Abstract
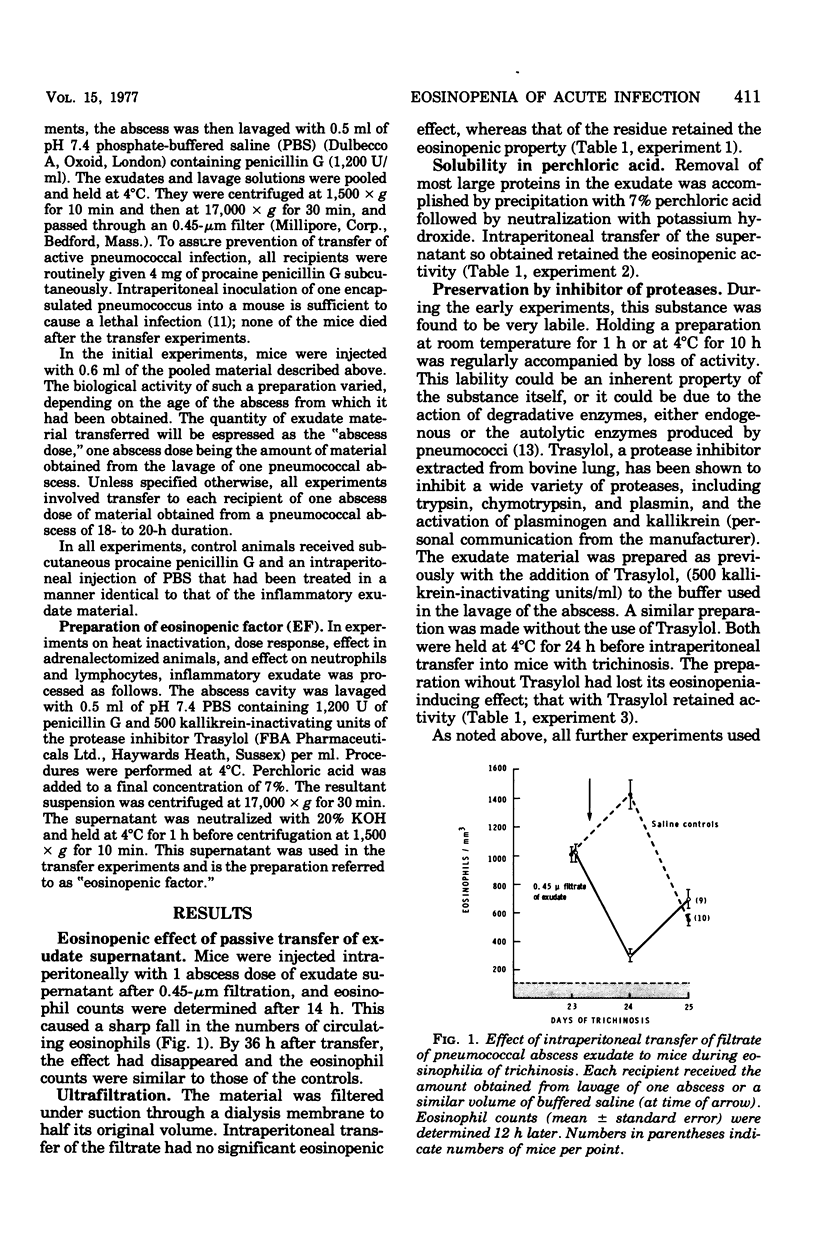
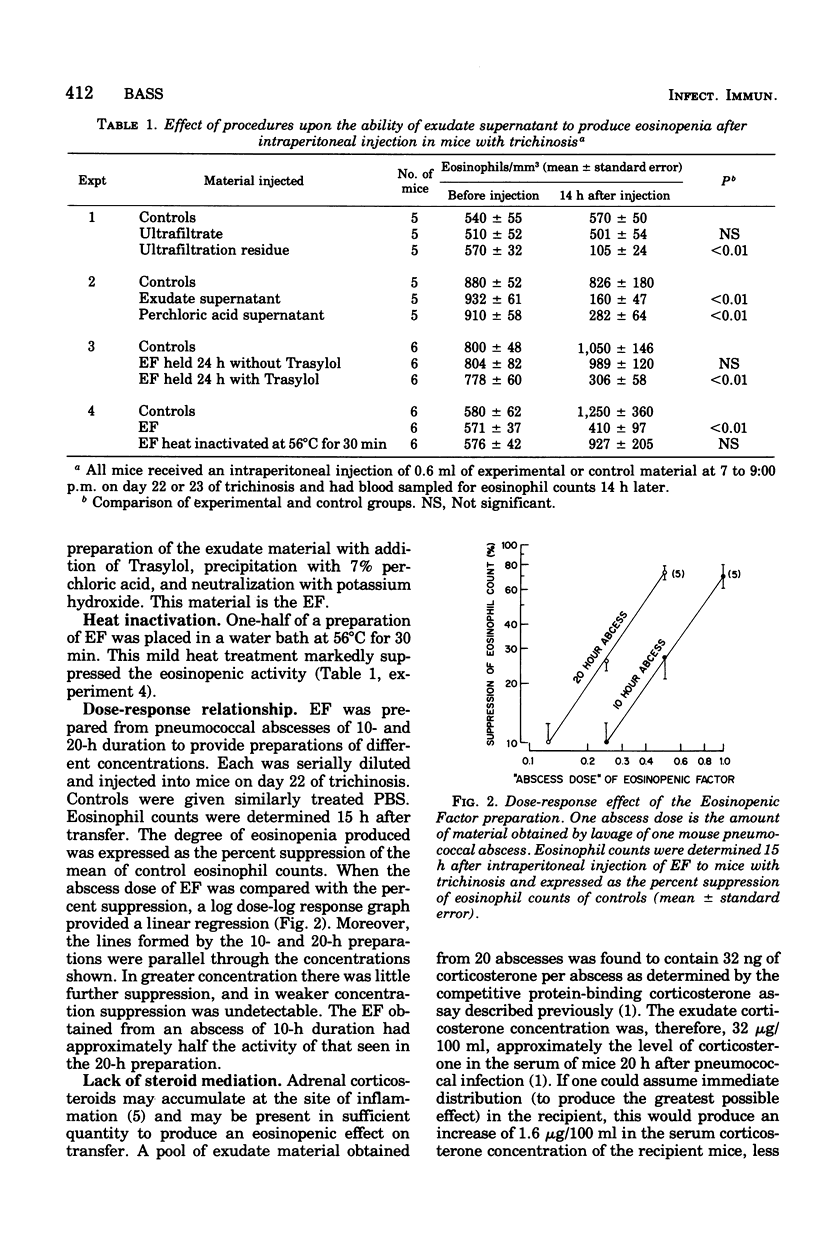
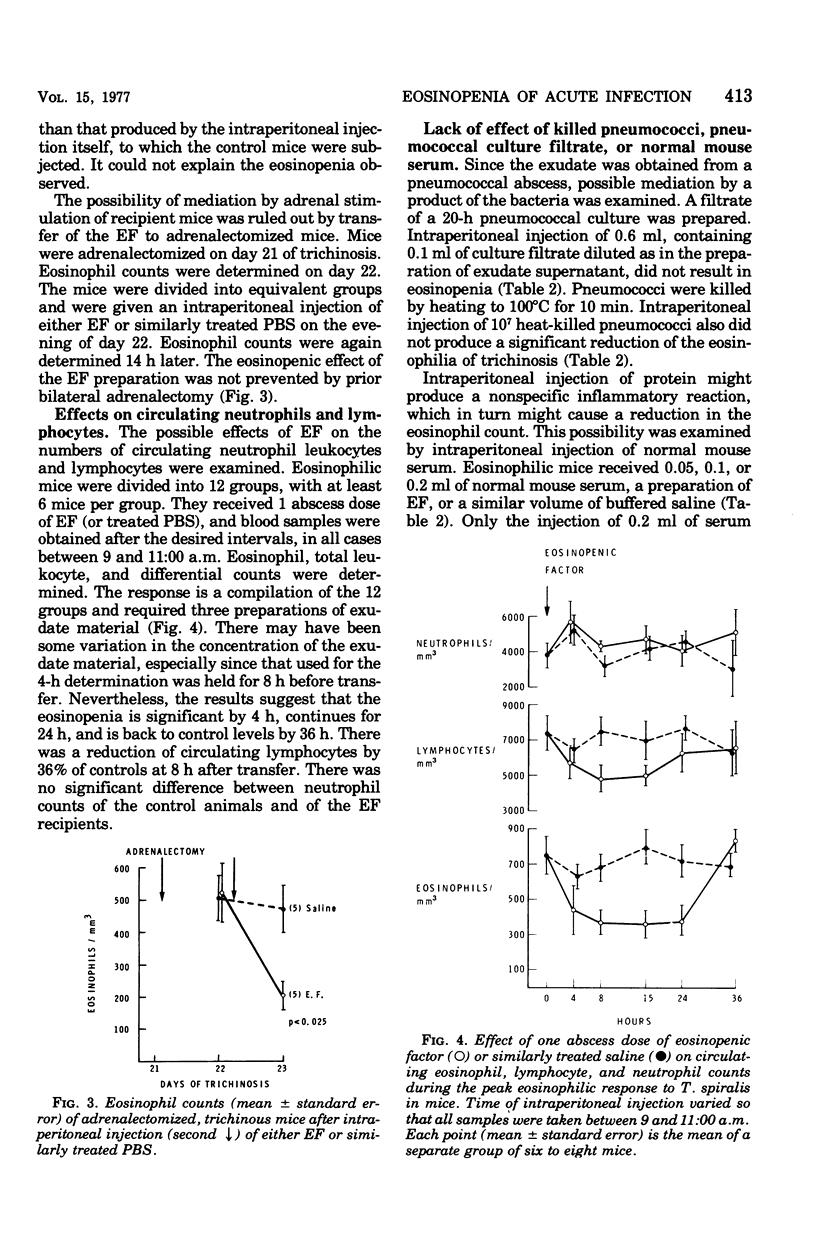
Studies of the eosinopenic effect of acute inflammation were conducted in mice previously rendered eosinophilic with trichinosis. Exudate removed from a pneumococcal abscess contained material (eosinopenic factor [EF]) capable of causing eosinopenia of 4- to 24-h duration when injected intraperitoneally into eosinophilic mice. The material passed through a 0.45-micronm filter, but was retained by a dialysis membrane capable of retaining protein molecules of greater than approximately 30,000 molecular weight. EF was soluble in 7% perchloric acid, was not destroyed by pneumococcal proteolytic enzymes in the presence of Trasylol, but was inactivated by heating to 56 degrees C for 30 min. EF was detectable in the exudate after 10 h and had reached its highest concentration after 20 h. When the effect of EF was expressed as a percent suppression of control eosinophil