Abstract
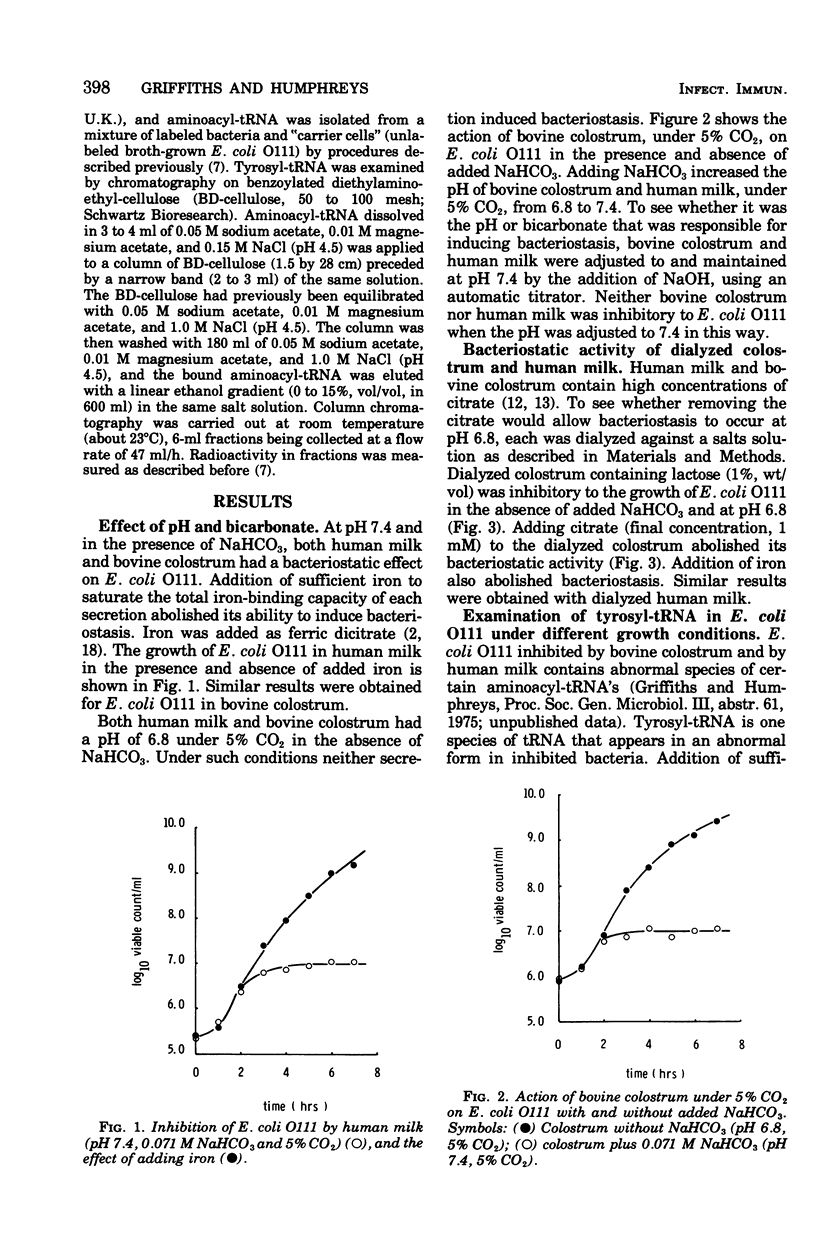
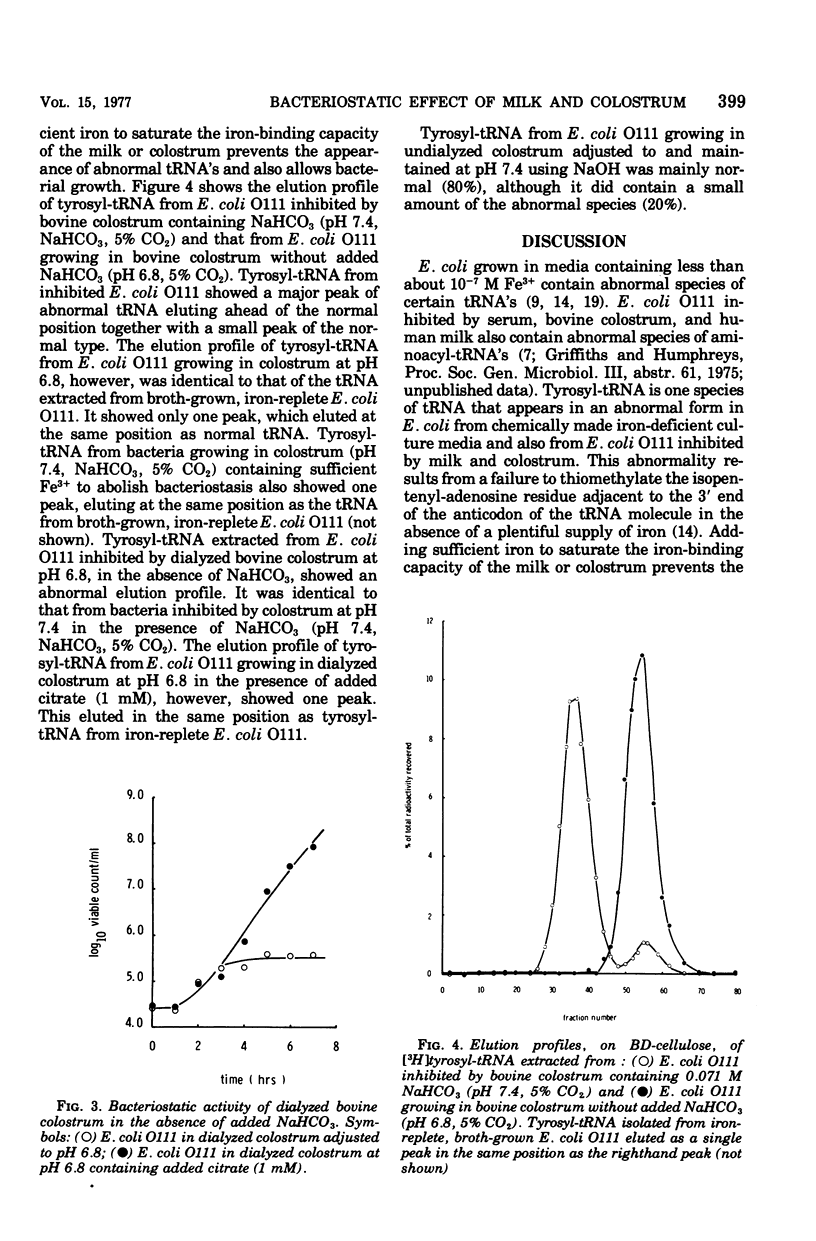
At pH 7.4 and in the presence of NaHCO3, human milk and bovine colostrum inhibited the growth of Escherichia coli O111. Adding sufficient iron to saturate the iron-binding capacity of the lactoferrin present in the milk or colostrum prevented bacteriostasis. At pH 6.8 neither molk nor colostrum inhibited E. coli 0111. Adjusting the pH to 7.4 with NaHCO3 resulted in the development of bacteriostatic activity. Adjusting the pH to 7.4 with NaOH was ineffective. Dialyzed colostrum and milk inhibited bacterial growth at pH 6.8 in the absence of added NaHCO3; addition of citrate or iron abolished bacteriostasis. The chromatographic elution profile of tyrosyl-transfer ribonucleic acid (tRNA) from iron-replete E. coli differs significantly from that of tyrosyl-tRNA from iron-deficient organisms. Examination of the elution profile tyrosyl-tRNA from E. coli 0111 growing in colostrum without added NaHCO3 showed that such bacteria were fully replete in iron. The nature of the elution profile of tyrosyl-tRNA also showed that iron was freely available to the bacteria when citrate was added to dialyzed colostrum but not available in its absence, even at pH 6.8. Results support the idea that the bacteriostatic action of milk and colostrum, due to the combined action of antibody and lactoferrin, depends on the addition of bicarbonate to counteract the iron-mobilizing effect of the citrate normally present in these secretions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P. Citrate-mediated exchange of FE3+ among tranferrin molecules. Biochem Biophys Res Commun. 1968 Jul 26;32(2):220–226. doi: 10.1016/0006-291x(68)90372-0. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bezkorovainy A., Zschocke R. H. Structure and function of transferrins. I. Physical, chemical, and iron-binding properties. Arzneimittelforschung. 1974 Apr;24(4):476–485. [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E. Abnormal phenylalanyl-tRNA found in serum inhibited Escherichia coli, strain 0111. FEBS Lett. 1972 Sep 1;25(1):159–164. doi: 10.1016/0014-5793(72)80476-9. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Mechanism of action of specific antiserum on Pasteurella septica. Selective inhibition of net macromolecular synthesis and its reversal by iron compounds. Eur J Biochem. 1971 Nov 11;23(1):69–76. doi: 10.1111/j.1432-1033.1971.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Hanson L. A., Winberg J. Breast milk and defence against infection in the newborn. Arch Dis Child. 1972 Dec;47(256):845–848. doi: 10.1136/adc.47.256.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez H., Skjold A. C., Hedgcoth C. Precursor relationship of phenylalanine transfer ribonucleic acid from Escherichia coli treated with chloramphenicol or starved for iron, methionine, or cysteine. J Bacteriol. 1975 Jan;121(1):44–54. doi: 10.1128/jb.121.1.44-54.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971 May 15;39(1):119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Peaker M., Linzell J. L. Citrate in milk: a harbinger of lactogenesis. Nature. 1975 Feb 6;253(5491):464–464. doi: 10.1038/253464a0. [DOI] [PubMed] [Google Scholar]
- Reiter B., Brock J. H., Steel E. D. Inhibition of Escherichia coli by bovine colostrum and post-colostral milk. II. The bacteriostatic effect of lactoferrin on a serum susceptible and serum resistant strain of E. coli. Immunology. 1975 Jan;28(1):83–95. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Gefter M. L. An iron-dependent modification of several transfer RNA species in Escherichia coli. J Mol Biol. 1969 Dec 28;46(3):581–584. doi: 10.1016/0022-2836(69)90197-1. [DOI] [PubMed] [Google Scholar]
- Schlabach M. R., Bates G. W. The synergistic binding of anions and Fe3+ by transferrin. Implications for the interlocking sites hypothesis. J Biol Chem. 1975 Mar 25;250(6):2182–2188. [PubMed] [Google Scholar]
- South M. A. Enteropathogenic Escherichia coli disease: new developments and perspectives. J Pediatr. 1971 Jul;79(1):1–11. doi: 10.1016/s0022-3476(71)80051-3. [DOI] [PubMed] [Google Scholar]


