Abstract
Spermine and spermidine act as neuromodulators upon binding to the extracellular site(s) of various ionotropic receptors, such as N-methyl-d-aspartate receptors. To gain access to the receptors, polyamines synthesized in neurons and astrocytes are stored in secretory vesicles and released upon depolarization. Although vesicular storage is mediated in an ATP-dependent, reserpine-sensitive fashion, the transporter responsible for this process remains unknown. SLC18B1 is the fourth member of the SLC18 transporter family, which includes vesicular monoamine transporters and vesicular acetylcholine transporter. Proteoliposomes containing purified human SLC18B1 protein actively transport spermine and spermidine by exchange of H+. SLC18B1 protein is predominantly expressed in the hippocampus and is associated with vesicles in astrocytes. SLC18B1 gene knockdown decreased both SLC18B1 protein and spermine/spermidine contents in astrocytes. These results indicated that SLC18B1 encodes a vesicular polyamine transporter (VPAT).
The polyamines, spermine (Spm) and spermidine (Spd), and their precursor, putrescine, are low molecular weight organic polycations that are synthesized from arginine and methionine and play essential roles in cell growth and differentiation in practically all living organisms1,2,3,4. Moreover, in the central nervous system, Spm and Spd play significant roles as second messengers in neurotransmission4,5,6. In neurons, intracellular Spm and Spd exhibit strong inward rectification upon binding to various ion channels, such as potassium channels, olfactory cyclic nucleotide-gated channels, and voltage-gated sodium channels, which may be involved in neurodegenerative diseases, such as Huntington's disease7,8,9,10,11. Extracellular Spm and Spd potentiate or block the N-methyl-d-aspartate (NMDA) receptor current upon binding to the specific binding sites at low and high concentrations, respectively5,6,12. As positive modulation of NMDA receptors may lead to enhanced Ca2+ entry, extracellular Spm and Spd may cause excitotoxicity under some pathological conditions5,6. Extracellular polyamines also potentiate the kainate receptor and the transient receptor potential cation channel subfamily V member 1 (TrpV1 or capsaicin receptor) and block the AMPA receptor upon binding to their specific sites13,14,15,16. Thus, polyamines interact with neurons intracellularly and extracellularly, and profoundly modulate neurotransmission, affecting higher order brain functions, including synaptic plasticity, and have been implicated in neuropathological disorders17. To understand the physiological and pathological relevance of polyamine-mediated neuromodulation, it is important to elucidate the signaling pathways and mechanisms by which polyamines gain access to the target receptors.
The brain contains relatively high concentrations of polyamines18,19. Spm- and Spd-like immunoreactivities are predominantly present in glial cells, particularly astrocytes. Much lower levels of immunoreactivity are observed in neurons, except that prominent localization is observed in some specific neurons, such as neurosecretory neurons20,21. The polyamine synthesizing enzymes, ornithine decarboxylase and spermidine synthase, are primarily present in neurons and neuropil rather than glial cells22,23,24. Furthermore, it has been reported that intrastriatal infusion of NMDA causes rapid marked increases in extracellular Spm and Spd levels in the brain25. Ca2+-dependent Spm and Spd release from brain slices and synaptosomes were also observed upon K+ stimulation26,27. The released polyamines may be sequestered through high-affinity polyamine uptake in neurons and astrocytes27,28. Moreover, the membrane fraction (P2 fraction) from rat brain exhibits ATP-dependent uptake of Spm and Spd, which was sensitive to proton conductor and reserpine27. In analogy to chemical transmission by the classical neurotransmitters, such as glutamate and monoamines, it is therefore tempting to speculate that Spm and Spd are synthesized de novo, stored intracellularly, and exocytosed from neurons and/or astrocytes to modulate the receptors upon binding extracellularly. However, transporters involved in vesicular storage of polyamines have yet to be identified.
Vesicular neurotransmitter transporters are active transporters of neurotransmitters and are responsible for their vesicular storage and subsequent exocytosis29,30. Six classes of transporter have been identified to date: vesicular monoamine transporter (VMAT), vesicular acetylcholine transporter (VAChT), vesicular GABA transporter (VGAT), vesicular glutamate transporter (VGLUT), vesicular nucleotide transporter (VNUT), and vesicular aspartate transporter (VEAT)29,30. Identification of vesicular polyamine transporters, if present, may help to identify polyamine-secreting cells and storage vesicles for Spm and Spd, and ultimately to define the signaling cascade for polyamines. Among the above transporters, VMATs and VAChT belong to the SLC18 family and transport monoamines. Recently, a novel gene belonging to the SLC18 family, SLC18B1 (HGNC:21573), was reported although its function is not known31 (Supplementary Fig. 1). We postulated that this protein may function as a vesicular polyamine transporter. Here, we present evidence that the SLC18B1 protein fulfills the requirements for vesicular storage of Spm and Spd in the brain.
Results
SLC18B1 protein is an H+/polyamine antiporter
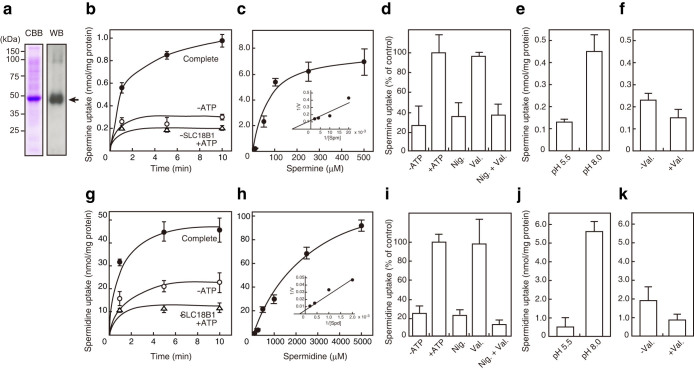
To test the working hypothesis that SLC18B1 encodes a vesicular polyamine transporter, the human SLC18B1 protein was expressed in High Five cells, solubilized from the membranes with detergent, and purified by Ni-NTA column chromatography. The purified protein fraction contained a major polypeptide with apparent molecular mass of 48 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after staining with Coomassie Brilliant Blue (Fig. 1a, left). The identity of the polypeptide as the SLC18B1 protein was confirmed by western blotting with anti-human SLC18B1 protein antibody (Fig. 1a, right). The purified, heterologously expressed SLC18B1 protein was co-reconstituted into liposomes with bacterial F-ATPase, and the uptake of radiolabeled polyamine was measured. Upon addition of ATP, the F-ATPase hydrolyzes ATP and pumps protons into the proteoliposomes, thereby establishing an electrochemical gradient of protons across the membrane29,30. Consequently, the proteoliposomes took up radiolabeled Spm in a manner that was dependent on time, ATP, and dose with Km and Vmax values of 94 μM and 8.6 nmol/min/mg protein, respectively (Figs. 1b and c). The uptake was significantly reduced in the absence of ATP or in the absence of SLC18B1 protein (Fig. 1b). The ATP-dependent uptake of Spm was also significantly reduced when nigericin was included in the assay medium containing K+, whereas valinomycin had no inhibitory effect. Addition of both nigericin and valinomycin concomitantly reduced uptake of Spm to that observed in the presence of nigericin alone (Fig. 1d). To confirm that ΔpH was the driving force, we prepared proteoliposomes containing only SLC18B1 protein as a protein source and examined Spm uptake. Imposing an artificial ΔpH gradient (inside acidic) by the pH jump procedure facilitated Spm uptake (Fig. 1e), while Δψ produced by valinomycin-mediated K+ diffusion potential did not (Fig. 1f). The proteoliposomes also took up radiolabeled Spd with Km and Vmax values of 4.2 mM and 170 nmol/min/mg protein, respectively, and with similar ionophore sensitivities (Figs. 1g–i). Imposing an artificial ΔpH (inside acidic) by the pH jump procedure also facilitated Spd uptake (Fig. 1j and k).
