Abstract
Introduction
Environmental effectors, such as ultraviolet radiation exposure, infection and stress, have been established as having a role in exacerbating lupus symptoms. However, unpredictable patterns of flare events still remain a mystery. Occupational effectors have also been suggested as having a contributing role; however, they are not widely researched. In this paper we report a pilot study designed to generate focus areas for future research regarding occupational exposures and systemic lupus erythematosus (SLE).
Methods
The study explored potential links between exposures and the occurrence of patient-reported flare events in 80 Australian women with SLE (American College of Rheumatology (ACR) criteria classified). Specifically, the study assessed the hypothesis that occupational exposure is associated with significant changes in the likelihood of lupus flares. Lifetime employment history was analysed with the Finnish Job Exposure Matrix (FINJEM), 40 different semiquantified exposure class estimates for a wide number of occupations based on probability of exposure (p≥5%=exposed) were analysed with the construction of negative binomial regression models to test relationships between occupational agents and flare days. A backward stepwise elimination was used to generate a parsimonious model.
Results
Significant associations were noted for exposure classes of manual handling burden, (p=0.02, incidence rate ratio (IRR) 1.01), Iron (p=0.00, IRR 1.37), wood dust (p=0.00, IRR 3.34) and asbestos (p=0.03, IRR 2.48).
Conclusion
Exposure assessment results indicated that occupations, such as nursing, with a high manual handling burden, posed increased risk to patients with SLE, however, the greatest risk was associated with wood dust and iron exposure with teachers and specialist labourers.
Key messages.
Occupational impacts needs to be considered when developing management plans and offering flare prevention advice.
Adjustment of work practises including adhering to personal protection devices and reducing amount of manual handling burden could lessen illness impacts over a patient's lifetime.
Background
Systemic lupus erythematosus (SLE), is an illness involving multiple organs and organ systems ranging from mild through to life threatening. It is characterised as being unpredictable due to differing patterns of disease symptom activities across and within diagnosed individuals over their lifetime.1 2 The characterisation of lupus flares is further complicated by the imperfect capacity of traditional lupus markers to capture mild flares not associated with changes in organ function or inflammatory markers,3 hence emphasising the need for incorporation of patient-reported symptom changes into disease activity assessment.
The interplay of endogenous and exogenous factors stimulating endocrine and immune tolerance is thought to manifest in either the heightening or suppression of immune system responses including increased pro-inflammatory cytokine production and immunoregulatory pathway reduction; clinically, this presents as periods of symptom quiescence and flares.4–6 Research data focusing upon the role of environmental interactions along with intrinsic factors, such as genetics, age and disease duration in the specific exploration of flare events are limited.1 2 4–7 The most researched and accepted flare effectors include ultraviolet radiation, infection, stress and a few pharmaceutical compounds.7–9 Occupational effectors, however, are not widely researched.
Employment histories of individuals often includes occupational changes, differing environments and multiple exposure sources over a lifetime.4 5 Additionally, new knowledge about adverse occupational exposures can lead to regulatory changes within occupational settings altering potential exposures within the same occupation or occupational setting. The demonstration of causal links between environmental exposures and symptoms is further obscured by the fact that environmental factors may induce disease only after prolonged lag-times or after cumulative effects of sub-threshold exposures. Additionally, the likelihood of demonstrating any link between environmental factors and disease flares is reduced by the adoption of insensitive flare assessment tools, emphasising the need to move beyond traditional disease activity assessment systems. These exposure assessment challenges have limited the ability to establish firm cause and effect models6; however, many studies have established systematic approaches to improving investigative processes focusing on lifetime occupational exposures through the use of Job Exposure Matrices.10–13 One of the most widely used is the Finnish Occupational Exposure Matrix (FINJEM).10
In this paper, we report a pilot study designed to generate future research focus areas regarding the role of occupational exposures and SLE. The study explored potential links between occupational exposures and the occurrence of flare events in an Australian SLE patient group. Specifically, the study assessed the hypothesis that occupational exposure is associated with significant changes in the likelihood of patient-perceived lupus flares.
Methods
The study was a retrospective analysis of a cohort of 80 Australian women diagnosed with SLE as defined by the American College of Rheumatology (ACR) classification criteria.14 Study participants completed a series of study-specific questionnaires and a clinical interview to examine lifestyle and occupational history, as well as their medical history with specific reference to their SLE management and flare history. Data were of a self-reporting nature based upon a novel flare definition.
The study underwent institutional review and approval processes according to the Declaration of Helsinki, 2008, revision.15
Study population
Patients from the Autoimmune Resource and Research Centre (ARRC) and Immunology clinics in New South Wales, Australia, were invited to participate in a study investigating lupus flares. All participants provided written consent to participation and review of their personal medical records, which were used to review medical histories and confirm SLE diagnosis via ACR classification guidelines.
Participants were public and private patients aged 18–80 years with a diagnosis of SLE for a minimum of 2 years. An SLE diagnosis date was obtained from the participant's health record. No gender-specific inclusion criteria were applied initially; however, due to low numbers of male respondents the study was limited to women.
Data collection
Participants completed study-specific postal questionnaires for assessment of medical, lifestyle and occupational history. Additionally, each participant attended a clinical assessment appointment where standard measures of health were undertaken along with a self-reported account of their SLE flare history for the preceding 12 months.
