Abstract
Background
In this study, we examined the effects of various enzymes on chemical conversions of ginsenosides in ginseng extract prepared by amylases.
Methods
Rapidase, Econase CE, Viscozyme, Ultraflo L, and Cytolase PCL5 were used for secondary enzymatic hydrolysis after amylase treatment of ginseng extract, and ginsenoside contents, skin permeability, and chemical compositions including total sugar, acidic polysaccharide, and polyphenols were determined on the hydrolyzed ginseng extract.
Results
Rapidase treatment significantly elevated total ginsenoside contents compared with the control (p < 0.05). In particular, deglycosylated ginsenosides including Rg3, which are known as bioactive compounds, were significantly increased after Rapidase treatment (p < 0.05). The Rapidase-treated group also increased the skin permeability of polyphenols compared with the control, showing the highest level of total sugar content among the enzyme treatment groups.
Conclusion
This result showed that Rapidase induced the conversion of ginsenoside glycosides to aglycones. Meanwhile, Cytolase PCL5 and Econase treatments led to a significant increase of uronic acid (acidic polysaccharide) level. Taken together, our data showed that the treatments of enzymes including Rapidase are useful for the conversion and increase of ginsenosides in ginseng extracts or products.
Keywords: enzymatic transformation, ginsenosides, Korean Red Ginseng extracts, Panax ginseng Meyer
1. Introduction
Ginseng (the roots of Panax ginseng Meyer, Araliaceae) has been usually used as a traditional herbal medicine in Asian countries. The major components of ginseng are ginsenosides, which are glycosides with a dammarane skeleton aglycone [1,2]. These ginsenosides have been reported to show various biological activities including anti-inflammatory [3] and antitumor effects [4,5]. The pharmacological actions of these ginsenosides have been explained by the biotransformation of ginsenosides by human intestinal bacteria [6–8].
Ginsenosides, glycosides with steroids or triterpenes as aglycones, are an important class of physiologically active compounds occurring in many herbs. Recent studies showed that the sugar chains of ginsenosides are closely related to the biological activity of ginsenosides, and modification of the sugar chains makes a difference to the biological activity [9–12].
Several studies have shown that removal of the glycosyl group in ginsenosides is required for enhancement of physiological action of ginsenosides [13]. Various transformation methods including mild acid hydrolysis [14], enzymatic conversion [15], and microbial conversion [16] have been used, but these chemical methods result in side reactions such as epimerization, hydration, and hydroxylation, and most microbial transformations do not reach a food-grade standard.
In our previous study [17], the treatment of enzymes such as Optidex and Viscozyme increased total sugar, uronic acid, polyphenol, and solid contents, and reduced the bitterness of red ginseng extract. In addition, conversions of ginsenosides were observed; Rb2 and Rc were converted into Rg3 or Rh2, and Rb1 was transformed into Rg3 following enzyme treatment. In this study, various hydrolytic enzymes were subsequently examined in red ginseng extract treated by amylase, with the purpose of increasing the amounts of ginsenoside metabolites as well as their conversions into aglycones. Therefore, we investigated the effects of each enzyme treatment on the chemical composition and the transformation of ginsenosides in red ginseng extract.
2. Materials and methods
2.1. Materials
Six-yr-old red ginseng was purchased at a ginseng market in Geumsan, Korea. Standard ginsenosides, including compound K, Rh2, Rh1, Rg5, Rk1, Rg2, Rg3, Rg1, Rf, Re, Road, Rb2, Rc, and Rb1, were purchased from Embo Laboratory in Daejeon, Korea. Spezyme prime, Optidex L-400 (Genencor International Inc., Palo Alto, CA, USA), Viscozyme (Novo Nordisk Ferment Ltd, Dittingen, Switzerland), Econase CE, Rapidase, Ultraflo L, and Cytolase PCL5 (obtained from Bision Biochem, Sungnam, Korea) were also used. The characteristics of enzymes are summarized in Table 1. All other chemicals were obtained from local suppliers and were of reagent grade.
Table 1.
