Abstract
Objectives
Growing evidence suggests that vitamin D plays a key role in the pathogenesis and progression of autoimmune diseases, including systemic lupus erythematosus (SLE). Recent studies have found an association between lower serum 25-hydroxyvitamin D (25(OH)D) levels and higher SLE activity. We studied the relationship between 25(OH)D levels and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score, and we assessed for the first time the role of vitamin D in predicting SLE flare-ups.
Methods
Serum 25(OH)D levels were measured in 170 patients with SLE who were prospectively followed up for 6 months (Plaquenil LUpus Systemic study, ClinicalTrials.gov number NCT00413361).
Results
The mean SLEDAI score was 2.03±2.43 and 12.3% patients had active disease (SLEDAI ≥6). The mean 25(OH)D level was 20.6±9.8 ng/mL. Deficiency (25(OH)D <10 ng/mL) was observed in 27 (15.9%), insufficiency (10≤25(OH)D<30) in 112 (65.9%) and optimal vitamin D status (25(OH)D≥30) in 31 (18.2%) patients. In multivariate analysis, female gender (p=0.018), absence of defined antiphospholipid syndrome (p=0.002) and higher creatinine clearance (p=0.004) were predictive of lower 25(OH)D levels. In multivariate analysis, lower 25(OH)D levels were associated with high SLE activity (p=0.02). Relapse-free survival rate was not statistically different according to the vitamin D status during the 6-month follow-up (p=0.22).
Conclusions
We found a low vitamin D status in the majority of patients with SLE, and a modest association between lower 25(OH)D levels and high disease activity. There was no association between baseline 25(OH)D levels and relapse-free survival rate.
Keywords: vitamin D, Systemic Lupus Erythematosus, Autoimmune Diseases, hydroxychloroquine
Key messages.
Suboptimal vitamin D status is found in more than 80% of patients with SLE.
Low vitamin D levels were are statistically associated with high disease activity.
Baseline vitamin D levels were not associated with relapse-free survival rate during a 6-month follow-up.
Introduction
1,25-Dihydroxyvitamin D (1,25(OH)2D), or calcitriol, the most active form of vitamin D, is a secosteroid hormone recognised as essential in calcium metabolism and bone homeostasis. Mounting evidence exists for a role of vitamin D in non-skeletal diseases, such as autoimmune diseases. Specifically, both in vitro and in vivo studies of vitamin D effects provide an immunological basis for potential beneficial effects of vitamin D in systemic lupus erythematosus (SLE).1 Studies using animal models of SLE demonstrated a preventive action of calcitriol or an analogue on SLE manifestations.2–5 Vitamin D and its synthetic analogues significantly reduced polyclonal and anti-dsDNA immunoglobulin G production by SLE peripheral blood mononuclear cells (PBMC) in vitro.6 Increased B cell activation was associated with decreased 25(OH)D levels in patients with SLE, suggesting that vitamin D deficiency may contribute to increased B cell activation in SLE.7 We have recently demonstrated that high-dose vitamin D supplementation provides beneficial immunological effects in vivo in patients with SLE with an increase of regulatory T cells and a decrease of Th17, Th1 and memory B cells.8 A suppressive action of vitamin D on the interferon α signature, considered as central in the pathophysiology of SLE, has been demonstrated in vitro9 and in vivo.7 Genetic studies have pointed out associations between allelic variation within vitamin D regulatory genes and susceptibility to SLE.10 11 Many epidemiological studies reported increased prevalence of vitamin D deficiency and insufficiency in patients with SLE,12–16 and recent studies have reported an association between lower serum 25(OH)D concentration and higher disease activity.9 13 16–19 To our knowledge, the role of 25(OH)D level in predicting SLE flare-ups has never been assessed.
The objectives of our study were (1) to assess the relationship between 25(OH)D levels and SLE disease activity, (2) to determine the predictive factors of vitamin D status and (3) to determine whether 25(OH)D levels predict disease flare-ups in the 6-month follow-up.
Patients and methods
Study population
This study is an ancillary study of the Plaquenil LUpus Systemic study (PLUS; ClinicalTrials.gov number NCT0041336), which is a randomised, double-blind, placebo-controlled, multicentre trial that was conducted from June 2007 through August 2010 at 37 centres in France.20 This research received the approval of the Saint-Louis Hospital ethic committee. All the patients involved signed a consent form.
Briefly, relevant inclusion criteria were adult patients with SLE according to ACR criteria,21 treated with hydroxychloroquine (HCQ) for at least 6 months. SLE had to be stable with no increase in the steroid dosage during the three previous weeks, a steroid dosage ≤0.5 mg/kg/day of prednisone or equivalent, no modifications of a possible immunosuppressant drug during the two previous months and a Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) ≤12. The main relevant exclusion criteria were estimated glomerular filtration rate lower than 60 mL/min/1.73 m², pregnancy and known or suspected non-adherence. Antiphospholipid syndrome (APS) was defined according to the Sydney criteria.22
The details regarding supplemental vitamin D intake were not available. Nevertheless, the usual daily vitamin D intake prescribed to prevent glucocorticoid-induced osteoporosis was comprised between 400 and 880 UI of vitamin D3.
