Abstract
Background
To investigate the antidiabetic effects of hydrolyzed ginseng extract (HGE) for Korean participants in an 8-wk, randomized, double-blinded, placebo-controlled clinical trial.
Methods
Impaired fasting glucose participants [fasting plasma glucose (FPG) ≥ 5.6mM or < 6.9mM] who had not been diagnosed with any disease and met the inclusion criteria were recruited for this study. The 23 participants were randomly divided into either the HGE (n = 12, 960 mg/d) or placebo (n = 11) group. Outcomes included measurements of efficacy (FPG, postprandial glucose, fasting plasma insulin, postprandial insulin, homeostatic model assessment-insulin resistance, and homeostatic model assessment-β) and safety (adverse events, laboratory tests, electrocardiogram, and vital signs).
Results
After 8 wk of HGE supplementation, FPG and postprandial glucose were significantly decreased in the HGE group compared to the placebo group. No clinically significant changes in any safety parameter were observed. Our study revealed that HGE is a potent antidiabetic agent that does not produce noticeable adverse effects.
Conclusion
HGE supplementation may be effective for treating impaired fasting glucose individuals.
Keywords: clinical trial, hydrolyzed ginseng extract, impaired fasting glucose, oral glucose tolerance test, Panax ginseng
1. Introduction
There is much concern about the dramatic increase in the population of type 2 diabetes patients. Both the recent prevalence rate and the estimated increase in incidence have become public health problems and create a serious burden on society. In order to identify an individual at increased risk of developing type 2 diabetes, the concept of impaired fasting glucose (IFG) has been introduced by the American Diabetes Association [1]. Individuals with IFG have fasting plasma glucose levels between 5.6 mmol/L and 6.9 mmol/L [2]. In addition to being more likely to develop diabetes in the near future, these people are at greater risk for cardiovascular disease [3,4]. Therefore, effective approaches to control blood glucose levels are urgently needed. Previous large-scale studies have demonstrated that lifestyle intervention is the best way to achieve this goal [5–7]. Pharmacotherapy is also used to manage individuals with IFG. Paradoxically, medications used to control blood glucose often cause metabolic side effects such as weight gain [8,9]. Thus, the development of alternative therapies is of paramount importance, and in this context, herbal extracts are among the most promising source of new treatments for the prevention of diabetes.
Ginseng (Panax ginseng Meyer) has been used as traditional medicine in the treatment of metabolic diseases, cancer, cardiovascular diseases, and other diseases in a number of Asian countries [10–12]. The bioactive constituents of ginseng include various saponins (ginsenosides) and nonsaponins, and the pharmacological activities of ginseng are mainly attributed to ginsenosides [13,14]. To date, 80 ginsenosides have been identified in ginseng. These ginsenosides are further biotransformed by intestinal bacteria, which increase intestinal absorption and bioactivity and diminish the toxicity of the metabolite compared to its parent compound [15,16]. In this regard, fermentation using microorganisms or treatment with an appropriate enzyme for the production of more effective compounds has been extensively studied.
In previous studies, pectinase was used for the biotransformation of ginsenosides in ginseng extract, and this process increased the level of bioactive compounds, including compound K (also known as IH-901), resulting in improved pharmacological functions [17,18]. Although the antidiabetic activities of ginseng have been well documented in animal [19] and human [20] studies, the improved effects of hydrolyzed ginseng on diabetic patients are not clear. Therefore, in this study, we investigated whether hydrolyzed ginseng extract (HGE) could be effective in reducing the risk of type 2 diabetes in individuals with IFG.