Characteristics of enzymes used to hydrolyze Korean Red Ginseng
| Enzyme | Source | Optimal temperature (°C) | Optimal pH | Main activity |
|---|---|---|---|---|
| Spezyme prime | Aspergillus niger | 85–90 | 6.2–6.5 | α-Amylase |
| Optidex L-400 | Geobacillus stearothermophilus | 58–65 | 4.0–4.5 | Glucoamylase |
| Econase CE | Trichoderma sp. | 55 | 4.0–5.5 | Cellulase |
| Rapidase | Aspergillus niger and Trichoderma longibrachiatum | 10–55 | 4.0–5.0 | pectinase, Hemicellulase, cellulase |
| Viscozyme | Aspergillus sp. | 40–50 | 3.3–5.5 | Arabinase, cellulase, β-Glucanase, hemicellulase, xylanase |
| Ultraflo L | Humicola insolens | 40 | 6.0 | β-Glucanase |
| Cytolase PCL5 | Aspergillus niger | 10–55 | 2.5–5.0 | Pectinase |
2.2. Preparation of Korean Red Ginseng extraction and enzymatic conversion of Korean Red Ginseng extraction
Red ginseng powder (200 g) was suspended in 1 L of distilled water, and the pH of the solution was adjusted to pH 6 with 2N NaOH. Spezyme prime (4 mL) was added to the red ginseng suspension. The red ginseng suspensions were incubated at 85°C for 12 h. Optidex L-400 (4 mL) was added to the suspensions followed by incubation at 60°C for 4 h after Spezyme treatment for 12 h. After hydrolysis, the reaction was terminated by boiling for 15 min [17].
The hydrolyzed mixtures were extracted twice with 3 L of ethanol under reflux in a water bath at 90°C for 2 h. The extract was then centrifuged at 10,000 × g for 30 min. This supernatant was evaporated to 10 brix. The concentrate was used for bioconversion with enzymes.
The concentrate was used as a substrate for enzymatic conversion by various enzymes. One wt% enzyme was added for a conversion reaction in optimal conditions as illustrated in Table 1. After the enzymatic conversion, the reaction was terminated by boiling for 15 min. The reaction was used in assays to determine chemical components such as polyphenols, total sugars, uronic acid, and ginsenosides.
2.3. Determination of chemical composition in Korean ginseng
The amount of total sugar was measured by the phenol–sulfuric acid method using glucose as the respective standard [18]. Uronic acid was estimated by the 3-phenylphenol method using galacturonic acid as the standard [19]. Total polyphenol content was determined by a modified Folin–Ciocalteu method of microscale using gallic acid as standard [20].
2.4. Analysis of ginsenoside contents
The solid-phase extraction sample (2 mL) was prepared by using the C18 ODS cartridge (Waters Associates, Milford, MA, USA) described by Lou et al [21]. The levels of 16 major ginsenosides were analyzed using a high performance liquid chromatography (HPLC)-based technique developed by Lee et al [22]. The HPLC system (Varian Prostar 200, Reno, NV, USA) was equipped with a quaternary solvent delivery system, an autosampler, and an UV detector. The column was an Imtakt Cadenza CD-C18 column (4.6 mm × 75 mm, Imtakt Co., Kyoto, Japan).
2.5. Skin permeability test
Skin permeation was determined by the method of Sonavane et al [23], with certain modifications. Male Sprague–Dawley rats, weighing 250–300 g (Nara Bio Animal Center, Seoul, Korea), were used for the study. The excised skin was mounted in a Franz-type diffusion cell. Then, 4.9 mL of 0.1M sodium phosphate buffer (pH 7.4) was used as a receptor medium, and 100 μL of ginseng sample was placed on the donor side. The receptor medium was kept at 37°C and stirred with a magnetic stirrer at 400 × g. The polyphenol content of the transports was determined by the Folin–Ciocalteu method [20].
2.6. Statistical analysis
In all cases, analyses were performed in triplicate, unless otherwise specified. These values were averaged and standard deviations were calculated. All data were analyzed by one-way analysis of variance and Duncan's multiple range tests using SPSS version 10.0 software (SPSS Inc., Chicago, IL, USA). The results were considered significant at p < 0.05.
3. Results and discussion
We previously reported that single use of Spezyme and Optidex, which usually act on the α-1,4 glycosidic bond, decreases the level of bitterness with an increase of sugar contents [17], and increases the yields of ginsenosides. To retain beneficial effects of taste and yield, ginseng extract was preferentially prepared by Spezyme and Optidex prior to treatment of the testing enzymes, which work on chemical bonds including β-1,4 glycosidic bonds resistant to amylases. Accordingly, we investigated the effects of five enzymes on the chemical composition and the transformation of ginsenosides in red ginseng extract prepared with Spezyme and Optidex.