At inclusion, all subjects had SLE activity assessment and blood examination including a centralised blood HCQ concentration measurement. Patients with blood HCQ concentration ranging between 100 and 750 ng/mL at inclusion, which is a range of concentrations that was previously identified as associated with a higher risk to develop flare-ups,23 were eligible for randomisation. Randomised patients received either the same dose of HCQ (group 1) or an increased dose aimed at achieving a blood HCQ concentration dosage above 1000 ng/mL (group 2). The maximal dosage of HCQ was 800 mg/day.
Follow-up
Randomised patients were followed up for 7 months. Assessments of SLE activity and side effects were performed in all patients at 1, 3, 5 and 7 months (M1, M3, M5 and M7 visit, respectively). The primary outcome measure was the occurrence of SLE flare-ups, as defined by the SELENA-SLEDAI flare composite score.24 Briefly, this score includes three elements: the SELENA-SLEDAI score; an assessment of new or worsening disease activity, medication changes and hospitalisations not captured with the use of the SLEDAI; and the score on the physician's global assessment visual analogue scale. Information about the use of this score was provided at each site before the study began.
We chose to analyse the serum sampled at M1 since (HCQ) were considered stable at that moment (patients had reached their target (HCQ)).
Serum 25(OH) vitamin D measurement
Blood samples were collected and processed immediately in each centre. After centrifugation at 2000g for 10 min, serum samples were stored at −80°C and thawed only once. Serum 25(OH)D was measured by means of a radio-immunoassay after simple extraction with acetonitrile (DiaSorin, Stillwater, Minnesota, USA), as described previously.25 The interassay and intraassay coefficients of variation were <7% and <5%, respectively, throughout the entire range of concentrations. The detection limit was 3 ng/mL. Samples with a measured concentration below 3 ng/mL were arbitrarily attributed a value of 2 ng/mL. The measurements were performed in a single laboratory, which participates in the DEQAS proficiency testing and finds results that fall within 10% of the all-laboratory trimmed mean of this International Quality Control. Vitamin D status was characterised as deficiency (<10 ng/mL), insufficiency (10≤25(OH)D<30 ng/mL) and optimal vitamin D status (≥30 ng/mL).
Statistical analysis
Predictive factors of serum 25(OH)D levels were identified via univariate analysis with a linear regression model for quantitative variables and with Mann–Whitney tests for qualitative variables. Variables with univariate p value <0.2 were included in a multivariate stepwise linear regression model. Model fit has been checked by visual inspection of the residuals. To assess the relationship between serum 25(OH)D levels and SELENA-SLEDAI score (score ≥6 vs <6), we performed a stepwise logistic regression. Variables included in the model were age, sex, HCQ levels, prednisone use and serum 25(OH)D levels. Association between vitamin D status (deficiency, insufficiency, optimal level) and relapse-free survival rate was tested in univariate analysis using the log-rank test. Relapse-free survival rate was calculated from M1 to the date of first flare-up. Patients alive without occurrence of flare-up at the date of last follow-up were censored at this date. To evaluate the effect of vitamin D status after adjustment for other potentially predictive variables, a multivariate Cox regression model was performed. Variables included in the multivariate Cox model were SELENA-SLEDAI score (≥6 vs <6), score on the physician's global assessment visual analogue scale (≥ median vs < median=0.11), C3, anti-dsDNA levels and vitamin D status. All tests were two-sided. p Values less than 0.05 were considered statistically significant. All analyses were performed with the SAS software V.9.2 (SAS Institute, Cary, North Carolina, USA).
Results
Study population
The study population included 170 of the 171 randomised patients (one patient had no sample at M1). Patient characteristics are listed in table 1. Of the 170 subjects, 148 (87%) were women, the mean age was 40±11 years and the median disease duration was 7.8 years (0.5–30.9). An APS was defined in 16% of the patients. The mean estimated creatinine clearance using Cockroft–Gault equation was 108±34 mL/min. The median SELENA-SLEDAI score was 1 (0–18). According to the design of the study, all the subjects were treated with HCQ. Other SLE medications included corticosteroids (55% with a mean dose of 8.0±4 mg/d) and immunosuppressant drugs (19%).
Table 1.
