Abstract
Purpose:Accumulating evidence indicates that glycyrrhizin (GZ) and its hydrolyzed metabolite 18-β glycyrrhetinic acid (GA) exhibit anti-inflammatory and anticancer activities. The objective of this study was to examine the in vitro cytotoxic activity of GA on human ovarian cancer A2780 cells.
Methods: A2780 cells were cultured in RPMI1640 containing 10% fetal bovine serum. Cells were treated with different doses of GA and cell viability and proliferation were detected by dye exclusion and 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assays. Apoptosis induction and expression of Fas and Fas ligand (FasL) were analyzed by flow cytometry.
Results: We observed that GA decreases cell viability and suppressed cells proliferation in a dose-dependent manner as detected by dye-exclusion and XTT assayes. In addition, our flow cytometry data show that GA not only induces apoptosis in A2780 cells but also upregulates both Fas and FasL on these cells in a dose-dependent manner.
Conclusion: we demonstrate that GA causes cell death in A2780 cells by inducing apoptosis.
Keywords: Glycyrrhetinic acid, Apoptosis, Ovarian Cancer
Introduction
Because of high mortality cancer is one of the most causes of human’s death. The conventional therapeutic procedures for cancer include surgery, chemotherapy and radiotherapy. However, these conventional treatments have many side effects and deficiencies which highlight the essential prospective for finding novel therapeutic approaches for cancer.1,2
Many new findings suggest that most of the cell growth regulating mechanisms are damaged in the process of cancer initiation and cell growth gets out of the body’s control. Apoptosis or programmed cell death is one of the fundamental mechanisms that regulates cell growth and death.3 Basically, apoptosis is activated by a cell surface receptor called Fas which binds to a ligand on the activating cell which is often a lymphocyte called Fas ligand (FasL).4
Interaction of Fas and FasL leads to activation of death domains which subsequently results in activation of caspase and cell death.4,5 Recent studies have demonstrated that many cancers became resistant to apoptosis. Of interest, apoptosis has been dysregulated in many ovarian cancers which includes nearly four percent of all cancers in women.6
In recent years, many studies have been conducted concerning the inhibitory effects of natural materials against cancers. In this regard, using compounds that are non-toxic to humans and have no side effects has attracted the attention of many researchers. Liquorice plant-derived compounds such as glycyrrhizin (GZ) have been shown to possess anti-inflammatory activities.7 18-ß glycyrrhetinic acid (GA), which is a five ring tri-terpenoid compound derived from the hydrolysis of GZ, has been shown to display anti-inflammatory and anti-tumoral effects.8,9 Accumulating evidence indicates that GA induces apoptosis in human gastric cancer, promyelocytic leukemia HL60 cells and hepatoma cells.10 On the other hand, in contrast to GZ which possesses pro-apoptosis effects in rat hepatocytes, GA inhibits it,11 indicating that it is uncertain whether the killing activity of GA is mediated by apoptosis executor caspases. Furthermore, the toxic and anti-proliferative effects of GA against human ovarian cancers have not been yet elucidated. The aim of the current study was therefore to examine the toxic effects of GA against A2780 cells in relation to Fas-mediated apoptosis.
Materials and Methods
Cell culture
The A2780 cell line (human ovarian carcinoma cell line) was purchased from Iran Pasteur Institute (Tehran, Iran) and were cultured in RPMI 1640 (Gibco, Manchester, UK) containing 10% FBS at 37°C in a humid incubator with 5% CO2. Cells were sub-cultured when they reached approximately 80% confluence.
Cell viability
To examine the effect of GA on cell viability of A2780 cells, 2x105 cells were cultured in the presence of different concentrations of GA(Sigma, Munich, Germany) in RPMI1640 containing 10% FBS for either 24 or 48 hours. Then cells viability was determined by dye exclusion assay and cells were counted using hemocytometer slides.