levels, there was a geometric dose response. Eosinopenia could not be ascribed to steroids present in the preparation, and the EF was effective in adrenalectomized animals. Eosinopenia was not induced by transfer of similarly treated heat-killed pneumococci, pneumococcal culture filtrate, or normal serum. The eosinopenia of acute infection may be the direct effect of a substance present at the site of acute inflammation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A. Behavior of eosinophil leukocytes in acute inflammation. I. Lack of dependence on adrenal function. J Clin Invest. 1975 Jun;55(6):1229–1236. doi: 10.1172/JCI108041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest. 1975 Oct;56(4):870–879. doi: 10.1172/JCI108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 36-1970. N Engl J Med. 1970 Aug 27;283(9):476–485. doi: 10.1056/NEJM197008272830910. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY T. F., BROWN H. E., BERLINER D. L. Metabolism of hydrocortisone during inflammation. Endocrinology. 1958 Apr;62(4):455–462. doi: 10.1210/endo-62-4-455. [DOI] [PubMed] [Google Scholar]
- HENRY W. L., Jr, OLINER L., RAMEY E. R. Relationship between actions of adrenocortical steroids and adrenomedullary hormones in the production of eosinopenia. Am J Physiol. 1953 Sep;174(3):455–458. doi: 10.1152/ajplegacy.1953.174.3.455. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Beeson P. B. Experimental observations on the eosinopenia induced by acute infection. Br J Exp Pathol. 1971 Apr;52(2):214–220. [PMC free article] [PubMed] [Google Scholar]
- Roberts W. C., Liegler D. G., Carbone P. P. Endomyocardial disease and eosinophilia. A clinical and pathologic spectrum. Am J Med. 1969 Jan;46(1):28–42. doi: 10.1016/0002-9343(69)90055-2. [DOI] [PubMed] [Google Scholar]
- WEINER H. A., MORKOVIN D. Circulating blood eosinophils in acute infectious disease and the eosinopenic response. Am J Med. 1952 Jul;13(1):58–72. doi: 10.1016/0002-9343(52)90081-8. [DOI] [PubMed] [Google Scholar]