Characteristics of the 170 patients with SLE
| Characteristics | Patients (n=170) |
|---|---|
| Age [mean±SD] (year) | 40 [11] |
| Gender, female (n, %) | 148 (87) |
| Ethnicity | |
| Caucasian (n, %) | 122 (71.8) |
| African-American (n, %) | 28 (16.4) |
| Asian (n, %) | 18 (10.6) |
| Others (n, %) | 2 (1.2) |
| Mean BMI [±SD] | 25 [5] |
| Current smokers (n, %) | 41 (24) |
| Median disease duration [range] (year) | 7.9 [0.6–31.0] |
| Photosensitivity (n, %) | 102 (60) |
| Malar rash (n, %) | 80 (47,3) |
| Discoid lupus (n, %) | 20 (11,8) |
| Renal disorder (n, %) | 48 (28) |
| Mean creatinine clearance (mL/min) [±SD] | 108 [34] |
| Low levels of complement (C3) (n, %) | 20 (11.8) |
| Median PGA [range] | 0.11 [0–2.6] |
| Active disease (SELENA-SLEDAI ≥6) (n, %) | 15 (8.8) |
| Median SELENA-SLEDAI score [range] | 1 [0–18] |
| Associated APS (n, %) | 28 (16) |
| Treatment variables | |
| Ever use of prednisone (n, %) | 137 (80) |
| Current prednisone use (n, %) | 94 (55.3) |
| Actual prednisone dosage [mean±SD] (mg/day) | 8.7 [6.2] |
| Ever use of immunosuppressive drugs (n, %) | 69 (40) |
| Current use of immunosuppressive drugs (n, %) | 32 (19) |
| Current anticoagulant treatment (n, %) | 7 (4) |
| Mean blood HCQ concentration at M1 [±SD] (ng/mL) | 1035 [535] |
APS, antiphospholipid syndrome; BMI, body mass index; HCQ, hydroxychloroquine; PGA, physicians’ global assessment; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index; SLE, systemic lupus erythematosus.
Vitamin D status
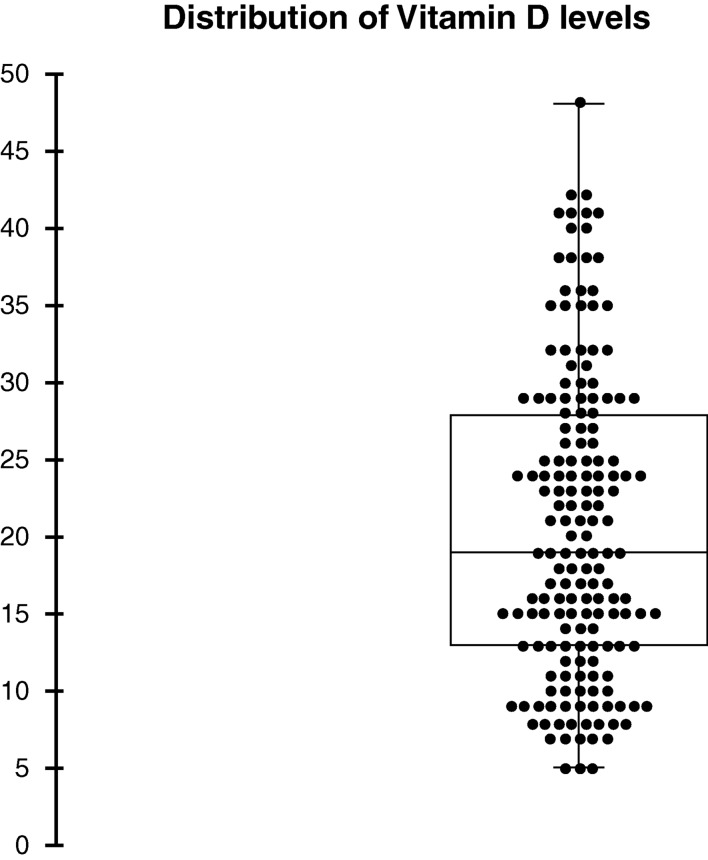
The mean serum 25(OH)D level was 20.6±9.8 ng/mL. In total, 27 (15.9%) subjects had vitamin D deficiency (25(OH)D<10 ng/mL), 112 (65.9%) had vitamin D insufficiency (10≤25(OH)D<30) and 31 (18.2%) had optimal vitamin D levels (25(OH)D≥30). The distribution of vitamin D levels is shown in figure 1.
Figure 1.
The central line marks the median value and the edges of the box mark the first and third quartiles. The vertical line issuing from the box extends to the minimum and maximum values.
In the univariate analysis (table 2), gender, age, body mass index (BMI), ethnicity, disease duration, photosensitivity, creatinine clearance, APS, anticoagulant treatment and season were associated with 25(OH)D levels with a p value <0.2 and were then included in the multivariate analysis. Of these, only female gender (p=0.018), an absence of defined APS (p=0.002) and higher creatinine clearance (p=0.004) were associated with lower 25(OH)D levels in multivariate analysis (table 3).
Table 2.