XTT assay
The assay detects the reductiom of XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) to formazon product which reflects the normal function of mitochondria. Thus, XTT assay (CCK-8, Dojindo, Tokyo, Japan) was employed to assess toxic effects of GA on A2780 cells. 4x103 cells were added to each well of 96 well plates (in duplicate) with or without different concentrations of GA in RPMI1640 containing 10% FBS. Cells were incubated at 37 °C in a humidified incubator with 5% Co2 for either 24 h or 48 h, and then 10µL of CCK-8 was added to each well and incubated again for 4 h. The optical density (OD) was measured at 450 nm using a microplate reader (Stat Fax, Palm City, FL). The absorbance of untreated cells was considered as 100%. Percent growth inhibition was calculated as previously reported. Percent of inhibition= 100-(test OD/untreated OD)x100).12
Apoptosis assay
In order to examine whether GA treatment could results in inducing of apoptosis, cells were treated with various concentrations of GA and apoptosis was examined using Annexin-V/PI kit (ebioscience, San Diego, CA). Cells were treated with different concentration of GA for 24h and stained for Annexin V and PI according to the manufacturer's instructions. Briefly, cells were harvested, washed and incubated with Annexin V and PI for 15 min in dark place. Next, after washing with 300 µL binding buffer, cells were gently resuspended in 300 µL of binding buffer, kept on ice and subjected to FACS analysis (FACS Calibur, Beckman Dickinson, San Jose, CA) within an hour. Flow cytometry data were analyzed by FCS Express software (De Novo Software, Los Angeles, CA).
Flow cytometry analysis of Fas receptor and Fas ligand expression
To examine the possible effects of GA on expression of Fas and FasL, we incubated the cells with different concentrations of GA for 24h. The levels of Fas and FasL expression on the cells surface of A2780 cells were determined by Flow cytometry. 5x105 cells were counted and washed three times with FCM buffer (PBS containing 0.1% BSA) and stained with either 10 µL of PE-conjugated control antibody or mouse anti-human Fas-PE or mouse anti-human FasL-PE (all from Biolegend, San Diego, CA) as previously reported.13 Cells then were washed three times with FCM buffer, fixed in 1% paraformaldehyde, and subjected to FACS analysis. Flow cytometry data were analyzed by FCS Express software.
Statistical analysis
Arithmetic means and standard deviations were calculated and statistical significance was defined as P≤0.05 using Student’s t-test.
Results
Effect of GA on cell viability and proliferation
First, we examined the effects of GA on cell viability of A2780 cells and observed that this licorice-derived compound is capable to decrease cell viability in a dose-dependent manner. As shown in Figure 1A, 50 µM of GA resulted in decreased cell viability to 35%. In addition, we exhibit that GA treatment for 48 h reduced the cell viability slightly more than that extent which observed during 24h of treatment. Moreover, the effect of GA on cell proliferation was evaluated using XTT assay. Our data show that GA inhibits cell proliferation in dose- and time-dependent manner (Figure 1B) confirming the data of cell counting assay.
Figure 1 .
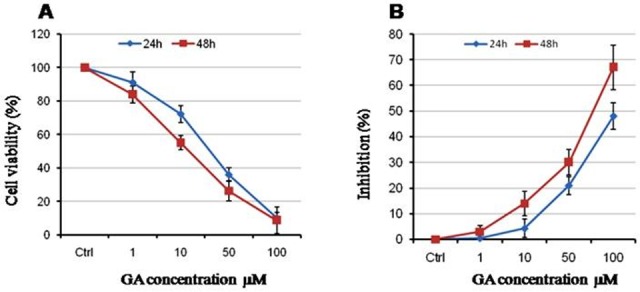
GA reduces cell viability and proliferation in a dose dependent manner. (A) Cells were incubated with increasing concentration of GA in RPMI1640 containing 10% FBS. The cell viability was determined by trypan blue dye exclusion assay. (B) The effect of GA on cell proliferation was detected by XTT assay. Data are represented of Mean ± SEM of three independent experiments. P<0.05
Effect of GA on inducing of apoptosis in A2780 cells
Having shown that GA reduces cell viability, we sought to examine the effect of GA on incusing of apoptosis in these ovarian cancer cells. To do this, cells were treated with various concentrations of GA and apoptosis was measured by detection of annexin V and PI using flow cytometry. As demonstrated in Figure 2, GA induced apoptosis in A2780 cells in a dose dependent manner.