Figure 1. SLC18B1 protein is an H+-coupled polyamine transporter.
(a) Purification of heterologously expressed human SLC18B1 protein. (left) Purified fraction (10 μg of protein) was analyzed by SDS-PAGE (11% gel) and visualized by staining with Coomassie Brilliant Blue. (right) A duplicate gel was analyzed by western blotting with anti-SLC18B1 antibody. The position of SLC18B1 protein is indicated by an arrow. (b) Time course of ATP-dependent radiolabeled Spm uptake by proteoliposomes containing reconstituted human SLC18B1 protein and bacterial F-ATPase. The reaction was started by adding radiolabeled Spm to a final concentration of 10 μM in the presence (filled circles) or absence (open circles) of 5 mM ATP. Spm uptake by proteoliposomes lacking SLC18B1 protein is also shown (open triangles). (c) Dose dependence of ATP-dependent Spm uptake by proteoliposomes containing reconstituted SLC18B1 protein and bacterial F-ATPase after 1 minute. A Lineweaver–Burk plot is shown in the inset. (d) The effects of ionophores on Spm uptake by proteoliposomes containing reconstituted SLC18B1 protein and bacterial F-ATPase. Ionophores were added at 2 μM to the reaction mixture in the presence of ATP, and the Spm uptake after 5 minutes is shown. (e) Spm uptake by the proteoliposomes containing reconstituted SLC18B1 protein was assayed by the pH jump method (acidic inside) as described in the Methods section. (f) Effects of membrane potential on Spm uptake. Proteoliposomes were reconstituted in the presence of 0.15 M NaCl and assayed in the reaction mixture containing 0.15 M KCl. (g) Time course of Spd uptake at 500 μM was measured as above instead of radiolabeled Spm at 10 μM. (h) Dose dependence of ATP-dependent Spd uptake by proteoliposomes containing reconstituted SLC18B1 protein and bacterial F-ATPase after 1 minute. A Lineweaver–Burk plot is shown in the inset. (i) The effects of ionophores on Spd uptake were examined as shown in Fig. 2d. (j) Spd uptake by proteoliposomes containing only SLC18B1 protein was examined as shown in Fig. 2e. (k) Effects of membrane potential on Spd uptake. Data are means ± SE; n = 3–6.
Pharmacology of SLC18B1 protein
Subsequently, we investigated the effects of reserpine and tetrabenazine, inhibitors for VMATs32,33, and vesamicol, an inhibitor of VAChT34, on SLC18B1-mediated Spm uptake. Furthermore, we compared the effects of these compounds on VMAT2 and VAChT under analogous conditions after purification and co-reconstitution with F-ATPase in liposomes (Supplementary Fig. 2). The results indicated that both reserpine and tetrabenazine inhibited ATP-dependent Spm uptake by SLC18B1 but their inhibitory potencies were weak compared with those for VMAT2 and VAChT (Figs. 2a–c). In contrast, vesamicol specifically inhibited VAChT-mediated acetylcholine transport and hardly affected the activities of SLC18B1 protein or VMAT2 (Figs. 2a–c).
Figure 2. Pharmacology and substrate specificity of SLC18B1 protein, VMAT2, and VAChT.
(a–c) The effects of reserpine, tetrabenazine, and vesamicol at 1 μM each on SLC18B1 protein-mediated Spm (a), VAMT2-mediated serotonin (b), and VAChT-mediated acetylcholine (c) uptake after 5 minutes. (d) ATP-dependent uptake of 10 μM Spm, 100 μM Spd, 10 μM serotonin, or 10 μM acetylcholine by SLC18B1 protein, VMAT2, and VAChT after 5 minutes. The values were corrected by subtracting the values in the absence of ATP. Control Spm, Spd, serotonin, and acetylcholine activities (100%) correspond to 0.62, 6.7, 0.35, and 0.34 nmol/mg protein, respectively. N.D.: no transport activity detected. (e) cis-Inhibition of Spm and Spd uptake after 1 minute. SLC18B1 protein-mediated uptake of 10 μM Spm and 500 μM Spd was measured in the absence or presence of the listed compounds at 1 mM and 5 mM, respectively. Data are means ± SE; n = 3–6.
Polyamine transport is a unique property of SLC18B1 protein
VMAT and VAChT are polyspecific in nature. In addition to typical substrates such as serotonin and acetylcholine, they also transport various other compounds, such as ethidium and tetraphenylphosphonium35,36. Before it could be concluded that SLC18B1 protein is a polyamine transporter, it was therefore necessary to confirm that VMATs and VAChT do not transport polyamines. As shown in Fig. 2d, we found that neither VMAT2 nor VAChT transport Spd and Spm. All three transporters tested took up serotonin to a similar extent, indicating that serotonin is a mutual transport substrate in the SLC18 family. In contrast, no acetylcholine uptake was observed with SLC18B1 protein and VMAT2.