Predictors of 25(OH)D levels in univariate analysis
| Parameter regression (standard error) (quantitative variable) | Median (range) vitamin D levels (binary variables) | p Value | |
|---|---|---|---|
| Age | 0.19 (0.07) | 0.007 | |
| Gender | |||
| Male | 24 (9–48) | 0.06 | |
| Female | 18 (5–42) | ||
| Ethnicity | |||
| Europe | 17 (5–42) | 0.19 | |
| Others | 20 (5–48) | ||
| BMI | −0.22 (0.14) | 0.12 | |
| Current smokers | |||
| No | 19 (5–48) | 0.64 | |
| Yes | 17 (5–42) | ||
| Season | |||
| Summer | 24 (8–41) | 0.001 | |
| Others | 17 (5–48) | ||
| Disease duration | 0.17 (0.11) | 0.12 | |
| Photosensitivity | |||
| No | 23 (7–42) | 0.04 | |
| Yes | 17 (5–48) | ||
| Malar rash | |||
| No | 20 (5–42) | 0.8 | |
| Yes | 18 (5–48) | ||
| Discoid lupus | |||
| No | 19 (5–48) | 0.8 | |
| Yes | 19 (5–41) | ||
| Creatinine clearance | −0.048 (0.02) | 0.03 | |
| Anti-dsDNA (Farr assay) | −0.014 (0.03) | 0.61 | |
| Anti-dsDNA (ELISA) | −0.006 (0.005) | 0.29 | |
| C3 levels | −1.17 (3.34) | 0.72 | |
| Associated APS | |||
| No | 18 (5–48) | 0.007 | |
| Yes | 25 (9–41) | ||
| Corticosteroid dosage | 0.03 (0.12) | 0.8 | |
| Ever immunosuppressant drugs use | |||
| No | 19 (5–42) | 0.24 | |
| Yes | 23 (5–48) | ||
| HCQ concentration | −0.0007 (0.001) | 0.66 | |
| Current anticoagulant treatment | |||
| No | 19 (5–48) | 0.1 | |
| Yes | 31 (15–40) | ||
25(OH)D, 25-hydroxyvitamin D; APS, antiphospholipid syndrome; BMI, body mass index; HCQ, hydroxychloroquine.
Table 3.
Predictors of lower 25(OH)D levels in multivariate analysis
| Variables | Parameter estimate (SE) | p Value |
|---|---|---|
| Higher creatinine clearance | −0.06 (0.02) | 0.004 |
| Female gender | −5.24 (2.15) | 0.018 |
| Absence of antiphospholipid syndrome | 6.25 (1.94) | 0.002 |
There was no statistically significant correlation between 25(OH)D levels and blood HCQ concentrations (p=0.66).
Relationship between 25(OH)D levels and SLE activity
The primary objective was to assess the relationship between 25(OH)D levels and SLE activity. Lower 25(OH)D levels were associated with significantly high SLE activity defined by a SELENA-SLEDAI score ≥6 (p=0.02), with an odd ratio of 0.93 (95% CI (0.873 to 0.989)). Prednisone use was also significantly associated with SLE activity (table 4).
Table 4.
Association of 25(OH)D levels and SLE activity (SELENA-SLEDAI ≥6) in multivariate analysis
| OR | 95% CI | p Value | |
|---|---|---|---|
| 25(OH)D levels | 0.93 | 0.87–0.99 | 0.02 |
| Prednisone use | 0.19 | 0.05–0.70 | 0.01 |
The other variables introduced in the model were not retained by the model.
SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment—Systemic Lupus Erythematosus Disease Activity Index26; SLE, systemic lupus erythematosus.
We then analysed the relationship between 25(OH)D levels and the risk of SLE flare-up. At M1, the mean 25(OH)D level was not significantly different in the 45 patients who developed a flare-up during the 6 months of follow-up and in the 125 who did not (19.3±9.6 vs 21.1±9.9 ng/mL, respectively). Relapse-free survival rate at 6 months was 63% in patients with vitamin D deficiency, 77% in patients with insufficiency and 77% in patients with optimal vitamin D status (p=0.22). We then adjusted for SELENA-SLEDAI (≥6 vs <6), physician's global assessment visual analogue scale (≥0.11 vs <0.11), C3 levels and anti-dsDNA levels. SELENA-SLEDAI at M1 (HR=3.14; 95% CI 1.54 to 6.4, p=0.002), physician's global assessment visual analogue scale (HR=2.78; 95% CI 1.4 to 5.5, p=0.003) and anti-dsDNA levels (HR=1.002; 95% CI 1.0001 to 1.004, p=0.04) were significantly predictive of relapse-free survival rate.
Discussion
The optimal vitamin D level is defined as greater than 30 ng/mL because lower levels are associated with deleterious compensatory secondary hyperparathyroidism.27 Consistently with other studies in SLE,12–16 18 28 we found a high proportion of patients with SLE with vitamin D deficiency and insufficiency (15.4% and 67.3%, respectively).