3.1. Total sugar content of the red ginseng extracts treated with various enzymes
The total sugar content of the red ginseng extracts is presented in Fig. 1. Rapidase showed the highest level of total sugar among the tested enzymes. It increased the amount of total sugar by around 25% compared with the control. The Ultraflo L treatment also showed a higher content of total sugar than the control without a significant difference with a Rapidase treatment (p < 0.05). Cytolase PCL5 showed a significant increase of total sugar content compared with the control, but Econase rather slightly decreases the content. Rapidase has been known to contain activities of pectinase, hemicellulase, and cellulase, suggesting that these enzymatic activities are involved in the further liberation of sugars after amylase treatment. This result showed further enzymatic treatment following amylase can promote the release of sugars from integrated compounds in red ginseng extract.
Fig. 1.
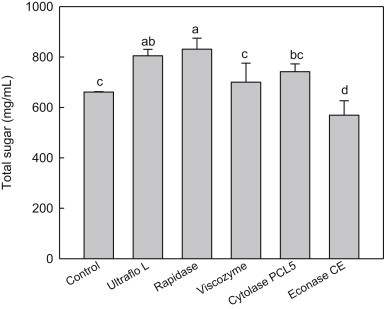
Total sugar contents of red ginseng extracts treated with various enzymes. The control is red ginseng extract prepared with Spezyme prime and Optidex L-400. Values are mean ± standard deviation (n = 3). Means with different letters are significantly different at p < 0.05 by Duncan's multiple range tests.
The major constituents of Korean ginseng are carbohydrates (60–70 g carbohydrate/100 g solid), which include starch, cellulose, and glycosides. Starch is a major component of ginseng carbohydrates [24]. The amylose content of ginseng starch varies from 15 g to 30 g amylose/100 g starch, depending on their year and grade [25]. Several studies suggested that the hydrolysis of these carbohydrates enhances the extraction of active compounds such as ginsenosides and shorter sugars [22,26]. The amylase treatment was shown to increase total sugar contents by hydrolyzing the starch in red ginseng [22]. In particular, α-amylase was used to extract saponins, oligosaccharides, and polysaccharides from fresh or dried roots of P. ginseng Meyer and Panax quinquefolius [27]. Tang [27] also reported that one or more of cellulases and hemicellulases were used to break down the cell walls of ginseng berries (e.g., P. ginseng Meyer or P. quinquefolius) to facilitate the extraction of triterpene saponins, oligosaccharides, and polysaccharides.
3.2. Acidic polysaccharide contents of red ginseng extracts treated with various enzymes
The uronic acid (as acidic polysaccharide) contents of red ginseng extracts are presented in Fig. 2. The order of enzymatic efficacy liberating uronic acid in the red ginseng extract preparations was as follows: Cytolase = Econase ≥ Ultraflo L ≥ Rapidase, Control, and Viscozyme. Cytolase and Econase treatments showed the most liberation of uronic acid by showing 11.9 mg/mL and 11.8 mg/mL, respectively. Rapidase and Ultraflo L also released more uronic acid content (9.9 mg/mL and 10.9 mg/mL, respectively) compared with the control showing only 8.2 mg/mL (Fig. 2).
Fig. 2.
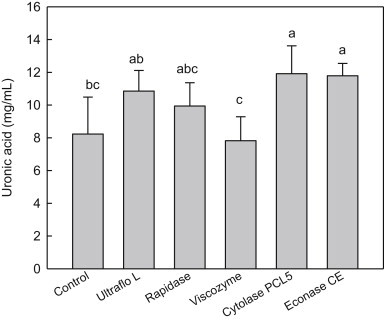
Uronic acid contents of red ginseng extracts treated with various enzymes. The control is red ginseng extract prepared with Spezyme prime and Optidex L-400. Values are mean ± standard deviation (n = 3). Means with different letters are significantly different at p < 0.05 by Duncan's multiple range tests.
This result suggested that additional treatment of enzymes after amylase increases the production of acidic polysaccharides such as uronic acid in red ginseng extract. The biological effects of acidic polysaccharides were observed in many studies. Acidic polysaccharide from Korean Red Ginseng was shown to have immunostimulating and antitumor activities with the activation of natural killer cells and nitric oxide production [28,29]. Toxohormone L-induced lipolysis was inhibited by acidic polysaccharides from ginseng root [25]. Acidic polysaccharides from Korean Red Ginseng modulated pancreatic lipase activity and caused a reduction of plasma triglyceride levels after oral administration of corn oil emulsion to rats, suggesting the involvement of pancreatic lipase in the reduction of lipolysis [30].