To strengthen the evidence regarding the polyspecific nature of SLC18B1 protein, we investigated its substrate specificity by cis-inhibition. As shown in Fig. 2e, the ATP-dependent uptake of radiolabeled Spm by SLC18B1 protein was inhibited by the addition of cold Spm, serotonin, and histamine to some extent, while putrescine, ornithine, dopamine, and acetylcholine had little effect. Both agmatine and noradrenaline produced slightly stimulatory effects. To our surprise, unlabeled excess Spd did not inhibit uptake of radiolabeled Spm but was stimulatory, even though the SLC18B1 protein transported Spd (Fig. 2e). Conversely, unlabeled excess Spm also did not inhibit the uptake of radiolabeled Spd, but had a stimulatory effect, as was the case with Spd and Spm transport (Fig. 2e). These results indicated that for SLC18B1 protein-mediated polyamine transport, the degree of cis-inhibition is not always consistent with transport. SLC18B1 protein was suggested to possess at least two different substrate binding sites for Spm and Spd: one prefers Spm and the other prefers Spd. Serotonin and histamine may share these two binding sites.
Localization of SLC18B1 protein
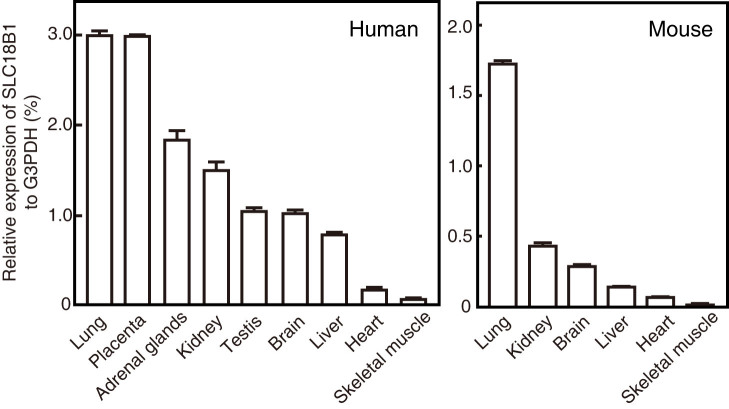
We analyzed tissue-dependent expression of human SLC18B1 by quantitative PCR. The results indicated that the SLC18B1 gene is widely expressed in various organs, including the lung, placenta, adrenal gland, liver, and brain (Fig. 3). A similar expression profile was obtained in mice, which was in good agreement with a previous report31.
Figure 3. Expression profile of SLC18B1.
Expression profiles of the human SLC18B1 and mouse ortholog were analyzed by quantitative PCR.Values relative to G3PDH are shown.
Then, we examined the localization of SLC18B1 protein in the rat brain using a polyclonal antibody specific to mouse SLC18B1 protein. The immunological specificity of the antibody was verified (Supplementary Fig. 3). The antibody recognized a 53-kDa protein in the P2 fraction of brain hippocampal homogenate. No immunoreactivity was observed when the antibody was preabsorbed with purified SLC18B1 protein (Fig. 4a). The antibodies also immunostained rat brain sections and showed strong immunoreactivity throughout brain tissue, particularly the hippocampus, cortex, and cerebellum (Fig. 4b and Supplementary Fig. 4). The immune signal for the SLC18B1 protein in the hippocampus was primarily colocalized with GFAP and not with NeuN or neurofilaments, suggesting that SLC18B1 protein is expressed in astrocytes and is roughly colocalized with synaptophysin, suggesting that some SLC18B1 protein is also associated with synaptic vesicles (Fig. 4c). In cultured neurons, SLC18B1 protein was partly colocalized with synaptophysin (Supplementary Fig. 5). However, it was also localized in other organelles.
Figure 4. Expression and localization of SLC18B1 protein in the rat brain.
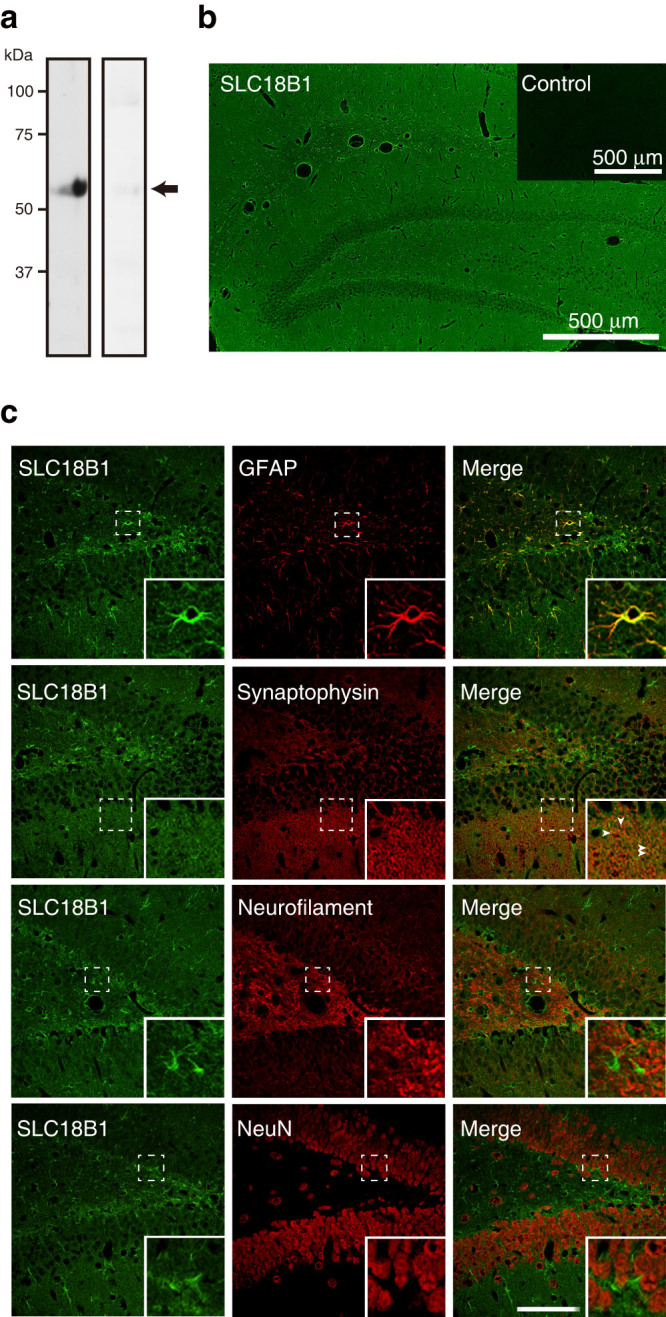
(a) Western blotting with purified antibodies indicated the presence of rat SLC18B1 protein in the P2 fraction of the hippocampus (30 μg protein) (left). The absorbed antibodies were used as a control (right). The position of SLC18B1 protein is indicated by an arrow. (b) Immunohistochemical localization of SLC18B1 protein in the hippocampal region. Images visualized by fluorescence deconvolution microscopy. Inset, control serum. Bar = 500 μm. (c) SLC18B1 was colocalized with GFAP. SLC18B1 was partially colocalized with synaptophysin in some areas of hippocampal sections (arrowheads). The brain slices were double immunostained with sets of antibodies against SLC18B1 and GFAP, synaptophysin, neurofilaments, or NeuN. Bar = 10 μm.