2. Materials and methods
2.1. Study design
This study was an 8-wk, randomized, double-blinded, placebo-controlled clinical trial. The randomization scheme was generated by a computerized procedure. Neither the investigators nor the participants knew the randomization code until the trial was completed and database locked. Participants who responded and met the entry criteria during a telephone screening interview were scheduled for a baseline visit. Participants were scheduled for a screening visit, during which the informed consent was reviewed and signed. At 0 wk and 8 wk, a 75-g oral glucose tolerance test (OGTT) was performed after an overnight fast. A catheter was inserted into a vein and blood samples were obtained prior to (0 min) and after (15 min, 30 min, 60 min, 90 min, and 120 min) consuming a 75-g glucose drink. During the 8-wk intervention period, participants were asked to continue their usual diets and to not take any other functional foods or dietary supplements. Participants were also asked to report for the assessment of any adverse events or any changes in lifestyle and eating patterns and to assess pill compliance.
2.2. Participants
The study participants were recruited from the Clinical Trial Center for Functional Foods at Chonbuk National University Hospital, Jeonbuk, Republic of Korea during 2009. IFG participants [fasting plasma glucose (FPG) ≥ 5.6mM and < 6.9mM] who had not been diagnosed with any disease and met the inclusion criteria were recruited for this study. Exclusion criteria for the study were: (1) abnormal lipid profile values; (2) acute/chronic inflammation; (3) treatment with corticosteroids within the past 4 wk; (4) cardiovascular disease, such as arrhythmia, heart failure, myocardial infarction, or a pacemaker; (5) allergic or hypersensitivity to any of the ingredients in the test products; (6) history of a disease that could interfere with the test products or impede their absorption, such as gastrointestinal disease (Crohn's Disease) or gastrointestinal surgery; (7) participation in any other clinical trials within the past 2 mo; (8) renal disease, such as acute/chronic renal failure or nephrotic syndrome; (9) abnormal hepatic function; (10) treatment by hypolipidemic drug therapy within the past 3 mo; (11) treatment by antipsychotic drug therapy within the past 2 mo; (12) a laboratory test, medical, or psychological conditions deemed by the investigators to interfere with successful participation in the study; (13) history of alcohol or substance abuse; or (14) pregnancy or breast feeding. All participants gave written informed consent prior to beginning the study. The protocol was approved by the Functional Foods Institutional Review Board (FFIRB) of Chonbuk National University Hospital (FFIRB number: 2009-02-001). The protocol was registered in www.clinicaltrials.gov (NCT01854164).
2.3. Test supplement
HGE was obtained from ILHWA Co. Ltd, (Guri, Republic of Korea), as described previously, with slight modifications [17]. The HGE contained 7.54 mg/g of Rg1, 1.87 mg/g of Re, 5.42 mg/g of Rb1, 0.29 mg/g of Rc, 0.36 mg/g of Rb2, and 0.70 mg/g of Rd. The compound K content in the HGE was 6.3 mg/g. It was administered as a capsule (480 mg/cap and 960 mg/d) composed of 30% HGE and 70% diluting agent (pumpkin seed oil, refined palm oil, yellow wax, and sorbic acid). The placebo capsules were composed primarily of powdered rice and pumpkin seed oil and were matched with regard to energy content, flavor, appearance, and dosage (Table 1).
Table 1.
Composition of test products provided/d
| Placebo supplement |
Hydrolyzed ginseng extract supplement |
||
|---|---|---|---|
| Component | Content (%) | Component | Content (%) |
| Powdered rice | 10 | Fermented ginseng extract | 30 |
| Pumpkin seeds oil | 65.84 | Pumpkin seeds oil | 55.4 |
| Refined palm oil | 15.44 | Refined palm oil | 9.0 |
| Yellow wax | 7.72 | Yellow wax | 4.6 |
| Sorbic acid | 1 | Sorbic acid | 1 |
| Total | 100 | Total | 100 |
All participants were instructed to take two capsules/d (one capsule each after breakfast and dinner). HGE and placebo capsules were packaged indistinguishably and labeled with the participant's number. Participants were instructed to bring all remaining supplements at each visit and were withdrawn from the study if the supplement consumption was > 70% of the prescribed dose.