3.3. Polyphenol of red ginseng extracts treated with various enzymes
Polyphenolic compounds are considered to be secondary metabolites synthesized in plants by a defense mechanism in response to various stress conditions [31]. Polyphenols from plants were known to present various biological activities such as antioxidative and anti-inflammatory effects.
As shown in Fig. 3, sequential enzyme treatment did not affect the content of polyphenols, showing a similar level to the control.
Fig. 3.
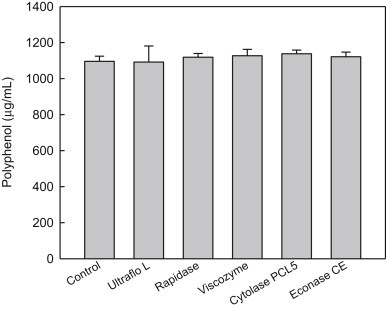
Polyphenol contents of red ginseng extracts treated with various enzymes. The control is red ginseng extract prepared with Spezyme prime and Optidex L-400. Values are mean ± standard deviation (n = 3). Means with different letters are significantly different at p < 0.05 by Duncan's multiple range tests.
Recently, carbohydrate-hydrolyzing enzymes, such as pectinase, cellulase, hemicellulase, and glucanase have been used to break the cell wall complex for the extraction of polyphenolics [32,33]. These enzymes were considered to disintegrate the plant cell wall matrix to facilitate polyphenol extraction [34]. However, our results did not exhibit a significant increase of polyphenols after enzymatic treatment on extract.
3.4. Ginsenoside composition of red ginseng extracts treated with various enzymes
The ginsenoside composition of red ginseng extracts is presented in Table 2. Rc was the most abundant in the control and Ultraflo L groups, but the other enzymatic treatment contained Rb1 as the highest ginsenoside. Meanwhile, ginsenoside Rh2 and compound K were not detected in all extracts. A total ginsenoside content generated by Rapidase was the highest among the enzyme treatments by showing 167.35 mg/mL. The treatment of other enzymes did not show a significant increase in total ginsenoside contents. In particular, deglycosylated ginsenoside metabolites such as Rh1, Rg5, Rk1, Rg2, and Rg3 were detected the most in Rapidase treatment. This result is correlated with the data (Fig. 1) showing a significant elevation of total sugar in Rapidase treatment, indicating that Rapidase allows the increase of deglycosylated ginsenosides by promoting the release of sugars linked to ginsenoside glycosides. Fig. 4 shows the contents of major ginsenoside contents. Contents of panaxadiols and panaxatriols in red ginseng extracts were also highest in Rapidase treatment (128.53 mg/mL and 32.36 mg/mL, respectively).
Table 2.
Ginsenoside contents of hydrolyzed red ginseng extracts using various enzymes
| Ginsenoside | Concentration (mg/mL) |
|||||
|---|---|---|---|---|---|---|
| Control | Ultraflo L | Rapidase | Viscozyme | Cytolase | Econase | |
| Rh1 | 1.26 ± 0.06 | 1.29 ± 0.09 | 1.35 ± 0.07 | 1.24 ± 0.07 | 1.31 ± 0.10 | 1.19 ± 0.05 |
| Rg5 + Rk1 | 4.27 ± 0.30 | 2.52 ± 0.13 | 6.46 ± 0.39 | 4.17 ± 0.20 | 5.27 ± 0.38 | 4.93 ± 0.15 |
| Rg2 | 3.96 ± 0.40 | 3.37 ± 0.27 | 5.03 ± 0.45 | 3.90 ± 0.30 | 4.44 ± 0.21 | 4.35 ± 0.26 |
| Rg3 | 6.10 ± 0.59 | 4.94 ± 0.30 | 8.42 ± 0.59 | 6.64 ± 0.46 | 7.12 ± 0.38 | 6.85 ± 0.48 |
| Rg1 | 3.05 ± 0.18 | 2.56 ± 0.18 | 4.25 ± 0.38 | 3.11 ± 0.24 | 3.58 ± 0.23 | 3.23 ± 0.26 |
| Rf | 3.11 ± 0.25 | 2.61 ± 0.15 | 3.98 ± 0.32 | 3.43 ± 0.23 | 3.40 ± 0.25 | 3.60 ± 0.22 |
| Re | 12.75 ± 0.38 | 9.65 ± 0.45 | 17.75 ± 1.24 | 12.50 ± 0.96 | 13.24 ± 0.75 | 12.93 ± 0.56 |
| Rd | 19.10 ± 0.76 | 14.54 ± 0.95 | 25.77 ± 1.55 | 17.80 ± 1.05 | 22.28 ± 1.02 | 18.39 ± 1.34 |