In cultured embryonic rat astrocytes, SLC18B1 protein was observed as a polypeptide with an apparent molecular mass of 53 kDa (Fig. 5a). Double labeling immunofluorescence microscopy showed that SLC18B1 immunoreactivity was present in particulate structures throughout the cells, which were roughly colocalized with VAMP2, a marker for secretory vesicles, but not with chromogranin A (CgA), a marker for dense granules, or with GM130, EEA1, markers of the Golgi apparatus and early endosome and PDI, a marker for endoplasmic reticulum, respectively (Fig. 5b and c). We further characterized the SLC18B1-containing organelles by sucrose density gradient centrifugation after homogenization of astrocytes. VAMP2, a secretory granule marker, and V-ATPase immunoreactivity were observed in fractions 1–3 of higher density and fractions 6–9 of lower density, whereas cathepsin D, a lysosome marker, was located in fractions 1–2 of higher density. As expected, SLC18B1 protein was mainly present in fractions 4–10, whereas secretogranin II as well as CgA, both markers of dense granules, were located in fractions 1–2 and 1–3, respectively, with higher density (Fig. 5d).
Figure 5. Localization of SLC18B1 protein in cultured astrocytes.

(a) Western blotting with purified antibodies indicated the presence of rat SLC18B1 protein in the membrane of hippocampal astrocytes (40 μg protein) (left). The absorbed antibodies were used as a control (right). The position of SLC18B1 protein is marked by an arrow. (b) Indirect immunofluorescence microscopy revealed that SLC18B1 was expressed in primary cultured rat astrocytes. Inset, control staining with normal serum. Bar = 10 μm. (c) SLC18B1 immunofluorescence was present in the vesicular component of cultured hippocampal astrocytes. Astrocytes were double immunostained with sets of antibodies against SLC18B1 and VAMP2, CgA, GM130, EEA1 or PDI. Bar = 10 μm. VAMP2 and CgA (red) were used as vesicular markers. (d) Sucrose density gradient analysis of SLC18B1 localization. The astrocyte membrane fraction was subjected to continuous sucrose density gradient centrifugation, and fractionated into 10 fractions from the bottom. Each fraction (50 μL) was solubilized with SDS sample buffer and immunoblotted with antibodies against SLC18B1, VAMP2, secretogranin II, chromogranin A (CgA), V-ATPase subunit A, and cathepsin D.
Finally, we investigated whether SLC18B1 protein is responsible for the vesicular storage of polyamines. As cultured astrocytes possess appreciable amounts of Spm and Spd, we attempted to determine the localization of endogenous Spm and Spd in cultured astrocytes. RNAi-mediated knockdown of the SLC18B1 gene expression in cultured astrocytes decreased the levels of SLC18B1 mRNA as determined by real-time PCR and the respective protein levels as shown by western blotting and immunohistochemistry (Figs. 6a–c). As shown in Fig. 6c, immunological counterparts of Spm and Spd showed a punctate distribution throughout astrocytes. In agreement with the decrease in mRNA and protein levels (Fig. 6a and 6b), the treatment also decreased immunological counterparts of Spm and Spd, suggesting that SLC18B1 protein is responsible for the vesicular storage of polyamines (Fig. 6c).
Figure 6. The effects of SLC18B1 gene knockdown on the endogenous expression of SLC18B1 and Spd/Spm in cultured astrocytes.

SLC18B1 gene knockdown was carried out as described in Materials and Methods. (a and b) Gene expression as measured by quantitative PCR (a) and SLC18B1 protein as measured by western blotting analysis (b). Expressions of VAMP2 and V-ATPase subunit A are also shown. (c) Immunohistochemical detection of SLC18B1 protein and Spm/Spd. Bar = 10 μm.
Discussion
In the present study, we explored and identified the transporter that is responsible for vesicular storage of Spm and Spd in mammals.
SLC18B1 encodes vesicular polyamine transporter
Biochemical studies with the heterologously expressed purified protein clearly demonstrated that the SLC18B1 protein, a novel isoform of the VMAT family, acts as an H+/Spm or Spd antiporter. The transport characteristics are similar to the ATP-dependent Spm and Spd uptake observed in crude synaptic vesicles27. These characteristics include the kinetic parameters, driving force, and effects of reserpine, basic amino acids, and amines27. Among the members of the SLC18 family, polyamine transport is a unique property of the SLC18B1 protein. Furthermore, as discussed in detail later, the SLC18B1 protein is localized to small vesicles in astrocytes and neurons. Taken together, the observations indicated that the SLC18B1 protein functions as a vesicular polyamine transporter (VPAT) responsible for vesicular storage of Spm and Spd. It is noteworthy that this is the first example of a vesicular polyamine transporter in mammals reported to date.
It has been shown that acidic organelles from CHO cells accumulate polyamines in a vacuolar ATPase-dependent manner37. Furthermore, insulin granules of islet β cells and mast cell granules are also known to accumulate polyamines38,39. Preliminary experiments indicated that VPAT is expressed in islets and mast cells, supporting our conclusion.