2.4. Outcome measures
Efficacy assessments included the following: glucose [FPG, plasma glucose during OGTT (postprandial plasma glucose: PPG), glucose incremental area under the curve (iAUC), and glucose maximum concentration (Cmax)], insulin [fasting plasma insulin (FPI), plasma insulin during OGTT (postprandial plasma insulin: PPI), insulin iAUC, and insulin Cmax], homeostatic model assessment (HOMA)–insulin resistance (IR), and HOMA-β, glycated albumin, fructosamine, and HbA1c. The glucose and insulin iAUCs during the OGTT were determined by the trapezoidal method.
Safety assessments included the following: electrocardiogram, hematology, and laboratory tests (white blood cells, red blood cells, hemoglobin, hematocrit, platelet count, total protein, albumin, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, creatinine, etc.), pulse rate, and blood pressure, along with a personal report and were recorded at every visits.
Blood samples were analyzed on a Hitachi 7600-110 analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan).
2.5. Statistical analysis
Statistical analysis was performed using SAS version 9.2 (SAS Institute, Chicago, IL. USA). Data are shown as the mean values and standard deviation. A Chi-square test was performed to determine differences at baseline in frequencies of categorized variables between the groups. The Student paired t test was used for continuous measures to assess differences between prior to and after the 8-wk intervention period. A linear mixed-effects model was applied to repeated measures data for each continuous outcome variable and data. Fixed effects included treatment group, treatment visit, and the interaction between treatment group and visit. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Participants
Among the 100 participants screened, 77 participants were excluded due to laboratory test results consistent with the exclusion criteria. The remaining 23 participants fulfilled the study criteria and were distributed into two groups, HGE (n = 12, 960 mg/day) and placebo (n = 11).
One participant from the HGE group and two participants from the placebo group withdrew from the study due to personal reasons. Twenty participants (11 in HGE group and 9 in placebo group) were able to finish the study (Fig. 1).
Fig. 1.

CONSORT diagram showing the study flow during the 8-wk intervention.
3.2. Participant characteristics
General characteristics of the participants are shown in Table 2. There were no significant differences in baseline characteristics between the HGE and placebo groups, such as age, sex, weight, height, and FPG.
Table 2.
Demographic characteristics of the study participants
| Placebo group (n = 9) | HGE group (n = 11) | p1) | |
|---|---|---|---|
| Age (y) | 44.56 ± 10.48 | 50.45 ± 12.36 | 0.271 |
| Weight (kg) | 68.00 ± 12.75 | 67.00 ± 11.43 | 0.855 |
| Height (cm) | 165.33 ± 7.89 | 167.18 ± 11.33 | 0.684 |
| Sex (M/F) | 4/5 | 6/5 | 1.0002) |
| FPG (mg/dL) | 105.56 ± 6.54 | 109.36 ± 6.96 | 0.227 |
| FPG (mmol/L) | 5.9 ± 0.4 | 6.1 ± 0.4 | 0.227 |
All values are presented as mean ± standard deviation
FPG, fasting plasma glucose. HGE, hydrolyzed ginseng extract
Analyzed by independent t-test and p-value compared to the placebo group
Analyzed by Fisher's exact test
3.3. Safety evaluation
At each of the clinic visits by the study participants, the investigators determined whether there were any adverse events experienced since the previous visit. No moderate or serious adverse events were reported during the 8-wk study period. The evaluation was also expanded to include laboratory tests, electrocardiogram, and vital signs (blood pressure and pulse). The results of the clinical tests were in the normal range, so no participants withdrew because of adverse events.
3.4. Efficacy evaluation
Changes in FPG, PPG, glucose iAUC, glucose Cmax, FPI, PPI, insulin iAUC, and insulin Cmax prior to and after the 8-wk intervention period are shown in Figs. 2, 3. After the 8-wk intervention, statistically significant differences were found in FPG (p = 0.017) and PPG60min (p = 0.01). PPG30min (p = 0.059), FPI (p = 0.063), and PPI60min (p = 0.077) showed a tendency to improve more than the placebo group, although there were no significant differences between the groups.
