Abstract
The P2X7 receptor (P2X7R) is an ATP-gated cation channel that is abundantly expressed in monocytes/macrophages. P2X7R activation by ATP results in various cellular responses including Ca2+ influx, membrane pore formation, and cytokine secretion. Since P2X7R has low affinity for ATP, high concentrations of ATP (in the mM range) are generally required to activate this receptor in vitro. Functional expression of P2X7R has been detected in monocytes/macrophages obtained from different animal species including humans, rodents, dogs, and bovines, but so far it has not been detected in swine (Sus scrofa). In this study, we investigated the expression and functions of P2X7R in swine macrophages, which were isolated from mixed primary cultures of swine kidney or liver tissue. The P2X7R mRNA and protein expression observed in the swine macrophages was comparable to that seen in a c-myc-immortalized mouse kidney-derived clonal macrophage cell line (KM-1). However, extracellular ATP did not induce P2X7R-dependent sustained Ca2+ influx, membrane pore formation, or the secretion of the bioactive cytokine interleukin-1β in the swine macrophages, whereas these responses were clearly observed in the mouse KM-1 cells after stimulation with millimolar concentrations of ATP as a positive control. These findings suggest that the ATP/P2X7R pathway is impaired in swine macrophages at least in the culture conditions used in the present study.
Keywords: Swine macrophages, P2X7 receptor, Ca2+ influx, Membrane pore formation, Interleukin-1β
1. Introduction
Extracellular ATP exerts a variety of biological actions by activating purinergic P2 receptors. Two types of P2 receptors, ligand-gated P2X ion channels and G protein-coupled P2Y receptors, have been identified [1,2]. The P2X7 receptor (P2X7R) is a member of the P2X subfamily, and its activation by ATP opens cation channels that are permeable to several cations such as K+, Na+, and Ca2+. Although ATP is considered to be a selective endogenous ligand of P2X7R, P2X7R has low affinity for ATP, and thus, high concentrations of ATP (in the mM range) are necessary to induce P2X7R-dependent cellular responses in vitro [3].
Since P2X7R is abundantly expressed in monocyte/macrophage-lineage cells, its functions are implicated in the regulation of the innate immune system [4]. In particular, P2X7R plays a key role in the activation of the inflammasome and the subsequent production and release of the bioactive form of interleukin-1β (IL-1β), a potent inflammatory cytokine [5]. Functional expression of P2X7R has been detected in monocytes/macrophages obtained from various animal species including humans [6], rodents [3], dogs [7], and bovines [8], but so far it has not been detected in swine (Sus scrofa).
A previous study showed the functional expression of P2X7R in swine ovarian theca cells [9]. In this study, to understand the role of P2X7R in the innate immune defense of swine, we aim to study the expression and function of P2X7R in swine macrophages, which were obtained from mixed primary cultures of swine kidney or liver tissues [10]. Despite the presence of P2X7R mRNA and protein, extracellular ATP failed to induce P2X7R-dependent cellular responses in the swine macrophages. This suggests that ATP does not sufficiently activate P2X7R in swine macrophages cultured in vitro.
2. Materials and methods
2.1. Materials
ATP, BzATP, lipopolysaccharide (LPS), LPC (1-palmitoyl-sn-glycero-3-phosphocholine), and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO). A438079 was purchased from Tocris (Bristol, UK). Biotinylated anti-mouse IL-1β (BAF401) and anti-swine IL-1β (BAF681) antibodies were purchased from R&D Systems (Minneapolis, MN). Horseradish peroxidase (HRP)–streptavidin conjugate was purchased from Zymed (South San Francisco, CA). Anti-P2X7R rabbit polyclonal (Epitope: KIRKEFPKTQGQYSGFKYPY from the C-terminus of rat P2X7R, mouse-18/20 and swine-15/20 residues identical) and goat polyclonal (Epitope: YETNKVTRIQSMNY from the N-terminus of human P2X7R, mouse-13/14 and swine-14/14 residues identical) antibodies were purchased from Alomone Labs (Jerusalem, Israel) and Covalab (Villeurbanne, France), respectively. Anti-actin mouse monoclonal antibody was purchased from Chemicon International (Temecula, CA). HRP-conjugated rabbit anti-goat, goat anti-rabbit, and goat anti-mouse immunoglobulins antibodies were purchased from ICN Pharmaceutical, Inc. (Aurora, OH).