| Rb2 | 12.75 ± 0.89 | 9.10 ± 0.35 | 14.55 ± 0.44 | 11.31 ± 0.54 | 12.17 ± 0.65 | 11.30 ± 0.70 |
| Rc | 37.40 ± 1.68 | 27.10 ± 1.33 | 35.48 ± 1.42 | 27.19 ± 1.11 | 27.48 ± 1.57 | 27.44 ± 1.65 |
| Rb1 | 35.56 ± 2.31 | 24.73 ± 1.73 | 44.32 ± 3.10 | 33.62 ± 1.98 | 36.32 ± 2.18 | 33.61 ± 1.34 |
| Compound K | 0 | 0 | 0 | 0 | 0 | 0 |
| Rh2 | 0 | 0 | 0 | 0 | 0 | 0 |
Fig. 4.
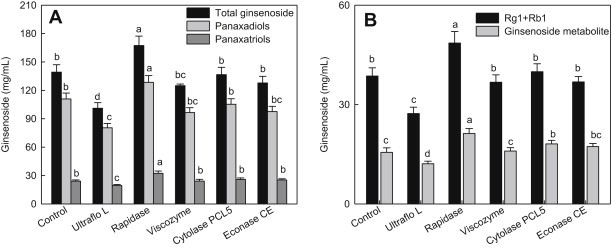
Total ginsenoside, panaxadiols, and panaxatriols (A), and sum of Rg1 and Rb2 and metabolite contents (B) of red ginseng extracts treated with various enzymes. The control is red ginseng extract prepared with Spezyme prime and Optidex L-400. Values are means ± standard deviation (n = 3). Means with different letters are significantly different at p < 0.05 by Duncan's multiple range tests.
Ginsenoside Rg3, Rg5, Rg2, Rg4, Rh2, Rh3, Rh1, and Rh4 have been shown to have special physiological activities: Rh2, Rh3, Rg3, and Rh1 have anticancer properties without side effects; and Rg3 and Rg2 have antithrombus effects. However, these ginsenosides have some difficulties in availability because of low levels in ginseng [35].
Ginsenosides are usually metabolized by human intestinal bacteria to deglycosylated forms, which are more readily absorbed in the bloodstream and act as biologically active compounds [36].
Among these deglycosylated ginsenosides, Rg3 exerts many pharmacological activities such as tumor-suppressing [37], antimetastatic [38], anticarcinogenic [39], hepatoprotective [40], neuroprotective [41], and vasodilating effects [42]. However, the concentration of ginsenoside Rg3 is extremely low in normal ginseng [43]. Thus, the increase of ginsenoside Rg3 level would be very important for the development of health-oriented products. In addition, many studies have been performed, aiming at the increase of minor active ginsenosides such as Rg3 via conversions of major ginsenosides contained abundantly [16,21,22]. Our data, which showed an increase of aglycones including Rg3 with Rapidase treatment (Table 2), suggest that use of Rapidase in the enzymatic process on ginseng extract will be useful in the development of ginseng products with the objective of increasing active compounds.
Kohda and Tanaka [44] reported crude preparations of several glycoside hydrolases for the hydrolysis of ginseng ginsenosides; cellulase and amylase exhibited very low hydrolytic activities, whereas pectinase, naringinase, and hesperidinase had much higher activities for hydrolyzing ginsenosides.
3.5. Skin permeability of Rapidase-treated red ginseng extract
A permeability study of Rapidase-treated red ginseng extract in rat skin was conducted by using Franz diffusion cells. The polyphenol contents of the samples transported through the rat skin was significantly increased over time (Fig. 5). The skin permeability of the red ginseng extract treated with Rapidase was higher than that of the control. In particular, after 4 h, the skin permeability of the red ginseng extract treated with Rapidase showed a significant increase (p < 0.05) compared with that of the control. Although total polyphenol contents are similar in the presence or absence of Rapidase treatment, Rapidase treatment showed a significant improvement of skin permeability. This result suggests that Rapidase can also act on polyphenol glycosides to produce aglycone forms of polyphenols.