Occupational exposure assessment
The FINJEM was used to estimate likely occupational exposures. Participant's full occupational histories were documented with a job calendar that collected information related to job titles, industries, performed tasks and time measures of employment inclusive of average daily hours, and start and end dates for each job.
To categorise job titles into appropriate occupational groups and to estimate individual occupational exposures, industry and job titles were first classified according to the Australian New Zealand Standard Classification of Occupations (ANZSCO),16 then translated into the 3-digit Finnish occupational codes used within the FINJEM.10 Occupational coding was performed by two independent coders; any disagreement of original ANZSCO code assignment and FINJEM cross-codes was discussed with reference to performed task descriptors to establish consensus final FINJEM code.
The FINJEM, developed by the Finnish Institute of Occupational Health contains probability of exposure to a range of chemical, physical and ergonomic domains for each occupational code. This method has been used extensively in epidemiological studies internationally and provides a standardised tool for quantified and semi-quantified exposure estimates for a wide number of occupations.10 11 Agent exposures, based on probability of exposure (p<5%=non-exposed; p≥5%=exposed)16 for chemical (n=50), physical (n=9) and ergonomic (n=8) domains were quantified in the following formats:
single agents using the probability of exposure
dichotomised single agents using a 5% cutpoint
combined agents based on chemical groups using highest probability of exposure, for example, solvents, combustion products, dusts and other12
dichotomised combined agents using a 5% cutpoint.
To correct for the correlation between age and total work years, employment hours were standardised with division by age.
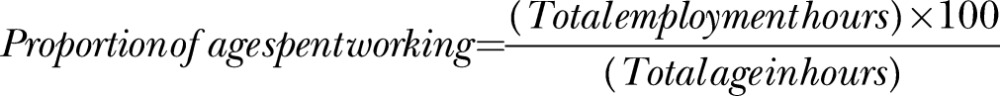 |
Clinical assessment
Participants attended a clinical assessment appointment where surveys were reviewed for missing data; clinical measurements of height and weight taken; and SLE flare history was documented from a patient perspective. Flare histories were collected via a structured interview reviewing the preceding 12 months. The same medical researcher completed all health assessments and flare interviews. Given that this process was not administered by a physician in the context of a full clinical assessment, traditional tools for assessing disease activity (eg, BILAG) were not employed.
Flare assessment outcomes
The flare definition used within this study was from a patient perspective. This decision was made because of this study's focus upon patient health experiences, the limited sensitivity of traditional activity markers, the potential for heightened sensitivity for mild flares using patient-reported symptoms, and the study's resource limitations. It was also dictated by the retrospective nature of the study over a12-month period, and the lack of standardised clinical assessment tools within individual patient health records. To standardise flare description within the cohort, a flare definition best describing the patient experience of an illness with periods of symptom quiescence and exacerbation was chosen and was drawn from another chronic autoimmune illness characterised by relapses and remissions, multiple sclerosis.
The appearance of a new clinical sign/symptom or the clinical worsening of a previous sign/symptom that had been stable for at least the previous 30 days and which persisted for a minimum of 24 hours17
To reduce potential bias, the flare data-collection process was standardised by the researcher following a scripted interview, inclusive of flare definition and a scripted example given for clarification. A series of 15 questions regarding the participant's experience over the 12-month study was then asked without prompting of responses. All responses were documented and will appear in a subsequent paper documenting the lived patient flare experience in greater detail. Flare interview script is available as an online supplementary appendix.
A total count of flare days was calculated from self-reported length of flare events, and the estimated number of flares that had occurred within the preceding 12 months to interview. Final analysis did not include participants that reported a flaring state as ‘constant’, ‘365 days’ or ‘daily’.
Other risk factors
Data related to other perceived flare risk factors were collected via the posted study questionnaire including participant demographics, medical history, general health and well-being. Participants were also asked to nominate their socioeconomic (SES) category; categories offered were: ‘Above Australian Average’, ‘Australian Average’ and ‘Below Australian Average’. Current stress levels were recorded via a visual analogue scale, and quality of life was measured with a 7-point Likert scale as a final component part of a symptom checklist,18 With participant Body Mass Index (BMI) according to Australian Government Health Guidelines.19 Due to the regularly reported impact of ultraviolet (UV) radiation as a trigger to SLE flare, hours spent outdoors were calculated as a yearly average from participants’ nominated weekday and weekend outdoor hours. Current smoking status was captured as a dichotomised ‘yes’, ‘no’ response.
Participant use of immune therapy medications (ITM) was also considered as being a flare-modifying factor. ITM included: methotrexate, hydroxychloroquine, prednisolone, imuran, intravenous immunoglobulin, dapsone, and cellcept considered as a single group to provide a surrogate marker for disease severe enough to warrant physician-initiated pharmacotherapy. Vitamin D supplementation was considered separately due to its reported properties of immune modulation.20 21
Statistical methods
Descriptive statistics summarised demographic and clinical characteristics, while negative binomial regression models were chosen to assess the relationship between occupational agents and flare days. Covariates considered were: participant age; disease duration; time spent outdoors; educational level; SES; BMI; stress; quality of life (QOL); total work hours; number of jobs and use of vitamin D and ITM.