Figure 2 .
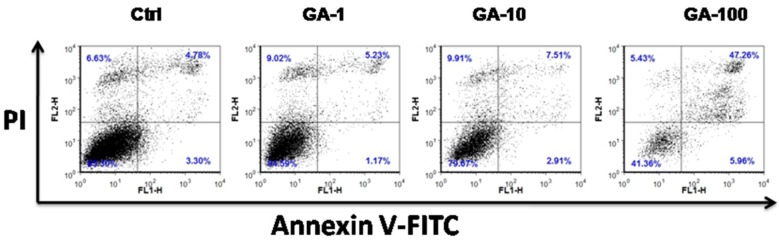
GA induces apoptosis in A2780 cells. Cells were incubated with increasing concentration of GA in RPMI1640 containing 10% FBS for 24h. Then, cells were harvested, washed with cold PBS, incubated with 5µL of Annexin V and 10µL PI for 15 min in dark. 300 µL of binding buffer was added and analyzed by flow cytometry within 1h. Representative data from three independent experiments is shown.
GA upregulates expression of both Fas and FasL on the cell surface of A2780 cells
One of the hallmarks of apoptosis is the increase in the expression of Fas and Fas ligand receptor in the surface of apoptotic cells during the cell death process.4 We examined the effect of GA on the expression of Fas on cell surface of A2782 cells and found that Fas is expressed at a low level of cell membrane of these cells and GA upregulates it in a dose-dependent manner (Figure 3A). Interestingly, we observed that FasL is not expressed on the cell surface of A2780 cells, but its expression enhanced when the cells were treated with GA (Figure 3B).
Figure 3 .
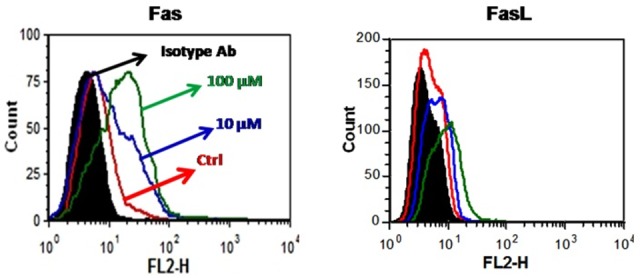
GA upregulates Fas and FasL on A2780 cells. Cells were incubated with increasing concentration of GA in RPMI1640 containing 10% FBS for 24h. Then, cells were harvested, washed with cold FCM buffer, incubated with 10µL of either PE-conjugated isotype antibody, mouse anti-human Fas-PE or mouse anti-human FasL-PE for 45 min on 4°C. Cells then washed three times with FCM buffer and analyzed by flow cytometry. Representative data from three independent experiments is shown.
Discussion
Dysregulation of apoptosis has been shown to be involved in initiation, progression and drug-resistance in many tumors including ovarian cancers.3,6 Many studies have recently focused on the role of apoptosis in intervention of lethal properties of anti-cancer drugs in neoplastic cells.14,15 Certain plants and their derivatives have exhibited cytotoxic and chemoprevention activities on tumor cells which mainly act through induction of apoptosis.16-18 Licorice-derived compound, GA has been shown to exhibits a wide range of biological properties including anti-inflammatory and cytotoxic activities.7-9 However, the potential toxic effects of GA on ovarian cancers has not yet been elucidated. In this study, the effects of GA on A2780 cells, a well-known ovarian cancer cell, was investigated. Our results indicate that this GA has a high potential to inhibit the growth of A2780 cells in the micromolar doses. GA induces apoptosis and lead to an increase in the expression of Fas and FasL on the cell surface of these cells.
The emerging body of evidence suggests that GA reveals anti-inflammatory and anti-cancer properties.16,18 Moreover, treatment with GA inactivates cellular Bcl-2 and induces apoptosis in Kaposi sarcoma-associated herpes virus-infected B lymphocyte.19 Conversely, Ishiwata S et al, reported that both GZ and GA did not induce apoptosis in human T cell lines. In contrast to GA, combination of GZ and anti-Fas antibody was capable to enhance apoptosis in these cells,20 indicating that the effects of GA in inducing of apoptosis is still unclear. In the current study, we demonstrate that GA leads to a significant reduction of both cell viability and proliferation of A2780 cells.