Fig. 2.
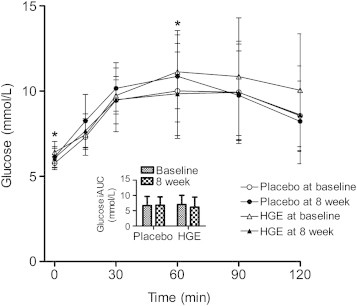
Blood glucose level and incremental area under the curve (iAUC) obtained during the oral glucose tolerance test prior to and after treatment with hydrolyzed ginseng extract or placebo. All values are presented as mean ± standard deviation. Derived from generalized linear model adjusted for baseline and p-value compared to the placebo group. *p < 0.05
Fig. 3.

Blood insulin level and incremental area under the curve (iAUC) obtained during the oral glucose tolerance test prior to and after treatment with hydrolyzed ginseng extract or placebo. All values are presented as mean ± standard deviation. Derived from generalized linear model adjusted for baseline.
Changes in HOMA–IR and HOMA-β prior to and after the 8-wk intervention period are shown in Table 3. After the 8-wk intervention, there were no significant differences between the groups.
Table 3.
Homeostatic model assessment (HOMA)–insulin resistance (IR) and HOMA-β level obtained prior to and after treatment with hydrolyzed ginseng extract (HGE) or placebo
| Placebo group (n = 9) |
HGE group (n = 11) |
p2) | |||||
|---|---|---|---|---|---|---|---|
| 0 week | 8 week | p1) | 0 week | 8 week | p1) | ||
| HOMA-IR | 1.87 ± 0.91 | 2.70 ± 2.10 | 0.173 | 2.73 ± 2.11 | 1.91 ± 1.08 | 0.193 | 0.137 |
| HOMA-β | 61.16 ± 24.45 | 74.68 ± 48.41 | 0.316 | 62.60 ± 37.15 | 57.18 ± 36.01 | 0.638 | 0.282 |
All values are presented as mean ± standard deviation
Analyzed by paired t-test and p-value compared to baseline visit
Derived from generalized linear model adjusted for baseline and p-value compared to placebo group
4. Discussion
The objective of this study was to determine the effect of HGE supplementation on the glycemic control in patients with IFG individuals and consequently be a possible nutritional approach to reducing the incidence of type 2 diabetes development. Although ginseng has been reported to possess antidiabetic activities in type 2 diabetic animals [21,22] and patients [23,24], the effect of HGE on type 2 diabetic patients or high-risk individuals has not been reported. After HGE supplementation, impaired fasting glucose levels returned to near-physiological levels after 8 wk of supplementation.
In general, deglycosylated ginsenosides are more readily absorbed by the human intestinal tract than their counterparts and have greater pharmacological functions. Nevertheless, these forms of ginsenosides are difficult to obtain because of their lower natural abundance in ginseng extract. Additionally, it is very difficult to synthesize deglycosylated ginsenosides chemically in order to develop them into a therapeutic medicine. In this regard, various attempts, including microbial conversion and enzymatic catalysis, have been made to enrich ginsenosides with less sugar. In this study, we used an enzymatic catalysis method because this approach has substantial advantages such as high selectivity, mild reaction conditions, and environmental compatibility. Pectinase is an enzyme able to degrade pectic substances by hydrolyzing the ester bond between galacturonic acid and methanol or by cleaving the glycosidic bonds of specific polymers [22]. Indeed, Jin et al [17] used pectinase to hydrolyze ginsenosides and found that compound K is more readily absorbed from HGE compared to non-HGE in human individuals. Compound K has received increasing attention because various pharmacologic actions including anticancer [25], anti-inflammation [26], and antidiabetes [27] were shown to be mediated by this compound. Using pectinase-hydrolyzed ginseng extract, Ramesh et al [28] found an improved antioxidant status and minimized occurrence of oxidative stress-related disorders in aged rats. Moreover, Yuan et al [29,30] reported that pectinase-processed ginseng radix had antidiabetic and hypolipidemic effects in high fat diet-fed ICR mice. Taken together, pectinase seems to be an effective tool to transform ginsenosides into deglycosylated ginsenosides, thereby enhancing the bioavailability and functionality of ginseng.