Structural and functional properties of VPAT
Mammalian VPAT is completely different from known polyamine transporters found in bacteria, yeast, and plants40,41. Among the structural aspects deduced from its primary amino acid sequence, a notable difference between VPAT and other SLC18 members is the lack of a large luminal loop between transmembrane domain (TMD) 1 and TMD2, which suggests a functional difference31. It is generally accepted that conserved charged residues in transmembrane domains play critical functional roles in many transporters. In the case of rat VMAT2, replacement of Asp33, Lys139, Asp400, or Asp427 markedly decreased serotonin transport activity42,43. These residues are located in the first, second, tenth, and eleventh TMD, and are conserved in VMATs and VAChT. Similar results were reported for rat VMAT1 and VAChT44,45,46. The presence of these charged amino acid residues that are important for activity in VMATs and VAChT indicated that these transporters share the same mechanism for transport. In contrast, these four amino acid residues are not conserved in VPAT31. VPAT has five charged amino acid residues in the TMDs, i.e., Glu83 in TMD2, Arg137 and Asp140 in TMD4, Asp255 in TMD7, and Glu356 in TMD10. Among these amino acid residues, Arg137 and Asp255 are conserved in all SLC18 members. Rat VAChT still retained transport activity if Glu309 was replaced with Gln (corresponding to Asp255 in VPAT), indicating that this residue does not have a critical role in VAChT47. Furthermore, Spm uptake by VPAT was not sensitive to vesamicol, an inhibitor of VAChT, but was moderately sensitive to the VMAT inhibitors reserpine and tetrabenazine. The amino acid residues responsible for inhibition by these drugs in VMAT and VAChT are not present in VPAT. For example, Phe335 is important for vesamicol binding to rat VAChT; the corresponding residue in VPAT is alanine48. Based on conservation of catalytically important residues, we suggest that the transport mechanism of VPAT is somewhat different from those of VMATs and VAChT although VPAT and VMAT2 share similar inhibitor sensitivity.
All transporters belonging to the SLC18 family can recognize monoamines as substrates but only VPAT can recognize monoamines, diamines, triamines, and tetraamines as substrates. Spm and Spd seem to have separate binding sites as VPAT transports Spm and Spd irrespective of the presence of the other polyamine. Furthermore, it is noteworthy that histamine and serotonin may bind to both binding site(s), as histamine inhibits transport of Spm and Spd to a similar extent (Fig. 3e). Inhibition of polyamine transport by histamine suggests that VPAT transports histamine and acts as a vesicular histamine transporter. Histamine granules in mast cells contain Spm and Spd, supporting this suggestion39. However, it is accepted that VMAT2 transports histamine and its genetic deletion results in the total loss of vesicular histamine in mast cells, indicating that VMAT2 is responsible for vesicular accumulation and release of histamine49. Further studies are underway in our laboratory to examine whether VPAT is expressed in mast cells, and if so, whether VPAT is involved in vesicular storage of histamine, Spm, and Spd in mast cells.
Through systematic analyses of the transport properties of VPAT, VMAT2, and VAChT, we found that VAChT transports serotonin. It has long been believed that VAChT specifically transports acetylcholine32,35. However, VAChT was shown to be polyspecific in nature36. The results of the present study further support this conclusion. Taken together, the present results indicate that all members of the SLC18 family are polyspecific, and that VPAT is unique because it possesses transport ability for divalent and trivalent amines. The possible contributions of VAChT and VPAT to serotonergic chemical transmission are interesting topics for further study, and reevaluation of classical monoaminergic chemical transmission will be necessary.
Spm and Spd as possible neuro/gliotransmitters
The identification of VPAT has helped to partially determine the novel signaling pathway(s) that polyamines undergo:vesicular storage, exocytosis, followed by binding to target receptors in postsynaptic membranes.
It should be noted that glia, particularly astrocytes, control synaptic plasticity and are involved in the regulation of higher brain functions, such as learning and memory50. d-Serine plays an essential role in these regulatory processes: d-serine is stored in synaptic-like microvesicles (SLMV), and vesicular d-serine is released in a Ca2+-dependent manner from astrocytes upon AMPA-type receptor stimulation and acts as a co-agonist of NMDA receptors upon binding to the specific site50. Moreover, vesicular d-serine transport activity was recently detected in astrocytic SLMV, which is energetically coupled with vacuolar H+-ATPase and actually behaves as an H+/d-serine antiporter51. Thus, the physiological relevance and the mode of action of d-serine seem to be very similar to those of Spm and Spd shown here. It is possible that postsynaptic stimulation triggers the polyaminergic and d-serinergic responses of astrocytes, leading to enhanced gliotransmission.
In conclusion, a seventh vesicular neurotransmitter transporter, VPAT, which is responsible for vesicular storage of polyamines, has been identified. Through identification and initial characterization of VPAT, the mechanism by which polyamines are stored and released from polyamine-secreting cells was partially clarified.
Methods
cDNA
cDNA of human SLC18B1 (Accession No. NM052831) and mouse SLC18B1 (Accession No. NM183116) were cloned by PCR.
Expression of the SLC18B1 gene
Human or mouse total RNAs from several tissues were purchased from Clontech. cDNA was generated from total RNA using a reverse transcriptase kit (Toyobo) using 1 μg of total RNA as the template. The resulting cDNA pool was used for real-time PCR. Real-time PCR was carried out with 400 nmol/L of specific forward and reverse primers and 5 units/μL of SYBR Premix Ex Taq (TaKaRa). Thirty-five cycles of amplification were performed with denaturation at 95°C for 15 s and annealing/extension at 60°C for 30 s. The primer sets used were as follows: for detection of human SLC18B1, 5′-ctggcatccttggtatttggaa-3′, 5′-caataaatactggcccatctggaa-3′; for mouse SLC18B1, 5′-tcgagtgggcagcagctatg-3′, 5′-ccagagctctcaaggctaggtgtc-3′; and for rat SLC18B1, 5′-acggaataagtacgctgggacttg-3′, 5′-tagctgctgcccactcgaaac-3′. The expression of SLC18B1 was evaluated relative to the mRNA expression of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (G3PDH).
Purification of SLC18B1 protein
Recombinant baculovirus containing human SLC18B1 cDNA was constructed using the Bac-to-Bac baculovirus expression system (Invitrogen) according to the manufacturer's protocol. Human SLC18B1 cDNA was amplified by PCR using the primers 5′-caccatggaggcgctgggtgac-3′ and 5′-actaggtttcattaggcaagag-3′. High Five cells were infected by recombinant baculoviruses at a multiplicity of infection (MOI) of 1 and cultured for a further 48 hours.