Xiu-ying Xiao et al analyzed the effects of seven licorice-derived components including GA and GZ on gastric cancer cell such as MKN-28, AGS, and MKN-45 cells. They observed that licochalcone A was the most cytotoxic component of the extract and showed the highest effect on incusing apoptosis and inhibiting cell growth.21 In contrast to our data, this previous report showed that GA was only toxic to gastric cell lines when the cells were treated with a high dose of GA.21 However, we demonstrate, herein, that a much lower dose of GA inhibits A2780 cells growth and proliferation. Conversely, another study has reported that GA is not capable to induce apoptosis in rat hepatocyets11 These discrepancies could be explained by difference in the cells line and experimental conditions.
Fas/FasL interaction is involved in signaling transduction pathway of apoptosis and its role in chemotherapy-induced apoptosis of a number of tumors have been already investigated.20,22
Fas is also known a tumor necrosis factor receptor superfamily member 6 is a glycosylated cell surface molecules, which localized on the cell surface and cytoplasm of many cells and its upregulation during induction of apoptosis has been displayed in many tumors cells.23
Ligation of Fas to FasL induces receptor oligomerization and formation of death-receptor signaling complex resulting to activation of a series of caspase enzymes activation which in turn leads in cells death.4 Our study show that GA upregulates Fas expression on cell surface of A2780 and that GA-mediated Fas upregulation may contribute, at least partially to cell death of A2780 cells following incubation with GA expression. In accordance with the results obtained in A2780 cells, a previously published study has clearly shown that Fas expression is significantly lower in malignant ovarian epithelial neoplasms than benign ovarian epithelial lesions, indicating that a decreased sensitivity to Fas-mediated apoptosis could be contributed to ovarian epithelial carcinogenesis.24 Noteworthy, it has been shown that FasL-expressing tumor cells kill immune cells which have a high level of Fas on their cells surface.25 Having shown that GA upregulates expression of both FasL and Fas on the cells membrane of A2780 cells we postulate that the interaction of elevated Fas and FasL leads to induction of apoptosis in GA-treated A2780 cells.
Conclusion
Finally, we conclude that GA shows cytotoxic activities against A2780 cells by induction of apoptosis and that GA-induced apoptosis is at least partially initiated by interaction of Fas/FasL as GA treatment increases both Fas and FasL expression on the cell surface of A2780 cells.
Acknowledgements
This work was supported by a grant from Kurdistan University of Medical Sciences to AJ.
Conflict of Interest
We declare that we have no competing interests.
References
- 1.Morrison J, Swanton A, Collins S, Kehoe S. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2007;4:CD005343. doi: 10.1002/14651858.CD005343.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Shylasree TS, Bryant A, Athavale R. Chemotherapy and/or radiotherapy in combination with surgery for ovarian carcinosarcoma. Cochrane Database Syst Rev. 2013;28(2) doi: 10.1002/14651858.CD006246.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature . 2001;411(6835):342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 4.Abrahams VM, Kamsteeg M, Mor G. The Fas/Fas ligand system and cancer: immune privilege and apoptosis. Mol Biotechnol . 2003;25(1):19–30. doi: 10.1385/MB:25:1:19. [DOI] [PubMed] [Google Scholar]