Regarding the factors associated with vitamin D deficiency, female gender, higher creatinine clearance and absence of defined APS were associated with lower 25(OH)D levels in multivariate analysis. Female gender is a classical risk factor for hypovitaminosis D in the general population29–31 and in patients with SLE.16 26 This might be explained by differences in outdoor activities or clothing,30 lower body surface area32 and androgen-related differences in vitamin D binding protein levels, in the precursor production by the skin or in the 25-hydroxylation by the liver.29 Levels of serum 25(OH)D are known to decrease with decreasing glomerular filtration rate in patients with chronic kidney diseases.31 33 However, in the cohort of the Third National Health and Nutrition Examination Survey (NHANES III), the adjusted mean serum 25(OH)D was higher in patients with mildly decreased kidney function (60<eGFR <89 mL/min/1.73 m²) compared with patients with normal kidney function (eGFR≥90 mL/min/1.73 m²).31 Since an eGFR <60 mL/min/1.73 m² was an exclusion criteria in our study, our findings are consistent with these results.31 Unexpectedly, we found that the presence of an APS is independently associated with higher 25(OH)D levels. Other studies reported lower vitamin D levels in patients with APS than in healthy controls,34–36 and lower vitamin D levels in SLE associated APS than in patients with primary APS.34 To our knowledge, our study is the only one that compared 25(OH)D levels between patients with SPE with versus without associated APS. This result raised the question of an interaction between vitamin K antagonists and vitamin D. We did not find evidence for this hypothesis in the literature; instead, warfarin is a potent ligand of nuclear receptor SXR, thereby activating CYP3A4, which is involved in the catabolism of calcitriol.37 Additionally, we did not find any association between anticoagulant treatment by vitamin K antagonists and 25(OH)D levels.
We did not find an association between corticosteroid use or dose and vitamin D status. To our knowledge, this association has never been assessed in SLE. Corticosteroid use is thought to increase the catabolism of vitamin D by activating the expression of the nuclear receptor SXR or NR1I2, which induces the destruction of 25(OH)D and 1,25(OH)2D through the expression of CYP3A4.27 38 Nevertheless, in our study, patients had to receive a steroid dosage lower than 0.5 mg/kg/d, and the mean steroid dosage was low, meaning that we cannot exclude that a higher steroid dosage would have been associated with lower 25(OH)D levels.
HCQ, an important medication in SLE,39 40 is associated with higher bone mineral density in patients with SLE, suggesting an effect of HCQ on calcium and bone metabolism.41 42 HCQ may inhibit the conversion of 25(OH)D to 1,25(OH)2D, but the precise mechanism remains unknown.43–45 In one of the first studies devoted to vitamin D status in SLE, 1,25(OH)2D levels were found to be lower in patients treated with HCQ, while 25(OH)D levels were not significantly different.28 By contrast, Ruiz-Irastorza et al26 concluded that treatment with HCQ independently predicted (p=0.014) higher levels of 25(OH)D. We were not able to assess this relationship since all of our patients were treated with HCQ. However, our study is the first to explore the relationship between the blood HCQ concentrations and the serum 25(OH)D levels, and we did not find any correlation.
We found that lower 25(OH)D levels were associated with high disease activity, which is consistent with most previous studies.9 13 16–19 42 46 47 The absence of association found by some studies14 15 26 48–50 might be explained by some bias: the low number of subjects,14 15 the very low disease activity50 and the heterogeneity of treatments, particularly HCQ.49 The association between lower vitamin D levels and high SELENA-SLEDAI score suggests that vitamin D may be beneficial in preventing and attenuating some manifestations of SLE. As previously stated, mounting evidence exists for beneficial effects of vitamin D in SLE. The clinical relevance of the modest association between vitamin D and high disease activity can be questioned. Moreover, all of the studies to date reporting this association were cross-sectional so that we cannot be certain that vitamin D status affects disease activity rather than vice versa. It has been proposed that VDR hyperexpression in activated lymphocytes induced by SLE activity results in increased turnover of vitamin D14 so that vitamin D deficiency could possibly serve as a biomarker of disease flare.51 Additionally, active patients with SLE might better respect photoprotection.
Our study assesses the relationship between 25(OH)D levels and the risk of further SLE flare-ups during a 6-month follow-up. We did not find an association between baseline vitamin D levels and relapse-free survival rate. It can be argued that 6 months may be a too short period to evaluate disease flare. This finding may also reflect the dual effects of sunlight exposure in SLE: ultraviolet B rays are the main vitamin D source in the absence of vitamin D supplements, and on the same time a possible trigger of a SLE flare-up. Another explanation might be that most subjects in our study had deficient or insufficient 25(OH)D levels making it difficult to capture the effect of 25(OH)D status. The threshold of 30 ng/mL is essential for bone health, but the serum 25(OH)D level needed for optimal health is unknown.52 We have shown that beneficial immunological effects of vitamin D in patients with SLE in vivo were more pronounced with a mean 25(OH)D level of 51.4 ng/mL than with a mean 25(OH)D level of 41.5 ng/mL.8 Finally, the lack of association of vitamin D levels with relapse-free survival may be explained by the absence of clinically meaningful association between vitamin D and SLE activity.