A backward stepwise approach was used including FINJEM agents and covariates of interest. FINJEM agents were independent variables of either semiquantified exposure values or dichotomised agents’ scores of positive exposure. Agents and covariates with significance at the p≤0.05 level in univariate analysis were included in multivariate models to test interactions with flare days (outcome) as a continuous variable; backward stepwise elimination was used to generate a parsimonious model. The final multivariate model retained the covariates diagnosis years (p=0.02), total work hours (p=0.03), and QOL (p<0.01). Age (p=0.10) was also retained within the model as a control variable. All normality assumptions were verified by inspection of probability plots and histograms of residuals, with Shapiro–Wilk test p>0.05. Associations were noted with significance at level of p≤0.05, expressed as incidence rate ratios (IRR) with 95% CIs.
All analysis was performed with the use of STATA V.11.0 (StataCorp LP, College Station, Texas, USA).
Results
An audit of 159 individual health records was performed, and of the reviewed records, documented evidence of ≥4 out of 11 ACR criteria was confirmed in 83 participants. Three participants reported an illness activity state of ‘constant’ flare and were excluded.
For the remaining 80 participants, demographic data are shown in table 1. Self-reported flares for the focus year ranged from 0 (no flares) to 52 flares, with a mean number of 6.8 (SD 2.1). Flare day counts ranged from 0 to 240 days (mean 29.2, SD8.9) with two participants experiencing a long time period within hospital for management of a major adverse health event (renal crisis). The three most frequently reported flare symptoms were joint and muscle pain (70%), fatigue (67.5%) and skin rash (31.3%).
Table 1.
Demographic and clinical characteristics
| n=80 | Mean | SD | n | % | |
|---|---|---|---|---|---|
| Age | 48 | 13.5 | Ethnic background | ||
| Diagnosis years | 7.7 | 6.2 | Caucasian | 78 | 97.5 |
| Quality of life* | 4 | 1.3 | Asian | 2 | 2.5 |
| Health VAS score† | 55 | 23 | Educational background | ||
| Stress VAS score‡ | 50 | 27 | Year 9 (15 years) | 8 | 10 |
| Work hours/day | 4.3 | 3.6 | School/Leaving Certificate | 24 | 30 |
| Number of jobs over lifetime | 6.9 | 3.8 | High school certificate | 2 | 2.5 |
| Proportion employment /age§ | 12.5 | 6.3 | Apprenticeship | 4 | 5 |
| N | % | Tertiary (university/college) | 36 | 45 | |
| Current employment status | Postgraduate studies | 6 | 7.5 | ||
| Full time | 14 | 17.5 | Socio economic status | ||
| Part-time | 22 | 27.5 | Above average | 9 | 11.25 |
| Homemaker or Homeworking | 12 | 15 | Average | 56 | 70 |
| Student+part-time | 1 | 1.25 | Below average | 15 | 18.75 |
| Retired | 20 | 25 | Body Mass Index | ||
| Unemployed | 1 | 1.25 | Underweight | 2 | 2.5 |
| Disabled | 16 | 20 | Normal | 21 | 26.25 |
| Total employment full or part time | 31 | 46.3 | Overweight | 33 | 41.25 |
| Clinical ACR SLE features | Obese | 24 | 30 | ||
| Malar rash | 57 | 71.3 | Smoking status | ||
| Discoid rash | 3 | 3.8 | Current smoker | 6 | 7.5 |
| Photosensitivity | 43 | 53.8 | Past smoker | 30 | 37.5 |
| Oral/nasal ulcers | 29 | 36.3 | Self-reported flare features | ||
| Arthritis | 63 | 78.8 | Joint and muscle pain | 56 | 70 |
| Serositis | 20 | 25.0 | Fatigue | 54 | 67.5 |
| Renal disorder | 37 | 46.3 | Rash | 25 | 31.25 |
| Neurological disorder | 33 | 41.3 | Headache | 19 | 23.75 |
| Haematological disorder | 39 | 48.8 | Fevers | 11 | 13.75 |
| Immunologic disorder | 27 | 33.8 | Brain fog/cognitive clouding | 11 | 13.75 |
| Antinuclear antibody | 73 | 91.3 | Joint swelling | 11 | 13.75 |
| Positive response to pharmaceutics | 75 | 93.8 | Gastrointestinal problems | 10 | 12.5 |
| Immune therapy medications | 67 | 83.8 | n=80 | Mean | SD |
| Vitamin D supplementation | 42 | 52.5 | Flare number (year) | 6.8 | 2.1 |
| Flare days (year) | 29.2 | 8.9 | |||
*Quality of life categories. (1) ‘Excellent’, (2) ‘Good’, (3) ‘Moderately good’, (4) ‘Neither good nor bad’, (5) ‘Rather poor’, (6) ‘poor’, (7) ‘Extremely poor’.
†Health VAS score of current health (0) ‘Excellent’—(100) ‘Extremely Poor’.
‡Stress VAS score of current stress level (0) ‘Not stressed at all’—(100) ‘Highly stressed’.
§Proportioned lifetime length of employment/age, range (2–32% of total life).
ACR, American College of Rheumatology; SLE, systemic lupus erythematosus; VAS, visual analogue scale.
Nine patients experienced flares more frequently than monthly, and two on a weekly basis, which could be reflective of (1) events representing an inadequately controlled single resurfacing symptom or (2) flare events based upon the development of more than one lupus-related symptom. As the study was retrospective and from the patient perspective, matching individual symptoms with each reported flare event was not possible.