2.2. Swine kidney tissue dissociation and primary culture
Swine neonates (1–14-days-old crossbred pigs) were obtained from the animal facility at the National Institute of Livestock and Grassland Science, according to the institutional guidelines for animal experiments. After anesthesia had been induced and the animals had been euthanized, their kidneys were dissected out. After the removal of the fibrous renal capsule, the renal cortex was cut into small pieces, and the tissue pieces (3–5 g) were then forced through a nylon mesh (pore size: 500 µm) in phosphate-buffered saline (PBS) using a scraper. The minced tissue was digested by incubation with collagenase–dispase (Roche Diagnostics, Basel, Switzerland)/PBS solution (1 mg/ml) containing DNase I (Roche) (40 µg/ml) for 1 h at 37 °C. Then, the digested tissue fragments were collected and re-suspended in growth medium composed of Dulbecco’s modified Eagle’s medium (Sigma) containing 10% heat-inactivated fetal bovine serum (Sanko Junyaku Co., Ltd., Tokyo, Japan), supplemented with 100 µM β-mercaptoethanol (Sigma), 10 µg/ml insulin (Sigma), 100 µg/ml streptomycin (Life Technologies, Carlsbad, CA), 100 U/ml penicillin (Life Technologies), and 5 µg/ml Fungin (InvivoGen, San Diego, CA). The cell suspension was split into 10 T-75 tissue culture flasks (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) and cultured at 37 °C in a humidified atmosphere of 95% air/5% CO2. The culture medium was replaced every 3–4 days. After the cells had been cultured for 2–3 weeks, the cultured cells were harvested by treatment with Accumax™ (Innovative Cell Technologies, Inc., San Diego, CA), suspended in Cell Banker 1 (Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan), and kept frozen in liquid nitrogen for 1–5 months (the cells from each T-75 flask were stored in the same cryotube).
2.3. Isolation of macrophages from swine kidney mixed culture
The frozen swine kidney cells stored in each cryotube were rapidly thawed at 37 °C and split into three or five T-75 flasks. After being cultured for 7–10 days, a mixed monolayer cell sheet formed, and macrophage-like cells began to actively proliferate on the cell sheet. The proliferating macrophage-like cells were loosely attached to the cell sheet, and large numbers of macrophage-like cells (1×105–1.5×106 cells/T-75 flask) were harvested from the culture supernatant by centrifugation (1500 rpm for 5 min) every 3–4 days for 1–2 months.
2.4. Immunocytochemistry
Immunocytochemical analyses were performed as described previously [10,11]. Primary antibodies against cytokeratin 18 (CK18; Millipore Co., Billerica, MA), cytokeratin 19 (CK19; Progen, Heidelberg, Germany), α-smooth muscle actin (SMA; Progen), macrophage scavenger receptor MSR-A:CD204 (KT022; TransGenic, Inc., Kumamoto, Japan), Iba 1 (Wako Pure Chemical Industries, Ltd, Osaka, Japan), and CD172a (VMRD, Inc., Pullman, WA) were used.
2.5. Isolation of macrophages from swine liver mixed culture
Macrophages were also isolated from a mixed primary culture of swine liver tissue, as described previously [10].
2.6. Isolation and immortalization of mouse kidney-derived macrophages
Mouse kidney macrophages were isolated from a mixed primary culture of C57BL/6 mice kidney cells according to the protocol used to isolate macrophages from the swine kidney tissue, before being immortalized by retroviral transduction of human c-myc, as described in our previous studies [12]. The clonal macrophage cell line (KM-1) was established and routinely cultured with growth medium.
2.7. Reverse transcription-polymerase chain reaction (RT-PCR) analysis
RT-PCR analyzes were performed as described previously [13], with minor modifications. The following oligonucleotide primers were used: mouse P2X7R (NM011027): sense, 5′-GACAAACAAAGTCACCCGGAT-3′, and antisense, 5′-CGCTCACCAAAGCAAAGCTAAT-3′; swine P2X7R (XM001926804): sense, 5′-GACAAACAAAGTCACCCGGAT-3′, and antisense, 5′-CTTGTCACTCACCAAAGCAAAG-3′; swine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (NM001206359): sense, 5′-TCACCAGGGCTGCTTTTAAC-3′, and antisense, 5′-GATCTCGCTCCTGGAAGAT-3′. The amplified DNA fragments derived from mouse P2X7R, swine P2X7R, and swine GAPDH mRNA were 101, 106, and 192 bp (bp) long, respectively. A mouse β-actin primer (#G5740, Promega, Madison, WI) was also used, and the resultant 285 bp DNA fragment was amplified.