Fig. 5.
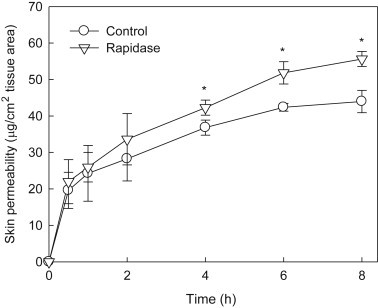
The skin permeability test using the Franz diffusion cell model of red ginseng extract. The skin permeability of the ginseng sample is expressed as μg of polyphenol/cm2 of tissue area. The red ginseng extract treated with Rapidase shows a significant difference compared with the control (*p < 0.05).
Recently, the study to maximize the bioactivity of plant extracts via the enzyme reaction has been performed in the cosmetic industry using natural compounds [45]. The bioactive ingredients of plants mostly include mixtures of compounds that are present in the form of aglycones and hydrophilic glycosides. However, glycosides have some difficulties in their application for skin cosmetics attributable to their low skin permeability. By contrast, aglycone, a hydrophobic polyphenol, can permeate human skin [46]. Wiechers [47] reported that low molecular weight contributes to easier skin penetration; there is a size limitation for chemical compounds and drugs to be absorbed across the human skin barrier. Therefore, Bos and Meinardi [48] reported that certain skin penetration enhancers have low molecular weight. Thus, the hydrolysis of glycoside ingredients into their aglycone forms has attracted attention as an effective means of enhancing the permeability and, consequently, bioactivity of extracts [45].
Most commercial ginseng products are produced from chemical processes such as solvent extractions and chromatographic purifications. These processes are complicated, costly, and are usually associated with low yields of active compounds such as ginsenosides, oligosaccharides, and polysaccharides. Enzymatic extraction was found to be an easy and rapid method for the separation and concentration of bioactive compounds. Therefore, Rapidase will be a major enzyme to enhance bioactive compounds in the development of health-oriented ginseng products via enzymatic processes.
Conflict of interest
All contributing authors declare that there is no conflicts of interest.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Ernst E. Panax ginseng: an overview of the clinical evidence. J Ginseng Res. 2010;34:259–263. [Google Scholar]
- 2.Furuya T., Kojima H., Syono K., Ishii T., Uotani K. Isolation of saponins and sapogenins from callus tissue of Panax ginseng. Chem Pharm Bull (Tokyo) 1973;21:98–101. doi: 10.1248/cpb.21.98. [DOI] [PubMed] [Google Scholar]
- 3.Park J.S., Park E.M., Kim D.H., Jung K., Jung J.S., Lee E.J., Hyun J.W., Kang J.L., Kim H.S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 2009;209:40–49. doi: 10.1016/j.jneuroim.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Surh Y.J., Na H.K., Lee J.Y., Keum Y.S. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(Suppl.):S38–S41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park D., Bae D.K., Jeon J.H., Lee J., Oh N., Yang G., Yang Y.H., Kim T.K., Song J., Lee S.H. Immunopotentiation and antitumor effects of a ginsenoside Rg(3)-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011;31:397–405. doi: 10.1016/j.etap.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.S., Kim J.J., Cho K.H., Jung W.S., Moon S.K., Park E.K., Kim D.H. Biotransformation of ginsenoside Rb1, crocin, amygdalin, geniposide, puerarin, ginsenoside Re, hesperidin, poncirin, glycyrrhizin, and baicalin by human fecal microflora and its relation to cytotoxicity against tumor cells. J Microbiol Biotechnol. 2008;18:1109–1114. [PubMed] [Google Scholar]
- 7.Bae E.-A., Han M.J., Choo M.-K., Park S.-Y., Kim D.-H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 8.Bae E.A., Kim N.Y., Myung J.H., Choo M.K., Kim D.H. Transformation of ginsenosides to compound K (IH-901) by lactic acid bacteria of human intestine. J Microbiol Biotechnol. 2003;13:9–14. [Google Scholar]