Participant mean age was 48 years with mean disease duration of 7.7 years (SD 6.2). Most participants were Caucasian (97.5%). Illness comorbidity was self-reported in 62.5% of participants with many participants reporting multiple concurrent illnesses. The cohort had a high representation of participants with educational level to advanced or vocational level, and above (57.5%). A majority (81.25%) reported a SES of either ‘Above Australian Average’ or ‘Australian Average’.
Employment status on a full-time, part-time, or student basis was reported by 46.25%; a further 15% reported being homemakers, 25% were retired and 20% reported being disabled or unable to work. No minimum job duration was set for reporting within job calendars; however, all participants self-selected to report jobs of 4 weeks or greater with the majority only reporting jobs held for 6 months or more. The mean number of jobs was 6.9 (range 1–19). The proportion of participants’ lives spent working ranged from 2% to 32% with a mean of 12.5% (SD 6.3).
Participants listed 587 occupations within industry groups of health, education, clerical or administrative jobs, and retail, with a smaller job number within manufacturing and labouring industries. Duplicate and similar occupations were merged into the final 301 participant occupations, and classified into ANZSCO major occupation groups, and are displayed with Australian Bureau of Statistics (ABS)22census reporting occupational groups for the same data year in table 2. This comparator was the closest available demographic resource to assess the occupational profile of the SLE participants, although the age range for this study differed slightly to that for the ABS database (age ≥18 years vs age ≥15 years, respectively). Of greatest interest is the lower percentage of ‘Professionals’ (12.3% compared to ABS 21.7%, p=0.07) and the higher percentage of ‘No formal occupation’ (17.3% compared to ABS 1.3%, p<0.01). However, the ANZSCO group of ‘No formal occupation’ included participant jobs of ‘homemaking’, ‘housewife’, ‘mother’ and ‘students’ of no specific industry (15%). Students specifying an industry or specific training job were classified within a standard ANZSCO occupation.
Table 2.
ANZSCO major occupation groups
| n=301 | n | % | % ABS 2006* |
|---|---|---|---|
| No formal occupation† | 52 | 17.3 | 1.3 |
| Managers | 18 | 6 | 7.6 |
| Professionals | 37 | 12.3 | 21.7 |
| Technicians and trade | 17 | 5.7 | 4.9 |
| Community and personal | 40 | 13.3 | 14.5 |
| Clerical and administration | 55 | 18.3 | 24.9 |
| Sales workers | 42 | 14 | 15.6 |
| Machinery operators | 6 | 2 | 1.2 |
| Unskilled and labourers | 34 | 11.3 | 8.5 |
*Australian Bureau of Statistics 2006 ANZSCO Occupational major groups for employed women 15 and above, Newcastle statistical area of NSW(22).
†No formal occupation included persons nominating ‘homemaking’, ‘housewife’, ‘mother’ or ‘student’ unspecified as job descriptor. Students that specified industry or a specific training were coded within appropriate ANZSCO major group.
ABS, Australian Bureau of Statistics; ANZSCO, Australian New Zealand Standard Classification of Occupations.
IRRs of increased participant total flare day events as a function of exposures (with 95% CI) assessed via FINJEM are presented in table 3. Flare day increases were demonstrated with lifetime occupational exposure to chemical agents of asbestos, iron, wood dust, including soft and hardwood subgroups, and the ergonomic agent group of manual handling (IRR>1.0, p>0.05).
Table 3.
FINJEM exposure and corresponding participant occupations of significance
| Exposure (n (%)) | IRR | 95% CI | p Value | FINJEM ocode | Participant occupation description (n) |
|---|---|---|---|---|---|
| Manual handling of burdens (69 (86.3)) | 1.01 | 1.00 to 1.02 | 0.02 | 32, 34, 36, 37, 38, 43, 91, 122, 231, 310, 540, 580, 651, 657, 673, 760, 781, 790, 800, 809, 814, 816, 820, 850, 851 | Nurses and nursing professions including midwives and institutional child care assistants (38), masseurs, child daycare centre staff (9), cashiers in shops and restaurants, retail shop personnel and shop supervisors (23), farm workers (2), motor truck and bus driver (3), postman (1), fitters, assemblers, machinists, carpenters, processed food workers, packers and warehousemen (16), labourers, firemen, security, home help, hospitality (restaurant and hotel waiters) (6), laundry workers and pressers (3). |
| Asbestos Dichotomised Asbestos (12 (15)) |
1.08 2.48 |
1.01 to 1.2 1.2 to 5.4 |
0.03 0.03 |
651, 660, 673, 680, 759, 781 | Fitter-assemblers, electricians, carpenters, painters, manufacturing, warehouseman (7) |
| Iron (11 (13.8)) | 1.37 | 1.2 to 1.5 | 0.00 | 52, 657, | Teachers and instructors (11), Assemblers and machinists (1) |
| Hardwood softwood wood dust (12 (15)) |
3.34 | 2.1 to 5.3 | 0.00 | 52, 673 | Teachers and instructors (11), carpenters (1) |
FINJEM, Finnish Job Exposure Matrix; IRR, incidence rate ratio.