- 5.Reichmann E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Biol . 2002;12(4):309–15. doi: 10.1016/s1044-579x(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 6.Ali AY, Farrand L, Kim JY, Byun S, Suh JY, Lee HJ. et al. Molecular determinants of ovarian cancer chemoresistance: new insights into an old conundrum. Ann N Y Acad Sci . 2012;1271:58–67. doi: 10.1111/j.1749-6632.2012.06734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhari S, Abdolmohammadi K, Panahi Y, Nikkhoo B, Peirmohammadi H, Rahmani MR. et al. Glycyrrhizin attenuates tissue injury and reduces neutrophil accumulation in experimental acute pancreatitis. Int J Clin Exp Pathol . 2014;7(1):101–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D, Song D, Guo G, Wang R, Lv J, Jing Y. et al. The synthesis of 18beta-glycyrrhetinic acid derivatives which have increased antiproliferative and apoptotic effects in leukemia cells. Bioorg Med Chem . 2007;15(16):5432–9. doi: 10.1016/j.bmc.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Kim YJ, Lee MS, Han ES, Lee SJ. 18beta-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci . 2008;83(13-14):481–9. doi: 10.1016/j.lfs.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Hibasami H, Iwase H, Yoshioka K, Takahashi H. Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int J Mol Med . 2006;17(2):215–9. [PubMed] [Google Scholar]
- 11.Gumpricht E, Dahl R, Devereaux MW, Sokol RJ. Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem . 2005;280(11):10556–63. doi: 10.1074/jbc.M411673200. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods . 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Fakhari S, Kalantar E, Nikzaban M, Hakhamneshi MS, Fathi F, Nikkhoo B. et al. Effect of Helicobacter pylori infection on stromal-derived factor-1/CXCR4 axis in bone marrow-derived mesenchymal stem cells. Adv Biomed Res . 2014;3:19. doi: 10.4103/2277-9175.124650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellarosa D, Ciucci A, Bullo A, Nardelli F, Manzini S, Maggi CA. et al. Apoptotic events in a human ovarian cancer cell line exposed to anthracyclines. J Pharmacol Exp Ther . 2001;296(2):276–83. [PubMed] [Google Scholar]
- 15.Duiker EW, Van Der Zee AG, De Graeff P, Boersma-Van Ek W, Hollema H, De Bock GH. et al. The extrinsic apoptosis pathway and its prognostic impact in ovarian cancer. Gynecol Oncol . 2010;116(3):549–55. doi: 10.1016/j.ygyno.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Kao TC, Shyu MH, Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J Agric Food Chem . 2010;58(15):8623–9. doi: 10.1021/jf101841r. [DOI] [PubMed] [Google Scholar]
- 17.Wang CY, Kao TC, Lo WH, Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-kappaB through PI3K p110delta and p110gamma inhibitions. J Agric Food Chem . 2011;59(14):7726–33. doi: 10.1021/jf2013265. [DOI] [PubMed] [Google Scholar]
- 18.Kuang P, Zhao W, Su W, Zhang Z, Zhang L, Liu J. et al. 18beta-glycyrrhetinic acid inhibits hepatocellular carcinoma development by reversing hepatic stellate cell-mediated immunosuppression in mice. Int J Cancer . 2013;132(8):1831–41. doi: 10.1002/ijc.27852. [DOI] [PubMed] [Google Scholar]
- 19.Curreli F, Friedman-Kien AE, Flore O. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J Clin Invest . 2005;115(3):642–52. doi: 10.1172/JCI23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishiwata S, Nakashita K, Ozawa Y, Niizeki M, Nozaki S, Tomioka Y. et al. Fas-mediated apoptosis is enhanced by glycyrrhizin without alteration of caspase-3-like activity. Biol Pharm Bull . 1999;22(11):1163–6. doi: 10.1248/bpb.22.1163. [DOI] [PubMed] [Google Scholar]
- 21.Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ. et al. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett . 2011;302(1):69–75. doi: 10.1016/j.canlet.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Ghahremani M, Foghi A, Dorrington JH. Activation of Fas ligand/receptor system kills ovarian cancer cell lines by an apoptotic mechanism. Gynecol Oncol . 1998;70(2):275–81. doi: 10.1006/gyno.1998.5091. [DOI] [PubMed] [Google Scholar]
- 23.Zusman I, Gurevich P, Gurevich E, Ben-Hur H. The immune system, apoptosis and apoptosis-related proteins in human ovarian tumors (a review) Int J Oncol . 2001;18(5):965–72. doi: 10.3892/ijo.18.5.965. [DOI] [PubMed] [Google Scholar]
- 24.Reed J, Hakam A, Nicosia SV, Coppola D. Significance of Fas receptor protein expression in epithelial ovarian cancer. Hum Pathol . 2005;36(9):971–6. doi: 10.1016/j.humpath.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D. et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells--a mechanism of immune evasion? Nat Med. 1996;2(12):1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]