Our data demonstrate that 8 wk of HGE supplementation causes a significant reduction in FPG (p = 0.017) and PPG60min (p = 0.01) in IFG individuals. Such reductions may be due to one or a combination of different mechanisms, including intestinal glucose absorption [31,32], insulin secretion from pancreatic β-cells [33], or peripheral glucose utilization [34]. After the supplementation of HGE, noticeable but not significant difference was found in the glucose level at an earlier time point (PPG30min, p = 0.059) during OGTT. This result suggests that HGE slows the absorption of glucose in the intestinal lumen. Also, our findings of significant decreases in FPG and PPG60min suggest one additional possibility, in which HGE improves glucose intolerance through increasing the insulin action on the target tissues responsible for glucose uptake. Moreover, FPI (p = 0.063) and PPI60min (p = 0.077) showed a tendency to improve in the HGE group compared to the placebo group. In supporting this possibility, ginsenosides CK and Rg1 have been reported to enhance insulin-mediated glucose uptake in 3T3-L1 adipocytes, which is related to the increased GLUT4 translocation [27,35]. Similarly, administration of HGE improves glucose homeostasis and insulin resistance state (or glucose and lipid parameters) in high fat diet-fed mice via activation of AMP-dependent protein kinase in muscle tissue [29,30]. In this study, however, there was no significant difference in HOMA-β, suggesting no effect on insulin secretion. In contrast to our results, studies reveal that ginseng significantly stimulates insulin release from pancreatic β-cells [36,37]. These discrepancies could be due to the differences in designs (human studies vs. animal studies) and materials (hydrolyzed ginseng vs. nonhydrolyzed ginseng) used in the studies. Because this was a relatively small-scale and short-term study, further large-scale and long-term studies are needed to fully evaluate the detailed mechanism of HGE.
We allowed participants to maintain their usual diet and activity without conducting surveys about their lifestyles. Therefore, the participants' diets and activity levels were not accurately controlled. For a more accurate study, the control of lifestyle factors, such as food intake and physical activity, is necessary. Despite this limitation, data from our study suggest that HGE is effective as a glucose-lowering agent. Thus, combined with lifestyle modification, the glucose-lowering effect of hydrolyzed ginseng will become more pronounced.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was supported by a grant from the Plant Diversity Research Center of the 21st Century Frontier Program, Republic of Korea (M106KD0110018-09K0401-01810). This study was conducted at the Clinical Trial Center for Functional Foods at Chonbuk National University Hospital.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.The Expert Committee on the Diagnosis CoDM Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl. 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 3.Barr E.L., Zimmet P.Z., Welborn T.A., Jolley D., Magliano D.J., Dunstan D.W., Cameron A.J., Dwyer T., Taylor H.R., Tonkin A.M. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116:151–157. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 4.Meigs J.B., Nathan D.M., D'Agostino R.B., Sr., Wilson P.W., the Framingham Offspring Study Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845–1850. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 5.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M., Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X., Hu Z.X., Lin J., Xiao J.Z., Cao H.B. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J., Lindström J., Eriksson J.G., Valle T.T., Hämäläinen H., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 8.Derosa G., Maffioli P. Thiazolidinediones plus metformin association on body weight in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;91:265–270. doi: 10.1016/j.diabres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Mitri J., Hamdy O. Diabetes medications and body weight. Expert Opin Drug Saf. 2009;8:573–584. doi: 10.1517/14740330903081725. [DOI] [PubMed] [Google Scholar]