The cells (1–2 × 108) were suspended in a buffer containing 20 mM Tris-HCl (pH 8.0), 0.1 M sodium acetate, 10% glycerol, 0.5 mM dithiothreitol, 10 μg/mL pepstatin A, and 10 μg/mL leupeptin, and disrupted by sonication with a TOMY UD200 tip sonifier. Cell lysates were centrifuged at 700 × g for 10 minutes to remove debris and the resultant supernatant was centrifuged at 160000 × g for 1 hour. The pellet (membrane fraction) was suspended in buffer containing 20 mM MOPS-Tris (pH 7.0), 10% glycerol, 10 μg/mL pepstatin A, and 10 μg/mL leupeptin at approximately 1.5 mg protein/mL, and was solubilized with 2% octyl glucoside. After centrifugation at 260000 × g for 30 minutes, the supernatant was taken and applied to 1 mL of Ni-NTA Superflow resin (Qiagen). After incubation for 4 hours at 4°C, the resin was washed with 20 mL of 20 mM MOPS-Tris (pH 7.0), 5 mM imidazole, 20% glycerol, and 1% octyl glucoside52. SLC18B1 protein was eluted from the resin with 3 mL of the same buffer containing 80 mM imidazole and could be stored at −80°C without loss of activity for at least a few months.
Reconstitution of SLC18B1 protein
Reconstitution of purified SLC18B1 protein into liposomes was carried out by the freeze-thaw method as described53. Briefly, 40 μg of SLC18B1 protein was mixed with 90 μg of FoF1-ATPase and asolectin liposomes (0.5 mg lipid), frozen at −80°C, and left at this temperature for at least 15 minutes. The mixture was thawed quickly by holding the sample tube in the hands and diluted 60-fold with reconstitution buffer containing 20 mM MOPS-Tris (pH 7.0), 0.1 M potassium chloride, and 5 mM magnesium acetate. Reconstituted proteoliposomes were sedimented by centrifugation at 200000 × g for 1 hour at 4°C, suspended in 0.2 mL of 20 mM MOPS-Tris (pH 7.0) containing 0.1 M potassium chloride and 5 mM magnesium acetate, and used within 1 day of preparation. Asolectin liposomes were prepared as follows: soybean lecithin (20 mg; Sigma Type IIS) was suspended in 2 mL of 20 mM MOPS-NaOH (pH 7.0) containing 1 mM dithiothreitol. The mixture was sonicated in a bath-type sonicator until clear, divided into small aliquots, and stored at −80°C until use.
Expression, purification, and reconstitution of VMAT2 and VAChT
The cDNAs of rat VMAT2 and human VAChT were described previously54,55. VMAT2 and VAChT protein fused with hydrophilic Escherichia coli protein YbeL (β-VAChT-β) were expressed in insect cells and E. coli C43 (DE3) cells, respectively. They were purified, reconstituted into proteoliposomes, and assayed in a manner analogous to that described for human SLC18B1 above54,55. The apparently higher molecular weight of β-VAChT-β on SDS-PAGE (~80 kDa) was due to fusion with YbeL (Supplementary Fig. 3).
Uptake of neurotransmitters by proteoliposomes
Transport assays were performed as described previously54,55. Proteoliposomes (1.5 μg total protein/assay) were suspended in 20 mM MOPS-Tris (pH 7.0), 5 mM magnesium acetate, and 0.1 M potassium chloride, and then incubated for 1 minute at 27°C. ATP was added to a final concentration of 5 mM and the mixture was incubated for a further 1 minute. The assay was initiated by addition of 10 μM [ 3H] Spm (0.5 MBq/μmol), 100 μM [ 3H] Spd (0.5 MBq/μmol), 10 μM [ 3H] serotonin (0.5 MBq/μmol), or 10 μM [ 3H] acetylcholine (0.5 MBq/μmol); 130 μL aliquots were taken at the times indicated and centrifuged through a Sephadex G-50 (fine) spin column at 760 × g for 2 minutes. Radioactivity in the eluate was measured with a liquid scintillation counter (PerkinElmer).
Hippocampal neuron and astrocyte cell culture
Hippocampal cultures were obtained from embryonic Wistar rats (E17) and C57BL/6 mice (E17). Briefly, after dissection, the hippocampi were dissociated by treatment with trypsin (0.25% w/v for 15 minutes at 37°C). Astrocyte cultures were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum in an atmosphere of 5% CO2/95% air at 37°C and grown to confluence. The medium was changed every 3–4 days. After reaching confluency, the cells were subcultured and used in experiments during the following 3 days. Neuron cultures were plated on poly-l-lysine and laminin-coated cell culture dishes and grown in Neurobasal medium (Invitrogen) containing B27 supplement (Invitrogen). The culture medium was exchanged every 2–3 days. Experiments were performed between 18 and 21 days in culture.
Antibodies
Site-specific polyclonal antibodies against human and mouse SLC18B1 were prepared by repeatedly injecting GST-fusion polypeptides encoding Met1–Thr70 of human SLC18B1 and Glu429–Thr457 of mouse SLC18B1 into rabbits, respectively. Subunit A of V-ATPase rabbit polyclonal antibody and anti-VMAT2 rabbit polyclonal antibody against synthetic peptide (Cys-Pro-Ile-Gly-Glu-Asp-Glu-Glu-Ser-Glu-Ser-Asp) prepared in-house were determined previously56. The following primary antibodies were purchased from the commercial sources indicated: anti-GFAP mouse monoclonal antibody (Thermo Fisher Scientific), anti-synaptophysin mouse monoclonal antibody (Progen), anti-160-kDa Neurofilament medium mouse monoclonal antibody (Abcam), anti-PDI mouse monoclonal antibody (Abcam), anti-NeuN mouse monoclonal antibody (Millipore), anti-VAMP2 mouse monoclonal antibody (Synaptic System), anti-CgA mouse monoclonal antibody (Affinity BioReagents), anti-GM130 mouse monoclonal antibody (BD Biosciences), anti-EEA1 mouse monoclonal antibody (BD Biosciences), anti-secretogranin II mouse monoclonal antibody (Abcam), anti-cathepsin D goat polyclonal antibody (Santa Cruz Biotechnology), anti-Spm/Spd rabbit polyclonal antibody (Novus Biologicals), and anti-VAChT rabbit polyclonal antibody (Abcam).
Immunohistochemistry
Indirect immunofluorescence microscopy was performed as described57. Brain samples were obtained from male Wistar rats or C57BL/6 mice perfused intracardially with 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Cultured cells on poly-l-lysine-coated coverslips were fixed with 4% paraformaldehyde in PBS for 30 minutes. The primary antibody against mouse SLC18B1 protein was diluted 1:200 (v/v) with PBS containing 0.5% (w/v) BSA and the sample was incubated for 1 hour at room temperature. Washing steps and secondary antibody treatment were performed as described57. The specimens were observed either under an Olympus FV300 confocal laser microscope (Olympus) or BIOZERO (Keyence).