In particular, the model estimated that patients with SLE engaged in educational occupations (11 participants, Ocode 52) had an increased risk of flare days associated with exposure to iron (IRR 1.37) and wood dust (IRR 3.34). Reported educational occupation job and task descriptors within the original job calendars were crosschecked for performed activities that could result in exposure to these agents. A large number of participants (69 (86.3%)) were involved in occupations with a manual handling burden involving lifting or carrying moderate (10 kg) to heavy (20 kg) objects. These occupations show small increased risks (IRR 1.01) but high significance (p=0.02), and are of interest due to the large number of participants exposed and the musculoskeletal nature of SLE symptoms. The model also identified asbestos exposure in labouring and manufacturing occupations. The increased risk of flare days was estimated at 2.48 (p=0.03), with 15% of the participants having occupational exposure and 58% of those having engaged in work as warehouse/storepersons. However, the ubiquitous nature of asbestos use within Australia over the past decades would indicate more widespread exposure.
Chemical agents such as solvents, aromatic hydrocarbons, heavy metals and pesticides, physical agents (ultraviolet radiation and hot environments) did not show any increased risk in this analysis. Additionally, occupations involving repetitive movements and difficult work positions also did not indicate increased risk. This is surprising, as lupus is a musculoskeletal illness with high prevalence of joint and muscular symptoms.
Discussion
This study was of an exploratory nature, designed to define patient-focused areas for future SLE flare and occupational research. Study data was retrospective and focused upon the participants’ health experiences and illness perspective. This along with the lack of standardised clinical assessment tools within individual patient health records prompted use of standardised flare assessment methods which did not use traditional clinical activity markers, however, potentially increased the detection of milder flares using patient-reported symptoms.
To explore impacts associated with single incident exposure, potential bioaccumulation, as well as health effects of chemical-admixing, the study took a wide approach considering lifetime exposure rather than limiting the occupational exposures to the study year. This approach increased the capacity for identifying potential exposure associations particularly in participants who had ceased work as a result of illness impacts or retirement.
Occupational data was cross-classified from Australian-specific codes into an occupational measure of potential exposure via the FINJEM. The study hypothesis that occupational exposure was associated with significant changes in the likelihood of lupus flares was supported by the significant regression results and relative risk for manual handling burden, asbestos, iron and wood dust. Future research directions were identified, in particular, exploring flare risk in occupations that involve regular manual handling and teaching. The finding that asbestos was associated with increased flares is of concern, as past asbestos use within Australia is widespread, leaving a long-term legacy of exposure from multiple sources including home and work environments.
Employment
Social trend patterns for Australian women, in employment and educational areas are changing with increases in higher education and paid part-time work.23 Overall employment rates (46.25%) were less than the Australian average of 52.6%.22 Participation in full-time work within our participants was also lower (17.5%) than the Australian average indicating a reduced capacity for the SLE patient to participate fully within the workforce. Most occupation studies focusing on SLE and other musculoskeletal illnesses investigate illness pathogenesis, physical limitations and work stress-related factors of work disability.4 24–30 Few studies specifically look at other exposure-related contributions to flare.30–32 Various rates of workforce disability have been reported with ranges of 5–58%.4 31 33 Within this study, self-reported disability was reported in 20% of participants; however, we did not investigate disability or reasons for workforce non-participation.
Disease severity and activity, sociodemographic and work-specific related factors have been identified as strong predictors of work disability. Specifically, SLE work disability related factors included age, race, education and SES as well as disease activity, length of illness and symptoms of pain, fatigue, anxiety and neurocognitive involvement.5 Patterns of moving in and out of the workforce, changing jobs and work hours are also reported. This pattern is also true for our study population with a mean number of 6.9 (range 1–19) jobs held over lifetimes, changes across different industries or transitions to part-time hours within similar industries.
Manual handling
Arthritis, as an SLE disease manifestation, ranges from 69% to 95%,1 34 35 and has been reported in 58% of flares.36 Joints in the knees and hands are often involved; however, nearly all joints can be affected causing varied degrees of mobility loss and pain. Arthritis within our study cohort was confirmed in 78.8% of participants and was self-reported as part of flare events in 70% of participants.
In addition to joint and muscular pain, fatigue (67.5%) was reported by the study population as a frequent flare symptom. Physical activity and fatigue associated with working an 8 h day is often reported as a common barrier to meeting physical work demands in people with musculoskeletal illness.4 26 32 37–39 Work task challenges relating to physical requirements that aggravate pain in joints or muscles, including typing, writing, hand-specific activities, prolonged static work positions, as well as lifting, pushing and carrying or moving loads, appear to be more problematic.26 40–43 Inflexibility of work hours, requirements for overtime, commuting, and the need for rest periods were also reported as impacting on fatigue levels and ultimately work capacity.4 32 42 The finding of an association between manual handling tasks as defined in FINJEM (multiple lifting or carrying of loads of 10 or 20 kg)10 and increased flare days within this study supports these findings.
The fact that a large proportion of our cohorts’ occupations were within nursing (32.2%), an occupation with a particularly high risk of musculoskeletal injury and aggravation,44–47 highlights the importance of considering modified work practises as a protective health strategy.
Iron and wood dust
While iron and wood dust were found to have a moderate increased flare risk, this finding should be examined in reference to the occupations specifically listed within the study cohort. As documented within table 3, in reference to iron and wood dust exposure and FINJEM codes, 11 participants reported having occupations within teaching professions (Ocode 52) while other participants reported other occupations relevant to these exposure agents, that is, panel beater-metal worker (Ocode 657, iron) and carpenter (Ocode 673, wood dust).