Given its relative safety, there is optimism that giving high doses of vitamin D will lead to better outcomes for patients with SLE. In the first interventional study published in SLE,50 no significant association was found between the variation of the 25(OH)D levels and the variation of the SLEDAI score. However, this study was not randomised and the supplementation modalities were at the discretion of the attending physician so that the level of 30 ng/mL, which is usually considered as sufficient, was obtained in less than 30% subjects. Abou-Raya et al53 conducted a randomised, double-blind, placebo-controlled trial of 2000 UI/day vitamin D3 supplementation for 12 months. In this trial, secondary outcome measure was the improvement in the SLEDAI score, and a significant improvement in disease activity was observed in the vitamin D group.53 In another prospective study, 763 patients with SLE with 25(OH) below 40 ng/mL among 1006 patients with SLE were supplemented with vitamin D2. A 20 ng/mL increase in 25(OH)D was associated with a significant decrease in mean SELENA-SLEDAI by 0.22, but the authors questioned the clinical relevance of this minor improvement.47
Our study was limited by a lack of details regarding the supplemental vitamin D intake and the single cross-sectional measurement of 25(OH)D levels. The study may be limited by the relatively low SLE activity in the patients included in the trial so that we cannot extrapolate our results in more active patients with SLE. The duration of follow-up can also be considered as a limitation. It was strengthened by its prospective design with follow-up every 2 months, the size of the population and the homogeneity of the patients in terms of treatment received.
In conclusion, vitamin D deficiency and insufficiency are highly prevalent in patients with SLE. There is a statistically significant association between vitamin D levels and high SLE activity, with dubious clinical consequences. Well-conducted trials are still needed to confirm or not that low vitamin D levels is a modifiable risk factor for morbidity in patients with SLE.
Acknowledgments
To the patients, to the Association France Lupus (AFL) for a grant for the PLUS study, to the Clinical Research Unit of Pitié-Salpêtrière Hospital that dealt with the methodological aspects, data management and monitoring of the PLUS study, to the sponsor of the PLUS study: «Assistance Publique—Hôpitaux de Paris», to the Société Nationale Française de Médecine Interne (SNFMI) for a research grant to YS, and to Sanofi, who provided the HCQ and placebo tablets for the PLUS study. The company had no role in the initiation, planning, conduct, data assembly, analysis or interpretation of the study.
Footnotes
Collaborators: Group of collaborators: Leonardo Astudillo, Cristina Belizna, Nadia Belmatoug, Olivier Benveniste, Audrey Benyamine, Holly Bezanahary, Patrick Blanco, Olivier Bletry, Bahram Bodaghi, Pierre Bourgeois, Benoît Brihaye, Emmanuel Chatelus, Richard Damade, Eric Daugas, Christian De-Gennes, Jean-François Delfraissy, Céline Delluc, Aurélien Delluc, Pierre Duhaut, Alain Dupuy, Isabelle Durieu, Hang-Korng EA, Dominique Farge, Christian Funck-Brentano, Frédérique Gandjbakhch, Justine Gellen-Dautremer, Bertrand Godeau, Cécile Goujard, Catherine Grandpeix, Claire Grange, Lamiae Grimaldi, Gaëlle Guettrot, Loïc Guillevin, Eric Hachulla, Jean-Robert Harle, Julien Haroche, Pierre Hausfater, Jean Jouquan, Gilles Kaplanski, Homa Keshtmand, Mehdi Khellaf, Olivier Lambotte, David Launay, Hervé Levesque, Olivier Lidove, Eric Liozon, Kim LY, Matthieu Mahevas, Kubéraka Mariampillai, Xavier Mariette, Alexis Mathian, Karin Mazodier, Marc Michel, Nathalie Morel, Luc Mouthon, Rokiya Ngack, Jacques Ninet, Eric Oksenhendler, Jean-Luc Pellegrin, Olivier Peyr, Anne-Marie Piette, Vincent Poindron, Jacques Pourrat, Fabienne Roux, David Saadoun, Sabrinel Sahali, Bernadette Saint-Marcoux, Françoise Sarrot-Reynauld, Jérémie Sellam, Damien Sene, Jacques Serratrice, Pascal Seve, Jean Sibilia, Claude Simon, Christelle Sordet, Benjamin Terrier, Salim Trad, Jean-François Viallard, Elisabeth Vidal, Bertrand Wechsler, Pierre-Jean Weiller, Noël Zahr.
Contributors: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The PLUS study was funded by a grant from the French PHRC 2005 Ministère de la santé; the Direction de la Recherche Clinique et du Développement provided logistic and administrative support.
Competing interest: None.
Data sharing statement: No additional data available.