- 9.Kim B.G., Shin K.S., Yoon T.J., Yu K.W., Ra K.S., Kim J.M., Kim S.Y., Suh H.J. Fermentation of Korean red ginseng by Lactobacillus plantarum M-2 and its immunological activities. Appl Biochem Biotechnol. 2011;165:1107–1119. doi: 10.1007/s12010-011-9328-6. [DOI] [PubMed] [Google Scholar]
- 10.Su J.-H., Xu J.-H., Lu W.-Y., Lin G.-Q. Enzymatic transformation of ginsenoside Rg3 to Rh2 using newly isolated Fusarium proliferatum ECU2042. J Mol Catal B Enzymat. 2006;38:113–118. [Google Scholar]
- 11.Popovich D.G., Kitts D.D. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry. 2004;65:337–344. doi: 10.1016/j.phytochem.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Bae S.H., Lee H.S., Kim M.R., Kim S.Y., Kim J.M., Suh H.J. Changes of ginsenoside content by mushroom mycelial fermentation in red ginseng extract. J Ginseng Res. 2011;35:235–242. doi: 10.5142/jgr.2011.35.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 14.Han B.H., Park M.H., Han Y.N., Woo L.K., Sankawa U., Yahara S., Tanaka O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982;44:146–149. doi: 10.1055/s-2007-971425. [DOI] [PubMed] [Google Scholar]
- 15.Ko S.R., Choi K.J., Uchida K., Suzuki Y. Enzymatic preparation of ginsenosides Rg2, Rh1, and F1 from protopanaxatriol-type ginseng saponin mixture. Planta Med. 2003;69:285–286. doi: 10.1055/s-2003-38476. [DOI] [PubMed] [Google Scholar]
- 16.Park C.S., Yoo M.H., Noh K.H., Oh D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 17.Kim B.-G., Choi S.Y., Suh H.J., Park H.J. Bitterness reduction and enzymatic transformation of ginsenosides from Korean red ginseng (Panax ginseng) extract. J Food Biochem. 2011;35:1267–1282. [Google Scholar]
- 18.Dubois M., Gilles K.A., Hamilton J.K., Rebers P., Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 19.Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 20.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 21.Lou D.-W., Saito Y., Jinno K. Solid-phase extraction and high-performance liquid chromatography for simultaneous determination of important bioactive ginsenosides in pharmaceutical preparations. Chromatographia. 2005;62:349–354. [Google Scholar]
- 22.Lee H.J., Jung E.Y., Lee H.-S., Kim B.-G., Kim J.H., Yoon T.J., Oh S.H., Suh H.J. Bioavailability of fermented Korean red ginseng. J Food Sci Nutr. 2009;14:201–207. [Google Scholar]
- 23.Sonavane G., Tomoda K., Sano A., Ohshima H., Terada H., Makino K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: effect of particle size. Colloids Surf B Biointerfaces. 2008;65:1–10. doi: 10.1016/j.colsurfb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Van Q., Nayak B., Reimer M., Jones P., Fulcher R., Rempel C. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. J Ethnopharmacol. 2009;125:487–493. doi: 10.1016/j.jep.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Oh S.H. Effect of fat soluble ginseng extract on lipolysis in vitro and in vivo. Korean Biochem J. 1984;17:209–214. [Google Scholar]
- 26.Ko S.-R., Suzuki Y., Suzuki K., Choi K.-J., Cho B.-G. Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem Pharm Bull (Tokyo) 2007;55:1522–1527. doi: 10.1248/cpb.55.1522. [DOI] [PubMed] [Google Scholar]
- 27.Tang Q.N. August 7, 2008. Inventor; Extraction of phytochemicals by enzymatic hydrolysis. WO2008092275 A1. [Google Scholar]
- 28.Park K.M., Kim Y.S., Jeong T.C., Joe C.O., Shin H.J., Lee Y.H., Nam K.Y., Park J.D. Nitric oxide is involved in the immunomodulating activities of acidic polysaccharide from Panax ginseng. Planta Med. 2001;67:122–126. doi: 10.1055/s-2001-11508. [DOI] [PubMed] [Google Scholar]
- 29.Du X.F., Jiang C.Z., Wu C.F., Won E.K., Choung S.Y. Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch Pharm Res. 2008;31:1153–1159. doi: 10.1007/s12272-001-1282-6. [DOI] [PubMed] [Google Scholar]