Published information on exposure sources in teaching are limited with a small amount of occupational health and safety information within woodworking or metalwork trade or technical teaching, but not for general teaching. The study population, while having 9.3% occupation coding of teaching, showed no individual documented sources to either iron or wood dust as a specific exposure of their job. Teaching occupational tasks noted within participant job calendars included class preparation and participation in science field trips including geology, agriculture and horticultural subspecialities, as well as preparation and participation of art and beauty trade instructional classes.
Increased mortality risk for systemic autoimmune illnesses including SLE has been previously linked to occupations involved with extensive exposure to the public, such as teaching. Increased risk associations, while not specific, has been suggested to be related to exposure to bacteria and virus including influenza, varicella and Epstein Barr virus.48 49 Within this study, flare activity risk association with exposure to microbiological agents, as assessed in FINJEM, was not analysed.
Asbestos
Asbestos as a FINJEM semiquantified exposure and as a dichotomised agent of positive exposure was identified within all models, estimating increased risk of flare days at 2.48 (p=0.03). Occupations identified within FINJEM Ocodes as having a likelihood of asbestos exposure were within labouring and manufacturing industries including electrician, carpenter and painter, however, the majority of participants had nominated jobs as warehouse storepersons or shelf stackers (7 (58.3%)). The association between asbestos exposure and flare risk increases is interesting, but creates interpretation difficulty due to the ubiquitous nature of asbestos use within Australia. While the biological plausibility of an SLE association could be questioned, there is experimental evidence that asbestos (a type of silicate) displays immune modulating effects that may increase risk for expression of autoimmune disease.50
Study strengths and weaknesses
Flare assessment via standard disease activity measures is resource intensive, relying on diagnostic and physician assessment, and was not possible for this pilot study; therefore, flares were assessed from a patient perspective with adherence to a standardised method which included a novel flare definition.17 The development of multiple disparate symptoms attributable to disease flares explains the small number of patients reporting flare frequency greater than monthly, and may have resulted in overestimation of flare frequency counts and calculated flare days; however, this overestimation would not be expected to introduce any systematic confounding influence.
Measurement bias within occupation exposure assessment was minimised by adopting a process of intracoding reliability with two independent researchers crosschecking occupational coding assignment. Lifetime occupational exposure can only be estimates as individual participant exposure measures were based upon job titles without weightings for individual job task descriptors or job environment, and without calculation of individual occupation time periods. Therefore, the classification of exposure or non-exposure may be erroneous, but again, this would be non-differential and, hence, would not bias the results.
FINJEM yields data for 40 different classes of exposure, hence, there is the potential for false positives due to multiple comparisons. There was no adjustment for this in the current analysis and, hence, the results must be seen as hypothesis generating.
It is unlikely that the findings reported here represent recall bias as participants were asked about their job history in the context of a wider project assessing numerous potential flare triggers and no discussion surrounding the association being tested. Additionally, the study analysed a lifetime job calendar for long-term and cumulative impacts.
Despite all gender and ethnic groups being invited to the study, the participating cohort was relatively homogeneous, with all being female and 98% reporting a Caucasian background. Participants were also asked about their parental heritage with a large majority reporting both parents to be of English or European Caucasian heritage. The lack of gender and ethnic diversity within the cohort would limit generalisability of the findings to other population mixes.
Implications for clinical practice
This study highlights the importance of considering occupational impacts within management regimes and exacerbation prevention advice. The inclusion of advice on adjusting work practises could serve to lessen illness impacts over a patient's lifetime.
Conclusion
Our study is different from other SLE occupational studies in that it focused on patient-reported flares and occupational exposures rather than work disability, with the aim of identifying potential occupational exposures associated with increased flare risk. The study findings provide insight into future research directions that will better inform appropriate protective occupational measures to reduce adverse health impacts.
While occupational exposure assessment had some limitations, the results indicated that occupations such as nursing, with a high manual handling burden, posed increased risk to patients with SLE, however, the greatest risk was associated with wood dust and iron exposure with teachers and specialist labourers. The findings reinforce the need to develop standardised and validated occupational research measurements with replication in other populations to further improve knowledge of SLE flare triggers.
Supplementary Material
Footnotes
Contributors: All listed authors were substantially involved in the conception of design, drafting and revising the work, final approval of the version to be published and agree to be accountable for the work. Analysis and interpretation of data was primarily undertaken by MLS, MG with assistance by GR.
Funding: This study forms part of the Environmental Determinants of Lupus Flare (EDOLF) PhD study, University of Newcastle. The study was funded via resources provided by the Autoimmune Resource Research Centre (Not-for-profit charity http://www.autoimmune.org.au) and the Val Badham Research Scholarship for Immunology, University of Newcastle Foundation.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: The University of Newcastle Human Research Ethics Committee: Approval Number, H-133-1105 and the Hunter New England Human Research Ethics Committee: Approval Number, 05/09/14/3.12.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Datasets used within this analysis form the basis of multiple analysis in regards to potential SLE flares, and are part of a large PhD thesis work (in progress). Requests for data sharing can be forwarded to the principle author.