2.8. Measurement of the intracellular Ca2+concentration ([Ca2+]i)
The cells were re-suspended in HEPES-buffered salt solution (HBSS; 145 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 20 mM HEPES, 10 mM glucose, and 0.01% BSA; pH 7.4), and the [Ca2+]i was measured at 30 °C by monitoring fura-2 fluorescence at 500 nm [excitation wavelengths of 340 (F340) and 380 nm (F380)] as described previously [12,13]. The ratio of the fluorescence intensities observed at the two above mentioned wavelengths (F340/F380) was used as an indicator of the [Ca2+]i. One milliliter of cell suspension containing 5×105 cells was used in each set of experiments.
2.9. Immunoblotting
The cells (3×105/well in 24-well plate) were primed with or without 1 µg/ml LPS for 4 h, and then the medium was replaced with 250 µl HBSS or Ca2+/Mg2+-free HBSS (Ca2+/Mg2+-free buffer) containing the indicated test reagents. After incubation at 37 °C for 30 min, the supernatants were collected, and the cells were lysed with 200 µl ice-cold lysis buffer, before IL-1β, P2X7R or actin was detected using immunoblotting, as described previously [14]. The target protein was revealed using ImmunoStar LD (Wako) and detected using a c-Digit Blot Scanner (LI-COR, Inc., Lincoln, NE).
2.10. Dye uptake assays
YO-PRO-1 or propidium iodide (PI) dye uptake was monitored in live cells cultured in HBSS at 37 °C using a fluorescence microscope and time-lapse recording, as described previously [14].
3. Results and discussion
3.1. Characterization of swine kidney-derived macrophages
Immunostaining demonstrated that almost all of the cells isolated from the mixed primary culture of swine kidney tissue were positive for macrophage markers (KT022, Iba-1, and CD172a), but negative for epithelial (CK18 and CK19) and mesenchymal (SMA) cell markers (Fig. 1A). The proportions of contaminating epithelial and mesenchymal cells comprised less than 1% by cell counting after immunostaining, suggesting that the purity of the macrophages was more than 99%. Semi-quantitative RT-PCR analyses were also performed to investigate the expression of P2X7R. In the KM-1 cells, an amplified 101-bp DNA fragment derived from mouse P2X7R mRNA was detected after 35 and 40 PCR cycles, but not after 30 PCR cycles (Fig. 1B). Similarly, an amplified 106-bp DNA fragment derived from swine P2X7R mRNA was detected in the swine kidney macrophages after 35 and 40 PCR cycles (Fig. 1B). These findings suggest that the expression level of P2X7R mRNA in swine kidney macrophages is comparable to that seen in KM-1 cells.
Fig. 1.
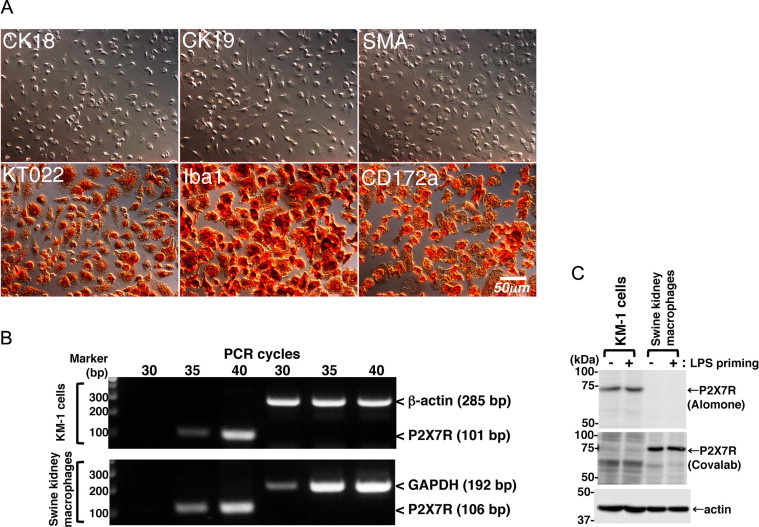
Characterization of swine kidney macrophages. Macrophage-like cells that had been isolated from a mixed primary culture of swine kidney tissue were found to be positive for macrophage markers (KT022, Iba1, and CD172a), but negative for epithelial (CK18 and CK19) and mesenchymal (SMA) markers (A). P2X7R mRNA expression was detected in the mouse KM-1 cells and swine kidney macrophages by RT-PCR (B). P2X7R protein expression was detected in KM-1 cells and swine kidney macrophages by immunoblotting using anti-P2X7R rabbit polyclonal (Alomone) and goat polyclonal (Covalab) antibodies, respectively (C). Equivalent protein loading in each lane was confirmed by immunoblotting with anti-actin antibody (C). All data shown are representative of two or three independent experiments.