- 10.Bao P.P., Lu W., Cui Y., Zheng Y., Gu K., Chen Z., Zheng W., Shu X.O. Ginseng and Ganoderma lucidum use after breast cancer diagnosis and quality of life: a report from the Shanghai Breast Cancer Survival Study. PloS One. 2012;7:e39343. doi: 10.1371/journal.pone.0039343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeds D.N., Patterson B.W., Okunade A., Holloszy J.O., Polonsky K.S., Klein S. Ginseng and ginsenoside Re do not improve β-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care. 2011;34:1071–1076. doi: 10.2337/dc10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H., Hou S.Z., Luo P., Zeng B., Wang J.R., Wong Y.F., Jiang Z.H., Liu L. Ginseng protects rodent hearts from acute myocardial ischemia-reperfusion injury through GR/ER-activated RISK pathway in an endothelial NOS-dependent mechanism. J Ethnopharmachol. 2011;135:287–298. doi: 10.1016/j.jep.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Taira S., Ikeda R., Yokota N., Osaka I., Sakamoto M., Kato M., Sahashi Y. Mass spectrometric imaging of ginsenosides localization in Panax ginseng root. Am J Chin Med. 2010;38:485–493. doi: 10.1142/S0192415X10008007. [DOI] [PubMed] [Google Scholar]
- 14.Wang C.Z., Ni M., Sun S., Li X.L., He H., Mehendale S.R., Yuan C.S. Detection of adulteration of notoginseng root extract with other panax species by quantitative HPLC coupled with PCA. J Agr Food Chem. 2009;57:2363–2367. doi: 10.1021/jf803320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H., Zhou M., Zhao A., Jia W. Traditional Chinese medicine: balancing the gut ecosystem. Phytother Res. 2009;23:1332–1335. doi: 10.1002/ptr.2590. [DOI] [PubMed] [Google Scholar]
- 16.Wang H.Y., Qi L.W., Wang C.Z., Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011;39:1103–1115. doi: 10.1142/S0192415X11009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H., Seo J.H., Uhm Y.K., Jung C.Y., Lee S.K., Yim S.V. Pharmacokinetic comparison of ginsenoside metabolite IH-901 from fermented and non-fermented ginseng in healthy Korean volunteers. J Ethnopharmacol. 2012;139:664–667. doi: 10.1016/j.jep.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Ramesh T., Kim S.W., Sung J.H., Hwang S.Y., Sohn S.H., Yoo S.K., Kim S.K. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol. 2012;47:77–84. doi: 10.1016/j.exger.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., Li W., Li X., Zhang M., Chen L., Zheng Y.N., Sun G.Z., Ruan C.C. Antidiabetic effects of malonyl ginsenosides from Panax ginseng on type 2 diabetic rats induced by high-fat diet and streptozotocin. J Ethnopharmacol. 2013;145:233–240. doi: 10.1016/j.jep.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 20.Ma S.W., Benzie I.F., Chu T.T., Fok B.S., Tomlinson B., Critchley L.A. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: results of a placebo-controlled human intervention trial. Diabetes Obes Metab. 2008;10:1125–1127. doi: 10.1111/j.1463-1326.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 21.Han G.C., Ko S.K., Sung J.H., Chung S.H. Compound K enhances insulin secretion with beneficial metabolic effects in db/db mice. J Agr Food Chem. 2007;55:10641–10648. doi: 10.1021/jf0722598. [DOI] [PubMed] [Google Scholar]
- 22.Jeon W.J., Oh J.S., Park M.S., Ji G.E. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytother Res. 2013;27:166–172. doi: 10.1002/ptr.4706. [DOI] [PubMed] [Google Scholar]