References
- 1.Barr SG, Zonana-Nacach A, Magder LS, et al. Patterns of disease activity in systemic lupus erythematosus. Arthritis Rheum 1999;42:2682–8. [DOI] [PubMed] [Google Scholar]
- 2.Bertoli AM, Fernandez M, Alarcon GS, et al. Systemic lupus erythematosus in a multiethnic US cohort LUMINA (XLI): factors predictive of self-reported work disability. Ann Rheum Dis 2007;66:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves G. C-reactive protein. Australian Prescriber 2007;30:74–6. [Google Scholar]
- 4.Allaire SH, Wei Li, LaValley MP. Work barriers experienced and job accommodations used by persons with arthritis and other rheumatic diseases. Rehabil Couns Bull 2003;46:147–56. [Google Scholar]
- 5.Baker K, Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology (Oxford) 2009;48:281–4. [DOI] [PubMed] [Google Scholar]
- 6.Parks CG, Cooper GS. Occupational exposures and risk of systemic lupus erythematosus: a review of the evidence and exposure assessment methods in population- and clinic-based studies. Lupus 2006;15:728–36. [DOI] [PubMed] [Google Scholar]
- 7.Zandman-Goddard G, Solomon M, Rosman Z, et al. Environment and lupus-related diseases. Lupus 2012;21:241–50. [DOI] [PubMed] [Google Scholar]
- 8.Cooper GS, Wither J, Bernatsky S, et al. Occupational and environmental exposures and risk of systemic lupus erythematosus: silica, sunlight, solvents. Rheumatology 2010;49:2172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess EV. Environmental chemicals and autoimmune disease: cause and effect. Toxicology 2002;181–182:65–70. [DOI] [PubMed] [Google Scholar]
- 10.Kauppinen T, Toikkanen J, Pukkala E. From cross-tabulations to multipurpose exposure information systems: a new job-exposure matrix. Am J Ind Med 1998;33:409–17. [DOI] [PubMed] [Google Scholar]
- 11.Kauppinen T, Uuksulainen S, Saalo A, et al. Trends of occupational exposure to chemical agents in Finland in 1950–2020. Ann Occup Hyg 2013;57:593–609. [DOI] [PubMed] [Google Scholar]
- 12.van Tongeren M, Kincl L, Richardson L, et al. Assessing occupational exposure to chemicals in an international epidemiological study of brain tumours. Ann Occup Hyg 2013;57:610–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teschke K, Olshan AF, Daniels JL, et al. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med 2002;59:575–93; discussion 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Rheumatology. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. 2005. [cited 2005 26 October 2005]. Classification criteria for diagnosis of Systemic Lupus Erythematosus]. http://www.rheumatology.org/practice/clinical/classification/sle/1997_update_of_the_1982_American_College_of_Rheumatology_Revised_Criteria_for_Classification_of_sle.pdf
- 15.World Medical Association. World Medical Association Declaration of Helsinki. 2008. [cited 25 November 2006]. Declaration of Helsinki 2008 revision. http://www.wma.net/en/30publications/10policies/b3/17c.pdf
- 16.Australian Bureau of Statistics. 1220.0 ANZCO—Australian and New Zealand Standard Classification of Occupations. 2013. [updated 24 June 2009; cited 2013 8 October 2013]; Comparison between ANZCO, ASCO 2nd edition and NZSCO 1999. http://www.abs.gov.au/ausstats/abs@.nsf
- 17.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–31. [DOI] [PubMed] [Google Scholar]
- 18.de Haes JO, Fayers M, Visser P, et al. The Rotterdam Symptom Checklist (RSCL): a manual. Manual of the validation of the RSCL ed. Groningen, The Netherlands: ISBN 90 72156382, 1996:38.
- 19.NSW Department of Health. New South Wales Population Health Survey 2010. In: Centre for Epidemiology and Research PHD, NSW Department of Health, editor.: NSW Government, AUSTRALIA, 2011.