Ethics approval: CPP Paris.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Astudillo Leonardo, Belizna Cristina, Belmatoug Nadia, Benveniste Olivier, Benyamine Audrey, Bezanahary Holly, Blanco Patrick, Bletry Olivier, Bodaghi Bahram, Bourgeois Pierre, Brihaye Benoît, Chatelus Emmanuel, Damade Richard, Daugas Eric, De-Gennes Christian, Delfraissy Jean-François, Delluc Céline, Delluc Aurélien, Duhaut Pierre, Dupuy Alain, Durieu Isabelle, EA Hang-Korng, Farge Dominique, Funck-Brentano Christian, Gandjbakhch Frédérique, Gellen-Dautremer Justine, Godeau Bertrand, Goujard Cécile, Grandpeix Catherine, Grange Claire, Grimaldi Lamiae, Guettrot Gaëlle, Guillevin Loïc, Hachulla Eric, Harle Jean-Robert, Haroche Julien, Hausfater Pierre, Jouquan Jean, Kaplanski Gilles, Keshtmand Homa, Khellaf Mehdi, Lambotte Olivier, Launay David, Levesque Hervé, Lidove Olivier, Liozon Eric, LY Kim, Mahevas Matthieu, Mariampillai Kubéraka, Mariette Xavier, Mathian Alexis, Mazodier Karin, Michel Marc, Morel Nathalie, Mouthon Luc, Ngack Rokiya, Ninet Jacques, Oksenhendler Eric, Pellegrin Jean-Luc, Peyr Olivier, Piette Anne-Marie, Poindron Vincent, Pourrat Jacques, Roux Fabienne, Saadoun David, Sahali Sabrinel, Saint-Marcoux Bernadette, Sarrot-Reynauld Françoise, Sellam Jérémie, Sene Damien, Serratrice Jacques, Seve Pascal, Sibilia Jean, Simon Claude, Sordet Christelle, Terrier Benjamin, Trad Salim, Viallard Jean-François, Vidal Elisabeth, Wechsler Bertrand, Weiller Pierre-Jean, and Zahr Noël
References
- 1.Schoindre Y, Terrier B, Kahn JE, et al. [Vitamin D and autoimmunity. First part: fundamental aspects]. Rev Med Interne 2012;33:80–6. [DOI] [PubMed] [Google Scholar]
- 2.Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 1992;12:143–8. [DOI] [PubMed] [Google Scholar]
- 3.Abe J, Nakamura K, Takita Y, et al. Prevention of immunological disorders in MRL/l mice by a new synthetic analogue of vitamin D3: 22-oxa-1 alpha, 25-dihydroxyvitamin D3. J Nutr Sci Vitaminol (Tokyo) 1990;36:21–31. [DOI] [PubMed] [Google Scholar]
- 4.Koizumi T, Nakao Y, Matsui T, et al. Effects of corticosteroid and 1,24R-dihydroxy-vitamin D3 administration on lymphoproliferation and autoimmune disease in MRL/MP-lpr/lpr mice. Int Arch Allergy Appl Immunol 1985;77:396–404. [DOI] [PubMed] [Google Scholar]
- 5.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J 2001;15:2579–85. [DOI] [PubMed] [Google Scholar]
- 6.Linker-Israeli M, Elstner E, Klinenberg JR, et al. Vitamin D(3) and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol 2001;99:82–93. [DOI] [PubMed] [Google Scholar]
- 7.Ritterhouse LL, Crowe SR, Niewold TB, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis 2011;70:1569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrier B, Derian N, Schoindre Y, et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther 2012;14:R221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Zvi I, Aranow C, Mackay M, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS ONE 2010;5:e9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozaki Y, Nomura S, Nagahama M, et al. Vitamin-D receptor genotype and renal disorder in Japanese patients with systemic lupus erythematosus. Nephron 2000;85:86–91. [DOI] [PubMed] [Google Scholar]
- 11.Huang CM, Wu MC, Wu JY, et al. Association of vitamin D receptor gene BsmI polymorphisms in Chinese patients with systemic lupus erythematosus. Lupus 2002;11:31–4. [DOI] [PubMed] [Google Scholar]
- 12.Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev 2006;5:114–17. [DOI] [PubMed] [Google Scholar]
- 13.Wu PW, Rhew EY, Dyer AR, et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum 2009;61:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller K, Kriegbaum NJ, Baslund B, et al. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D3 in patients with systemic lupus erythematosus. Clin Rheumatol 1995;14:397–400. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007;179:1634–47. [DOI] [PubMed] [Google Scholar]
- 16.Wright TB, Shults J, Leonard MB, et al. Hypovitaminosis D is associated with greater body mass index and disease activity in pediatric systemic lupus erythematosus. J Pediatr 2009; 155:260–5. [DOI] [PubMed] [Google Scholar]
- 17.Amital H, Szekanecz Z, Szucs G, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis 2010;69:1155–7. [DOI] [PubMed] [Google Scholar]
- 18.Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus 2008;17:6–10. [DOI] [PubMed] [Google Scholar]
- 19.Borba VZ, Vieira JG, Kasamatsu T, et al. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int 2009;20:427–33. [DOI] [PubMed] [Google Scholar]
- 20.Costedoat-Chalumeau N, Galicier L, Aumaitre O, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 2013;72:1786–92. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 22.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 23.Costedoat-Chalumeau N, Amoura Z, Hulot JS, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:3284–90. [DOI] [PubMed] [Google Scholar]
- 24.Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. [DOI] [PubMed] [Google Scholar]