For immunoperoxidase labeling, coronal sections of the mouse brain 30 μm thick were cut on a freezing microtome. Immunoperoxidase staining was performed on free-floating sections according to standard immunoperoxidase protocols as described58. The sections were photographed with BIOZERO (Keyence) and merged to obtain whole brain images using image-joint software BZ-Analyzer (Keyence).
RNAi
HiPerFect transfection reagent (Qiagen) was used for transfection of 10 nM AllStars negative control siRNA or rat SLC18B1 siRNA (Qiagen, SI01708707). SLC18B1 expression was assayed after 48 hours incubation.
Miscellaneous procedures
Transport assay by the synaptic vesicles, SDS-PAGE, and western blotting were performed as described59. Protein concentration was assayed using BSA as a standard60.
Data analysis
All numerical values are shown as the means ± SEM; n = 3–6, unless otherwise specified. Statistical significance was determined by Student's t test.
Author Contributions
M.H., T.M., H.O. and Y.M. designed the experiments, analyzed data and wrote the paper. M.H., T.M., Y.H., T.T., Y.H., S.M., H.O. and A.Y. performed the experiments.
Supplementary Material
Supplementaly dataset
Acknowledgments
This work was supported in part by Grants-in-Aid from the Japanese Ministry of Education, Science, Sports, and Culture for Scientific Research (A) (No. 25253008) and the Japan Science and Technology Agency for Japan-Israel Scientific Research Cooperation to Y.M., Program to Disseminate Tenure Tracking System, MEXT, Japan, Grants-in-Aid for Young Scientists (B) (No. 25860062) and Grants-in-Aid for Scientific Research on Innovative Areas (No. 26117514) to H.M., Grants-in-Aid for Scientific Research (C) (No. 26460067), the Uehara Memorial Foundation, the Takeda Science Foundation and the Smoking Research Foundation to T.M., and by Grants-in-Aid for Scientific Research (B) (No. 21370057) to H.O. We thank Dr. Nathan Nelson at Tel Aviv University for kind support during the study.
References
- Cohen S. S. A guide to the polyamines. Oxford University Press, New York, USA. (1998) [Google Scholar]
- Germer E. W. & Meyskens F. J. Jr Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 4, 781–792 (2004). [DOI] [PubMed] [Google Scholar]
- Medina M. A. et al. Biogenic amines and polyamines: similar biochemistry for different physiological mission and biomedical applications. Crit Rev Biochem Mol Bio 38, 23–59 (2003). [DOI] [PubMed] [Google Scholar]
- Casero R. A. Jr & Marton L. J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 6, 373–390 (2007). [DOI] [PubMed] [Google Scholar]
- Williams K. Modulation and Block of ion channels: a new biology of polyamines. Cell Signal. 9, 1–13 (1997). [DOI] [PubMed] [Google Scholar]
- Ogden K. K. & Traynelis S. F. New advances in NMDA receptor pharmacology. Trends Pharmacol. Sci. 32, 726–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker E. et al. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science 266, 1068–1072 (1994). [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N. & Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 (1994). [DOI] [PubMed] [Google Scholar]
- Bowie D. & Mayer M. L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 (1995). [DOI] [PubMed] [Google Scholar]
- Rozov A. & Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401, 594–598 (1999). [DOI] [PubMed] [Google Scholar]
- Huang C. J. & Moczydlowski E. Cytoplasmic polyamines as permeant blockers and modulators of the voltage-gated sodium channel. Biophys. J. 80, 1262–1279 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M. & Mayer M. L. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J. Physiol. 464, 131–163 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott D. D., Washburn M. S., Zhang S. & Dingledine R. J. Subunit-dependent modulation of kainate receptors by extracellular protons and polyamines. J. Neurosci. 23, 1179–1188 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern G. P., Wang X. & Miyares R. L. Polyamines are potent ligands for the capsaicin receptor TRPV1. J. Biol. Chem. 281, 8991–8995 (2006). [DOI] [PubMed] [Google Scholar]
- Quinn S. J. et al. The Ca2+-sensing receptor: a target for polyamines. Am. J. Physiol. 273, C1315–C1323 (1997). [DOI] [PubMed] [Google Scholar]
- Fleidervish I. A., Libman L., Katz E. & Gutnick M. J. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc. Nat.l Acad. Sci. USA 105, 18994–18999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori L. M. & Turecki G. Implication of the polyamine system in mental disorders. J. Psychiatry Neurosci. 33, 102–110 (2008). [PMC free article] [PubMed] [Google Scholar]
- Nishimura K., Shiina R., Kashiwagi K. & Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 139, 81–90 (2006). [DOI] [PubMed] [Google Scholar]
- Seiler N. Polyamine metabolism and function in brain. Neurochem. Int. 3, 95–110 (1981). [DOI] [PubMed] [Google Scholar]
- Laube G. & Veh R. W. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 19, 171–179 (1997). [DOI] [PubMed] [Google Scholar]
- Laube G., Bernstein H. G., Wolf G. & Veh R. W. Differential distribution of spermidine/spermine-like immunoreactivity in neurons of the adult rat brain. J Comp. Neurol. 444, 369–386 (2002). [DOI] [PubMed] [Google Scholar]
- Bernstein H. G. & Müller M. The cellular localization of the L-ornithine decarboxylase/polyamine system in normal and diseased central nervous systems. Prog. Neurobiol. 57, 485–505 (1999). [DOI] [PubMed] [Google Scholar]
- Krauss M. et al. Spermidine synthase is prominently expressed in the striatal patch compartment and in putative interneurones of the matrix compartment. J. Neurochem. 97, 174–189 (2006). [DOI] [PubMed] [Google Scholar]
- Krauss M. et al. Cellular and subcellular rat brain spermidine synthase expression patterns suggest region-specific roles for polyamines, including cerebellar pre-synaptic function. J. Neurochem. 103, 679–693 (2007). [DOI] [PubMed] [Google Scholar]
- Fage D., Voltz C., Scatton B. & Carter C. Selective release of spermine and spermidine from the ray striatum by N-methyl-D-aspartate receptor activation in vivo. J. Neurochem. 58, 2170–2175 (1992). [DOI] [PubMed] [Google Scholar]
- Harman R. J. & Shaw G. G. The spontaneous and evoked release of spermine from rat brain in vitro. Br. J. Pharmacol. 73, 165–174 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko T. et al. Polyamine transport, accumulation, and release in brain. J. Neurochem. 84, 610–617 (2003). [DOI] [PubMed] [Google Scholar]