Furthermore, the expression of P2X7R protein in KM-1 cells or swine kidney macrophages was confirmed by immunoblotting using two different anti-P2X7R antibodies (Fig. 1C). Anti-P2X7R Alomone antibody is known to react with mouse P2X7R despite a 90% homology with its epitope peptide [15]. However, this antibody failed to recognize swine P2X7R, possibly due to the lower (75%) sequence homology with the epitope peptide. Conversely, anti-P2X7R Covalab antibody recognized swine P2X7R (100% identical with its epitope peptide), while did not recognize mouse P2X7R (93% identical). Although the reason why Covalab antibody did not react with mouse P2X7R in our experimental conditions is unclear, this may be due to the use of relative lower antibody concentration (0.5 µg/ml) for immunoblotting. Further experiments are required to optimize the reaction conditions for the detection of mouse P2X7R by immunoblotting with Covalab antibody. Although several pro-inflammatory stimuli are reported to modulate P2X7R expression [16], LPS-priming did not affect the protein expression level of P2X7R in KM-1 cells and swine kidney macrophages (Fig. 1C).
3.2. ATP did not induce a sustained increase in the [Ca2+]i of swine kidney macrophages
Previous studies have demonstrated that millimolar concentrations of extracellular ATP induce sustained Ca2+ influx via the activation of P2X7R, while micromolar concentrations of ATP induce transient increases in the [Ca2+]i by activating other types of P2 receptor [12]. Indeed, in the mouse KM-1 cells ATP induced a sustained increase in the [Ca2+]i at a concentration of 4 mM, while it only induced a transient [Ca2+]i increase at a concentration of 0.5 mM (Fig. 2A). BzATP, a potent P2X7R agonist, also induced a sustained increase in the [Ca2+]i of the KM-1 cells (Fig. 2A). Pretreatment with A438079, a selective antagonist of P2X7R, clearly blocked the sustained [Ca2+]i increases induced by ATP and BzATP (Fig. 2A), suggesting that P2X7R activation is involved in the [Ca2+]i increases induced by ATP.
Fig. 2.
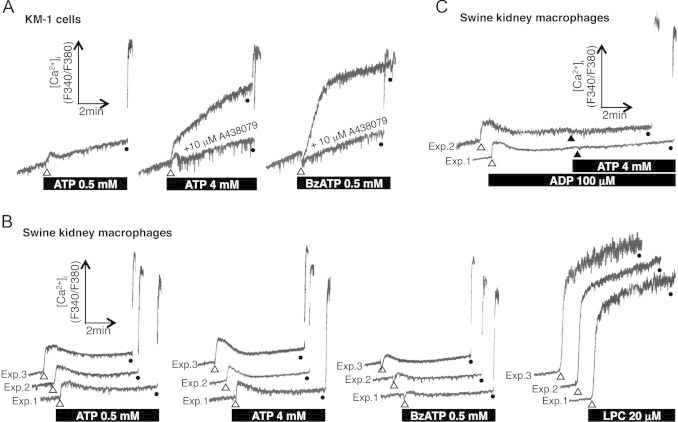
P2X7R-mediated sustained Ca2+ influx was not observed in the swine kidney macrophages. P2X7R agonists (ATP and BzATP) triggered sustained increases in the [Ca2+]i of the mouse KM-1 cells (A), but not in those of the swine kidney macrophages (B). Pretreatment with A438079 blocked the sustained [Ca2+]i increases seen in the KM-1 cells (A). LPC triggered a sustained increase in the [Ca2+]i of the swine kidney macrophages (B). Pretreatment with the P2Y receptor agonist ADP desensitized the Ca2+ response of the swine kidney macrophages to 4 mM ATP (C). Traces obtained from two or three independent experiments are shown (B, C). The cells were permeabilized by treatment with 0.2% Triton X-100, and the maximum fura-2 fluorescence was measured (closed circles).