- 23.Sievenpiper J.L., Sung M.K., Di Buono M., Seung-Lee K., Nam K.Y., Arnason J.T., Leiter L.A., Vuksan V. Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies. J Am Coll Nutr. 2006;25:100–107. doi: 10.1080/07315724.2006.10719519. [DOI] [PubMed] [Google Scholar]
- 24.Vuksan V., Stavro M.P., Sievenpiper J.L., Beljan-Zdravkovic U., Leiter L.A., Josse R.G., Xu Z. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diabetes Care. 2000;23:1221–1226. doi: 10.2337/diacare.23.9.1221. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.J., Ko W.G., Kim J.H., Sung J.H., Moon C.K., Lee B.H. Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochem Pharmacol. 2000;60:677–685. doi: 10.1016/s0006-2952(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 26.Choi K., Kim M., Ryu J., Choi C. Ginsenosides compound K and Rh(2) inhibit tumor necrosis factor-α-induced activation of the NF-κB and JNK pathways in human astroglial cells. Neurosci Lett. 2007;421:37–41. doi: 10.1016/j.neulet.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Yoon S.H., Han E.J., Sung J.H., Chung S.H. Anti-diabetic effects of compound K versus metformin versus compound K-metformin combination therapy in diabetic db/db mice. Biol Pharm Bull. 2007;30:2196–2200. doi: 10.1248/bpb.30.2196. [DOI] [PubMed] [Google Scholar]
- 28.Dichmann O., Engel U., Jensen D.B., Bilde T. Juxtatesticular seminoma. Br J Urol. 1990;66:324–325. doi: 10.1111/j.1464-410x.1990.tb14940.x. [DOI] [PubMed] [Google Scholar]
- 29.Yuan H.D., Kim J.T., Chung S.H. Pectinase-processed Ginseng radix (GINST) ameliorates hyperglycemia and hyperlipidemia in high fat diet-fed ICR mice. Biomol Ther. 2012;20:220–225. doi: 10.4062/biomolther.2012.20.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan H.D., Quan H.Y., Jung M.S., Kim S.J., Huang B., Kim do Y., Chung S.H. Anti-diabetic effect of pectinase-processed Ginseng radix (GINST) in high fat diet-fed ICR mice. J Ginseng Res. 2011;35:308–314. doi: 10.5142/jgr.2011.35.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onomura M., Tsukada H., Fukuda K., Hosokawa M., Nakamura H., Kodama M., Ohya M., Seino Y. Effects of Ginseng radix on sugar absorption in the small intestine. Am J Chin Med. 1999;27:347–354. doi: 10.1142/S0192415X99000392. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y., Ito Y., Konno C., Furuya T. Effects of tissue cultured ginseng on gastric secretion and pepsin activity. Yakugaku Zasshi. 1991;111:770–774. doi: 10.1248/yakushi1947.111.12_770. [In Japanese] [DOI] [PubMed] [Google Scholar]
- 33.Waki I., Kyo H., Yasuda M., Kimura M. Effects of a hypoglycemic component of ginseng radix on insulin biosynthesis in normal and diabetic animals. J Pharmacobiodyn. 1982;5:547–554. doi: 10.1248/bpb1978.5.547. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi Y., Takagi S., Miura T., Usami M., Kako M., Ishihara E., Yano H., Tanigawa K., Seino Y. Effect of ginseng radix on GLUT2 protein content in mouse liver in normal and epinephrine-induced hyperglycemic mice. Biol Pharma Bull. 1996;19:1238–1240. doi: 10.1248/bpb.19.1238. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y.C., Lin C.Y., Huang S.F., Lin H.C., Chang W.L., Chang T.C. Effect and mechanism of ginsenosides CK and Rg1 on stimulation of glucose uptake in 3T3-L1 adipocytes. J Agr Food Chem. 2010;58:6039–6047. doi: 10.1021/jf9034755. [DOI] [PubMed] [Google Scholar]
- 36.Kim K., Kim H.Y. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008;120:190–195. doi: 10.1016/j.jep.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Park S.M., Hong S.M., Sung S.R., Lee J.E., Kwon D.Y. Extracts of Rehmanniae radix, Ginseng radix and Scutellariae radix improve glucose-stimulated insulin secretion and β-cell proliferation through IRS2 induction. Genes Nutr. 2008;2:347–351. doi: 10.1007/s12263-007-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