- 30.Kwak Y.-S., Kyung J.-S., Kim J.S., Cho J.Y., Rhee M.-H. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010;33:468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 31.Dixon R.A., Paiva N.L. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sørensen H.R., Pedersen S., Viksø-Nielsen A., Meyer A.S. Efficiencies of designed enzyme combinations in releasing arabinose and xylose from wheat arabinoxylan in an industrial ethanol fermentation residue. Enzyme Microb Technol. 2005;36:773–784. [Google Scholar]
- 33.Landbo A.K., Meyer A.S. Enzyme-assisted extraction of antioxidative phenols from blackcurrant juice press residues (Ribes nigrum) J Agric Food Chem. 2001;49:3169–3177. doi: 10.1021/jf001443p. [DOI] [PubMed] [Google Scholar]
- 34.Le Bourvellec C., Guyot S., Renard C. Interactions between apple (Malus × domestica Borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohydr Polym. 2009;75:251–261. [Google Scholar]
- 35.Yu H., Zhang C., Lu M., Sun F., Fu Y., Jin F. Purification and characterization of new special ginsenosidase hydrolyzing multi-glycisides of protopanaxadiol ginsenosides, ginsenosidase type I. Chem Pharm Bull (Tokyo) 2007;55:231–235. doi: 10.1248/cpb.55.231. [DOI] [PubMed] [Google Scholar]
- 36.Bae E.-A., Park S.-Y., Kim D.-H. Constitutive-glucosidases hydrolyzing ginsenoside Rb-1 and Rb-2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 37.Shinkai K., Akedo H., Mukai M., Imamura F., Isoai A., Kobayashi M., Kitagawa I. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J Cancer Res. 1996;87:357–362. doi: 10.1111/j.1349-7006.1996.tb00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mochizuki M., Yoo Y.C., Matsuzawa K., Sato K., Saiki I., Tono-oka S., Samukawa K., Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20 (R)-and 20 (S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Guan Y., Zhou X., Sun L., Liu Y., He Q., Fu L., Mao Y. Anticarcinogenic effect of 20 (R)-ginsenoside Rg3 on induced hepatocellular carcinoma in rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36:217–220. [PubMed] [Google Scholar]
- 40.Lee H.-U., Bae E.-A., Han M.J., Kim D.-H. Hepatoprotective effect of 20(S)-ginsenosides Rg3 and its metabolite 20(S)-ginsenoside Rh2 on tert-butyl hydroperoxide-induced liver injury. Biol Pharm Bull. 2005;28:1992–1994. doi: 10.1248/bpb.28.1992. [DOI] [PubMed] [Google Scholar]
- 41.He B., Chen P., Yang J., Yun Y., Zhang X., Yang R., Shen Z. Neuroprotective effect of 20(R)-ginsenoside Rg(3) against transient focal cerebral ischemia in rats. Neurosci Lett. 2012;526:106–111. doi: 10.1016/j.neulet.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 42.Kim N.D., Kim E.M., Kang K.W., Cho M.K., Choi S.Y., Kim S.G. Ginsenoside Rg3 inhibits phenylephrine-induced vascular contraction through induction of nitric oxide synthase. Br J Pharmacol. 2003;140:661–670. doi: 10.1038/sj.bjp.0705490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng L.Q., Na J.R., Bang M.H., Kim M.K., Yang D.C. Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry. 2008;69:218–224. doi: 10.1016/j.phytochem.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 44.Kohda H., Tanaka O. Enzymatic hydrolysis of ginseng saponins and their related glycosides. Yakugaku Zasshi. 1975;95:246–249. doi: 10.1248/yakushi1947.95.2_246. [DOI] [PubMed] [Google Scholar]
- 45.Do Y.-K., Kim J.-M., Chang S.-M., Hwang J.-H., Kim W.-S. Enhancement of polyphenol bio-activities by enzyme reaction. J Mol Catal B Enzymat. 2009;56:173–178. [Google Scholar]
- 46.Miller N.J., Ruiz-Larrea M.B. Flavonoids and other plant phenols in the diet: their significance as antioxidants. J Nutr Environ Med. 2002;12:39–51. [Google Scholar]
- 47.Wiechers J.W. The barrier function of the skin in relation to percutaneous absorption of drugs. Pharm Weekbl Sci. 1989;11:185–198. doi: 10.1007/BF01959410. [DOI] [PubMed] [Google Scholar]
- 48.Bos J.D., Meinardi M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]