In contrast, no sustained [Ca2+]i increase was observed in the swine kidney macrophages stimulated with 4 mM ATP or 0.5 mM BzATP (Fig. 2B), suggesting that these treatments did not sufficiently activate P2X7R in the swine kidney macrophages. An LPC-induced sustained [Ca2+]i increase was detected in the swine kidney macrophages (Fig. 2B), which occurred in a P2X7R-independent manner [13]. In addition, ADP, a selective agonist of P2Y receptors, induced a transient [Ca2+]i increase, and pretreatment with ADP desensitized the Ca2+ response of the swine kidney macrophages to 4 mM ATP (Fig. 2C). This suggests that the [Ca2+]i changes induced by ATP are mediated by P2Y receptors, but not by P2X7R, in swine kidney macrophages.
3.3. ATP did not induce IL-1β maturation and release, or membrane pore formation in swine kidney macrophages
We next examined whether extracellular ATP triggers the production and release of mature IL-1β (mIL-1β) in LPS-primed swine kidney macrophages. In response to LPS pretreatment, the 33-kDa precursor form of IL-1β (pro-IL-1β) was synthesized and accumulated in the cytosol of both the mouse KM-1 cells and swine kidney macrophages (Fig. 3A, lysates, second and fourth panels). Additional stimulation with ATP triggered the production and release of the 17-kDa form of mIL-1β in the LPS-primed KM-1 cells (Fig. 3A, sup, first panel). The ATP-induced release of mIL-1β from these cells was enhanced when they were incubated in Ca2+/Mg2+-free buffer (Fig. 3A, sup, first panel) because these divalent cations steadily suppress the functions of P2X7R by directly modulating it [17]. Conversely, the ATP-induced release of mIL-1β from the KM-1 cells was inhibited by co-treatment with A438079 (Fig. 3B, sup). In contrast, no production or release of mIL-1β was detected in the LPS-primed swine kidney macrophages treated with ATP, even those incubated in Ca2+/Mg2+-free buffer (Fig. 3A, sup, third panel).
Fig. 3.
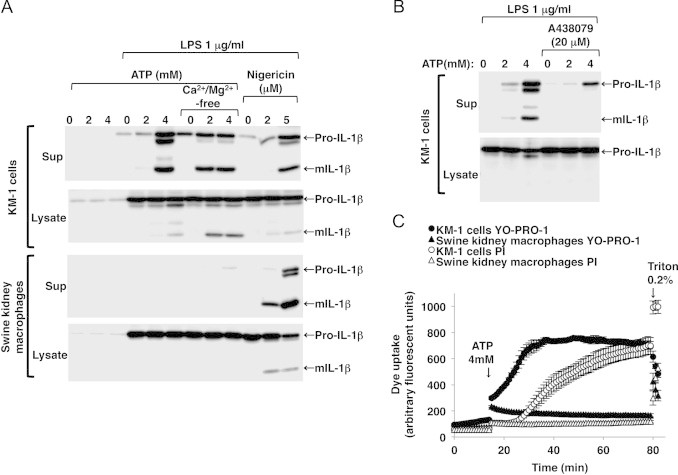
ATP-induced P2X7R-mediated maturation and release of IL-1β and membrane pore formation were not observed in the LPS-primed swine kidney macrophages. mIL-1β release was detected in the LPS-primed mouse KM-1 cells after ATP stimulation (A, sup, first panel). The ATP-induced mIL-1β release was enhanced when the cells were incubated in Ca2+/Mg2+-free buffer (A, sup, first panel), whereas it was inhibited by co-treatment with A438079 (B, sup). ATP-induced mIL-1β release was not detected in the LPS-primed swine kidney macrophages even when they were incubated in Ca2+/Mg2+-free buffer, whereas nigericin, a K+/H+ ionophore, triggered mIL-1β release (A, sup, third panel). Immunoblots are representative of at least three independent experiments. YO-PRO-1 uptake, which preceded PI uptake, was detected in the LPS-primed KM-1 cells after stimulation with 4 mM ATP (C, closed and open circles). Negligible ATP-induced YO-PRO-1 and PI uptake were observed in the LPS-primed swine kidney macrophages (C, closed and open triangles). Maximum dye uptake was estimated after permeabilizing the cells with 0.2% Triton-X100 (C). Fluorescence is expressed in arbitrary units, and the data are shown as mean±SEM values (n=3).
