.
In conclusion, we observed unprecedented endothelial retention (>95 %) at 1-h post-surgery, with long-term benefits on graft remodeling and function correlating with the pre-seeded BOEC.
Acknowledgments
The authors would like to acknowledge the University of Minnesota Experimental Surgical Service staff for surgical assistance, Jim Berry for ultrasonography measurements, Mark Roney for assistance with BOEC isolation, and Pat Schaeffer for histological sectioning/staining. The funding for these studies was provided by the NIH R01 HL083880 (to RTT).
Abbreviations
- BOEC
blood outgrowth endothelial cells
- TEVG
tissue-engineered vascular grafts
Contributor Information
Lee A. Meier, Department of Biomedical Engineering, University of Minnesota, 7-114 NHH, 312 Church St SE, Minneapolis, MN 55455, USA
Zeeshan H. Syedain, Department of Biomedical Engineering, University of Minnesota, 7-114 NHH, 312 Church St SE, Minneapolis, MN 55455, USA
Matthew T. Lahti, Experimental Surgical Services, University of Minnesota, Minneapolis, MN, USA
Sandra S. Johnson, Department of Biomedical Engineering, University of Minnesota, 7-114 NHH, 312 Church St SE, Minneapolis, MN 55455, USA
Minna H. Chen, Department of Chemical Engineering & Materials Science, University of Minnesota, Minneapolis, MN, USA
Robert P. Hebbel, Department of Medicine, University of Minnesota, Minneapolis, MN, USA
Robert T. Tranquillo, Email: tranquillo@umn.edu, Department of Biomedical Engineering, University of Minnesota, 7-114 NHH, 312 Church St SE, Minneapolis, MN 55455, USA; Department of Chemical Engineering & Materials Science, University of Minnesota, Minneapolis, MN, USA.
References
- 1.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108(22):9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tschoeke B, Flanagan TC, Koch S, Harwoko MS, Deichmann T, Ella V, Sachweh JS, Kellomaki M, Gries T, Schmitz-Rode T, Jockenhoevel S. Tissue-engineered small-caliber vascular graft based on a novel biodegradable composite fibrin-polylactide scaffold. Tissue Eng Part A. 2009;15(8):1909–1918. doi: 10.1089/ten.tea.2008.0499. [DOI] [PubMed] [Google Scholar]
- 3.Hashi CK, Derugin N, Janairo RR, Lee R, Schultz D, Lotz J, Li S. Antithrombogenic modification of small-diameter microfibrous vascular grafts. Arterioscler Thromb Vasc Biol. 2010;30(8):1621–1627. doi: 10.1161/ATVBAHA.110.208348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, Chu B, Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104(29):11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janairo RR, Henry JJ, Lee BL, Hashi CK, Derugin N, Lee R, Li S. Heparin-modified small-diameter nanofibrous vascular grafts. IEEE transactions on nanobioscience. 2012;11(1):22–27. doi: 10.1109/TNB.2012.2188926. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn JJ, Hinds MT. Endothelial outgrowth cells: function and performance in vascular grafts. Tissue engineering Part B, Reviews. 2013 doi: 10.1089/ten.TEB.2013.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroncek JD, Grant BS, Brown MA, Povsic TJ, Truskey GA, Reichert WM. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue engineering Part A. 2009;15(11):3473–3486. doi: 10.1089/ten.TEA.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei P, Milbauer LC, Enenstein J, Nguyen J, Pan W, Hebbel RP. Differential endothelial cell gene expression by African Americans versus Caucasian Americans: a possible contribution to health disparity in vascular disease and cancer. BMC medicine. 2011;9:2. doi: 10.1186/1741-7015-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Milbauer L, Wei P, Enenstein J, Jiang A, Hillery CA, Scott JP, Nelson SC, Bodempudi V, Topper JN, Yang RB, Hirsch B, Pan W, Hebbel RP. Genetic endothelial systems biology of sickle stroke risk. Blood. 2008;111(7):3872–3879. doi: 10.1182/blood-2007-06-097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32(3):714–722. doi: 10.1016/j.biomaterials.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syedain ZH, Meier LA, Lahti MT, Johnson SL, Tranquillo RT. Implantation of completely biological engineered arteries following decellularization into the sheep femoral artery. Tissue Eng Part A. doi: 10.1089/ten.tea.2013.0550. submitted. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjork JW, Johnson SL, Tranquillo RT. Ruthenium-catalyzed photo cross-linking of fibrin-based engineered tissue. Biomaterials. 2011;32(10):2479–2488. doi: 10.1016/j.biomaterials.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelson AD. P2Y12 antagonism: promises and challenges. Arterioscler Thromb Vasc Biol. 2008;28(3):s33–s38. doi: 10.1161/ATVBAHA.107.160689. [DOI] [PubMed] [Google Scholar]
- 15.Turner NA, Hall KT, Ball SG, Porter KE. Selective gene silencing of either MMP-2 or MMP-9 inhibits invasion of human saphenous vein smooth muscle cells. Atherosclerosis. 2007;193(1):36–43. doi: 10.1016/j.atherosclerosis.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, Lan YJ, Yeh CH, Stanford WL. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28(1):54–60. doi: 10.1161/ATVBAHA.107.147256. [DOI] [PubMed] [Google Scholar]
- 17.Tsihlis ND, Oustwani CS, Vavra AK, Jiang Q, Keefer LK, Kibbe MR. Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10. Cell Biochem Biophys. 2011;60(1–2):89–97. doi: 10.1007/s12013-011-9179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, Jensen TW, Roy EJ, Cha C, Devolder RJ, Kohman RE, Zhang BZ, Textor KB, Rund LA, Schook LB, Tong YW, Kong H. Tuning the non-equilibrium state of a drug-encapsulated poly(ethylene glycol) hydrogel for stem and progenitor cell mobilization. Biomaterials. 2011;32(7):2004–2012. doi: 10.1016/j.biomaterials.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kienstra KA, Hirschi KK. Vascular progenitor cell mobilization. Methods Mol Biol. 2012;904:155–164. doi: 10.1007/978-1-61779-943-3_13. [DOI] [PubMed] [Google Scholar]