- Dot J., Lluch M., Blanco I. & Rodríguez-Alvarez J. Polyamine uptake in cultured astrocytes: characterization and modulation by protein kinases. J. Neurochem. 75, 1917–1926 (2000). [DOI] [PubMed] [Google Scholar]
- Omote H., Miyaji T., Juge N. & Moriyama Y. Vesicular neurotransmitter transporter: bioenergetics and regulation of glutamate transport. Biochemistry 50, 5558–5565 (2011). [DOI] [PubMed] [Google Scholar]
- Omote H. & Moriyama Y. Vesicular neurotransmitter transporters: an approach for studying transporters with purified proteins. Physiology 28, 39–50 (2013). [DOI] [PubMed] [Google Scholar]
- Jacobsson J. A., Stephansson O. & Fredriksson R. C6ORF192 forms a unique evolutionary branch among solute carriers (SLC16, SLC17, and SLC18) and is abundantly expressed in several brain regions. J. Mol. Neurosci. 41, 230–242 (2010). [DOI] [PubMed] [Google Scholar]
- Schuldiner S. A molecular glimpse of vesicular monoamine transporters. J. Neurochem. 62, 2067–2078 (1994). [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Schäfer M. K., Weihe E. & Schütz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pfuegers Arch. 447, 636–640 (2004). [DOI] [PubMed] [Google Scholar]
- Parsons S. M. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 14, 2423–2434 (2000). [DOI] [PubMed] [Google Scholar]
- Yelin R. & Schuldiner S. The pharmacological profile of the vesicular monoamine transporter resembles that of multidrug transporters. FEBS Lett. 377, 201–207 (1995). [DOI] [PubMed] [Google Scholar]
- Bravo D. T., Kolmakova N. G. & Parsons S. M. New transport assay demonstrate vesicular acetylcholine transporter has many alternative substrates. Neurochem. Int. 47, 243–247 (2005). [DOI] [PubMed] [Google Scholar]
- Soulet D. et al. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J. Biol. Chem. 279, 49355–49366 (2004). [DOI] [PubMed] [Google Scholar]
- Hougaard D. M., Nielsen J. H. & Larsson L. I. Localization and biosynthesis of polyamines in insulin-producing cells. Biochem. J. 238, 43–47 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Faroldi G. et al. Polyamines are present in mast cell secretory granules and are important for granule homeostasis. PLoS One 5, e15071 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K. & Kashiwagi K. Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiol. Biochem. 48, 506–512 (2010). [DOI] [PubMed] [Google Scholar]
- Fujita M. et al. Natural variation in a polyamine transporter determines paraquat tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 109, 6343–6347 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merickel A., Rosandich P., Peter D. & Edwards R. H. Identification of residues involved in substrate recognition by a vesicular monoamine transporter. J. Biol. Chem. 270, 25798–25804 (1995). [DOI] [PubMed] [Google Scholar]
- Merickel A., Kaback H. R. & Edwards R. H. Charged residues in transmembrane domains II and XI of a vesicular monoamine transporter form a charge pair that promotes high affinity substrate recognition. J. Biol. Chem. 272, 5403–5408 (1997). [DOI] [PubMed] [Google Scholar]
- Steiner-Mordoch S., Shirvan A. & Schuldiner S. Modification of the pH profile and tetrabenazine sensitivity of rat VMAT1 by replacement of aspartate 404 with glutamate. J. Biol. Chem. 271, 13048–13054 (1996). [DOI] [PubMed] [Google Scholar]
- Bravo D. T., Kolmakova N. G. & Parsons S. M. Mutational and pH analysis of ionic residues in transmembrane domains of vesicular acetylcholine transporter. Biochemistry 44, 7955–7966 (2005). [DOI] [PubMed] [Google Scholar]
- Kim M. H. et al. Mutational analysis of aspartate residues in the transmembrane regions and cytoplasmic loops of rat vesicular acetylcholine transporter. J. Biol. Chem. 274, 673–680 (1999). [DOI] [PubMed] [Google Scholar]
- Khare P., Ojeda A. M., Chandrasekaran A. & Parsons S. M. Possible important pair of acidic residues in vesicular acetylcholine transporter. Biochemistry 49, 3049–3059 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda A. M., Kolmakova N. G. & Parsons S. M. Acetylcholine binding site in the vesicular acetylcholine transporter. Biochemistry 43, 11163–11174 (2004). [DOI] [PubMed] [Google Scholar]
- Travis E. R. et al. Differential quantal release of histamine and 5-hydroxytryptamine from mast cells of vesicular monoamine transporter 2 knockout mice. Proc. Natl. Acad. Sci. USA 97, 162–167 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G., Navarrete M. & Araque A. Tripartite synapses: astrocyte process and control synaptic information. Trends Neurosci. 32, 421–431 (2009). [DOI] [PubMed] [Google Scholar]
- Martineau M. et al. Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. J. Neurosci. 33, 3413–3423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K. et al. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 105, 5683–5686 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N. et al. Vesicular glutamate transporter contains two independent transport machineries. J. Biol. Chem. 281, 39499–39506 (2005). [DOI] [PubMed] [Google Scholar]
- Leviatan S., Sawada K., Moriyama Y. & Nelson N. Combinatorial method for overexpression of membrane proteins in Escherichia coli. J. Biol. Chem. 285, 23548–23556 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N. et al. Metabolic control of vesicular glutamate transport and release. Neuron 68, 99–112 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y. et al. Localization of N-ethylmaleimide-sensitive fusion protein in pinealocytes. Neuroreport 6, 1757–1760 (1995). [DOI] [PubMed] [Google Scholar]
- Hayashi M. et al. Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J. Biol. Chem. 278, 1966–1974 (2003). [DOI] [PubMed] [Google Scholar]
- Otsuka M. et al. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 102, 17923–17928 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y. & Yamamoto A. Vesicular glutamate transporter in microvesicles from bovine pineal glands. Driving force, mechanism of chloride anion activation, and substrate specificity. J. Biol. Chem. 270, 22314–22320 (1995). [DOI] [PubMed] [Google Scholar]
- Schaffner W. & Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56, 502–514 (1973). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementaly dataset