- 25.Hollis BW, Kamerud JQ, Selvaag SR, et al. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 1993;39:529–33. [PubMed] [Google Scholar]
- 26.Ruiz-Irastorza G, Egurbide MV, Olivares N, et al. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford) 2008;47:920–3. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 28.Huisman AM, White KP, Algra A, et al. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J Rheumatol 2001;28:2535–9. [PubMed] [Google Scholar]
- 29.Carnevale V, Modoni S, Pileri M, et al. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: seasonal and gender differences. Osteoporos Int 2001;12:1026–30. [DOI] [PubMed] [Google Scholar]
- 30.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–20. [DOI] [PubMed] [Google Scholar]
- 31.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int 2007;71:134–9. [DOI] [PubMed] [Google Scholar]
- 32.Pazaitou-Panayiotou K, Papapetrou PD, Chrisoulidou A, et al. Height, whole body surface area, gender, working outdoors, and sunbathing in previous summer are important determinants of serum 25-hydroxyvitamin D levels. Exp Clin Endocrinol Diabetes 2012;120:14–22. [DOI] [PubMed] [Google Scholar]
- 33.Urena-Torres P, Metzger M, Haymann JP, et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis 2011;58:544–53. [DOI] [PubMed] [Google Scholar]
- 34.Agmon-Levin N, Blank M, Zandman-Goddard G, et al. Vitamin D: an instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann Rheum Dis 2011;70:145–50. [DOI] [PubMed] [Google Scholar]
- 35.Andreoli L, Piantoni S, Dall'Ara F, et al. Vitamin D and antiphospholipid syndrome. Lupus 2012;21:736–40. [DOI] [PubMed] [Google Scholar]
- 36.Paupitz JA, Freire de Carvalho J, Caparbo VF, et al. Primary antiphospholipid syndrome in premenopausal women: low vitamin D, high fat mass and maintained bone mineral mass. Lupus 2010;19:1302–6. [DOI] [PubMed] [Google Scholar]
- 37.Rulcova A, Prokopova I, Krausova L, et al. Stereoselective interactions of warfarin enantiomers with Pregnane X nuclear (PXR) receptor in gene regulation of major drug-metabolizing cytochrome P450 enzymes. J Thromb Haemost 2010;8:2708–17. [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Assem M, Tay JC, et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest 2006;116:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costedoat-Chalumeau N, Amoura Z, Hulot JS, et al. Hydroxychloroquine in systemic lupus erythematosus. Lancet 2007;369:1257–8. [DOI] [PubMed] [Google Scholar]
- 40.Costedoat-Chalumeau N, Leroux G, Piette J-C, et al. Antimalarials and systemic lupus erythematosus. In: Lahita RG, Tsokos G, Buyon JP, Koike T. eds. Systemic lupus erythematosus. 5th edn Elsevier, 2010:1061–81. [Google Scholar]
- 41.Lakshminarayanan S, Walsh S, Mohanraj M, et al. Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol 2001; 28:102–8. [PubMed] [Google Scholar]
- 42.Mok CC, Mak A, Ma KM. Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 2005;14:106–12. [DOI] [PubMed] [Google Scholar]
- 43.Barre PE, Gascon-Barre M, Meakins JL, et al. Hydroxychloroquine treatment of hypercalcemia in a patient with sarcoidosis undergoing hemodialysis. Am J Med 1987;82:1259–62. [DOI] [PubMed] [Google Scholar]
- 44.Adams JS, Diz MM, Sharma OP. Effective reduction in the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia with short-course chloroquine therapy. Ann Intern Med 1989;111:437–8. [DOI] [PubMed] [Google Scholar]
- 45.O'Leary TJ, Jones G, Yip A, et al. The effects of chloroquine on serum 1,25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N Engl J Med 1986;315:727–30. [DOI] [PubMed] [Google Scholar]
- 46.Mok CC, Birmingham DJ, Leung HW, et al. Vitamin D levels in Chinese patients with systemic lupus erythematosus: relationship with disease activity, vascular risk factors and atherosclerosis. Rheumatology (Oxford) 2012;51:644–52. [DOI] [PubMed] [Google Scholar]
- 47.Petri M, Bello KJ, Fang H, et al. Vitamin D in SLE: modest association with disease activity and urine protein/creatinine ratio. Arthritis Rheum 2013;65:1865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orbach H, Zandman-Goddard G, Amital H, et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci 2007;1109:385–400. [DOI] [PubMed] [Google Scholar]
- 49.Toloza SM, Cole DE, Gladman DD, et al. Vitamin D insufficiency in a large female SLE cohort. Lupus 2010;19:13–19. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Irastorza G, Gordo S, Olivares N, et al. Changes in vitamin D levels in patients with systemic lupus erythematosus: Effects on fatigue, disease activity, and damage. Arthritis Care Res (Hoboken) 2010;62:1160–5. [DOI] [PubMed] [Google Scholar]
- 51.Birmingham DJ, Hebert LA, Song H, et al. Evidence that abnormally large seasonal declines in vitamin D status may trigger SLE flare in non-African Americans. Lupus 2012;21:855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souberbielle JC, Body JJ, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev 2010;9:709–15. [DOI] [PubMed] [Google Scholar]
- 53.Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol 2013;40:265–72. [DOI] [PubMed] [Google Scholar]