- 20.Borges MC, Martini LA, Rogero MM. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition 2011;27:399–404. [DOI] [PubMed] [Google Scholar]
- 21.Parravicini V, Caserta S. The immunomodulatory roles of vitamin D: new tricks for an old dog. Mol Interv 2010;10:204–8. [DOI] [PubMed] [Google Scholar]
- 22.Australian Bureau of Statistics. 2006 Census Table: Newcastle (NSW) Statistical District 20680- Occupation by Hours Worked by Sex—Newcastle (NSW) Canberra: Commonwealth of Australia, 2007. [updated 8 February 2008; cited 2013 13/10/2013]; Table of Occupational major Codes ANZCO. [Google Scholar]
- 23.Australian Bureau of Statistics. Australian social trends, 2006. Canberra: Australian Bureau of Statistics, Commonwealth of Australia, 2006. [updated 03 August 2007; cited 2013 10/10/2013]; Social trands in employment for Australian populations. [Google Scholar]
- 24.Cervera R, Khamashta MA, Hughes GR. The Euro-lupus project: epidemiology of systemic lupus erythematosus in Europe. Lupus 2009;18:869–74. [DOI] [PubMed] [Google Scholar]
- 25.Cooper GS. Occupational exposures and risk of rheumatoid arthritis: continued advances and opportunities for research. J Rheumatol 2008;35:950–2. [PubMed] [Google Scholar]
- 26.de Croon EM, Sluiter JK, Nijssen TF, et al. Work ability of Dutch employees with rheumatoid arthritis. Scand J Rheumatol 2005;34:277–83. [DOI] [PubMed] [Google Scholar]
- 27.Dooley MA, Hogan SL. Environmental epidemiology and risk factors for autoimmune disease. Curr Opin Rheumatol 2003;15:99–103. [DOI] [PubMed] [Google Scholar]
- 28.Gignac MA, Sutton D, Badley EM. Arthritis symptoms, the work environment, and the future: measuring perceived job strain among employed persons with arthritis. Arthritis Rheum 2007;57:738–47. [DOI] [PubMed] [Google Scholar]
- 29.Li XJ, Sundquist J, Sundquist K, et al. Occupational Risk Factors for Systemic Lupus Erythematosus: A Nationwide Study Based on Hospitalizations in Sweden. J Rheumatol 2012;39:743–51. [DOI] [PubMed] [Google Scholar]
- 30.Partridge AJ, Karlson EW, Daltroy LH, et al. Risk factors for early work disability in systemic lupus erythematosus: results from a multicenter study. Arthritis Rheum 1997;40:2199–206. [DOI] [PubMed] [Google Scholar]
- 31.Yelin E, Trupin L, Katz P, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum 2007;57:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Dhanhani AM, Gignac MA, Su J, et al. Work disability in systemic lupus erythematosus. Arthritis Rheum 2009;61:378–85. [DOI] [PubMed] [Google Scholar]
- 33.Baker K, Pope J, Fortin P, et al. Work disability in systemic lupus erythematosus is prevalent and associated with socio-demographic and disease related factors. Lupus 2009;18:1281–8. [DOI] [PubMed] [Google Scholar]
- 34.Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol 2009;23:495–506. [DOI] [PubMed] [Google Scholar]
- 35.Mok CC, Cheung MY, Ho LY, et al. Risk and predictors of work disability in Chinese patients with systemic lupus erythematosus. Lupus 2008;17:1103–7. [DOI] [PubMed] [Google Scholar]
- 36.Petri M, Singh S, Tesfasyone H, et al. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol 2009;36:2476–80. [DOI] [PubMed] [Google Scholar]
- 37.Varekamp I, Haafkens JA, Detaille SI, et al. Preventing work disability among employees with rheumatoid arthritis: what medical professionals can learn from the patients’ perspective. Arthritis Rheum 2005;53:965–72. [DOI] [PubMed] [Google Scholar]
- 38.Young A, Dixey J, Kulinskaya E, et al. Which patients stop working because of rheumatoid arthritis? Results of five years’ follow up in 732 patients from the Early RA Study (ERAS). Ann Rheum Dis 2002;61:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerner DJ, Amick BC, III, Malspeis S, et al. A national survey of health-related work limitations among employed persons in the United States. Disabil Rehabil 2000;22:225–32. [DOI] [PubMed] [Google Scholar]
- 40.Gignac MA, Cao X, Tang K, et al. Examination of arthritis-related work place activity limitations and intermittent disability over four-and-a-half years and its relationship to job modifications and outcomes. Arthritis Care Res 2011;63:953–62. [DOI] [PubMed] [Google Scholar]
- 41.Arkela-Kautiainen M, Kauppi M, Heikkila S, et al. Evaluation of the arthritis impact measurement scales (AIMS2) in Finnish patients with rheumatoid arthritis. Scand J Rheumatol 2003;32:300–5. [DOI] [PubMed] [Google Scholar]
- 42.Mancuso CA, Paget SA, Charlson ME. Adaptations made by rheumatoid arthritis patients to continue working: a pilot study of workplace challenges and successful adaptations. Arthritis Care Res 2000;13:89–99. [PubMed] [Google Scholar]
- 43.Robinson HS, Walters K. Patterns of work—rheumatoid arthritis. Int Rehabil Med 1979;1:121–5. [DOI] [PubMed] [Google Scholar]
- 44.Chiou ST, Chiang JH, Huang N, et al. Health issues among nurses in Taiwanese hospitals: National survey. Int J Nurs Stud 2013;50:1377–84. [DOI] [PubMed] [Google Scholar]
- 45.Chung YC, Hung CT, Li SF, et al. Risk of musculoskeletal disorder among Taiwanese nurses cohort: a nationwide population-based study. BMC Musculoskelet Disord 2013;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yassi A, Lockhart K. Work-relatedness of low back pain in nursing personnel: a systematic review. Int J Occup Environ Health 2013;19:223–44. [DOI] [PubMed] [Google Scholar]
- 47.Sembajwe G, Tveito TH, Hopcia K, et al. Psychosocial stress and multi-site musculoskeletal pain a cross-sectional survey of patient care workers. Workplace Health Saf 2013;61:117–25. [DOI] [PubMed] [Google Scholar]
- 48.Gold LS, Ward MH, Dosemeci M, et al. Systemic autoimmune disease mortality and occupational exposures. Arthritis Rheum 2007;56:3189–201. [DOI] [PubMed] [Google Scholar]
- 49.Walsh SJ, DeChello LM. Excess autoimmune disease mortality among school teachers. J Rheumatol 2001;28:1537–45. [PubMed] [Google Scholar]
- 50.Otsuki T, Maeda M, Murakami S, et al. Immunological effects of silica and asbestos. Cell Mol Immunol 2007;4:261–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