The prolonged activation of P2X7R by ATP causes membrane pore dilation followed by cytolysis in monocytes/macrophages. Thus, we examined whether ATP triggers membrane pore formation in swine kidney macrophages. To this end, P2X7R-mediated pore formation and the resultant cytolysis were assessed using YO-PRO-1 and PI uptake, respectively. In LPS-primed mouse KM-1 cells, stimulation with 4 mM ATP elicited maximum YO-PRO-1 uptake within 20 min, which preceded PI uptake (Fig. 3C). However, no ATP-induced YO-PRO-1 or PI uptake was observed in the LPS-primed swine kidney macrophages (Fig. 3C). The data also suggest that cationic dyes (YO-PRO-1 and PI) do not pass through plasma membranes even after ATP stimulation. Given the presence of separate P2X7R-mediated permeation pathways for cationic and anionic dyes [18], it still remains unclear whether the anionic dyes are taken up on ATP stimulation in LPS-primed swine kidney macrophages.
3.4. ATP did not induce P2X7R-mediated cellular responses in swine liver-derived macrophages
We further tested the effect of extracellular ATP in swine liver-derived macrophages. ATP-induced P2X7R-mediated sustained Ca2+ influx (Fig. 4A), and the maturation and release of IL-1β (B) were also not observed in swine liver macrophages despite the presence of P2X7R mRNA (C) and protein (D).
Fig. 4.
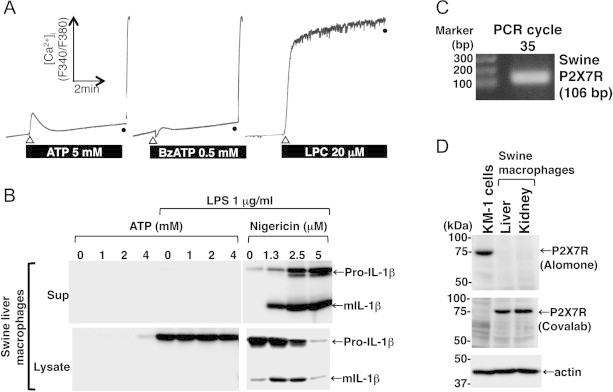
ATP-induced P2X7R-mediated sustained Ca2+ influx, and IL-1β maturation and release were not observed in the swine liver-derived macrophages despite the fact that they expressed P2X7R. P2X7R agonists (ATP and BzATP) failed to trigger sustained increases in the [Ca2+]i of the swine liver macrophages, whereas LPC triggered a sustained [Ca2+]i increase (A). ATP-induced mIL-1β release was not detected in the LPS-primed swine liver macrophages, whereas nigericin triggered mIL-1β release (B). P2X7R mRNA and protein expression was detected in the swine liver macrophages by RT-PCR (C) and by immunoblotting (D), respectively. Equivalent protein loading in each lane was confirmed by immunoblotting with anti-actin antibody (D). All data shown are representative of two or three independent experiments.
3.5. Conclusion
ATP-induced P2X7R-mediated cellular events, such as sustained Ca2+ influx, the maturation and release of IL-1β, and membrane pore formation, were not observed in swine kidney and liver-derived macrophages stimulated with ATP despite the fact that they express P2X7R mRNA and protein. The data raise the possibility that P2X7R is non-functional in these swine macrophages at least under the culture conditions used in the present study, although amino acid sequence of swine P2X7R is 88% and 79% similar to the sequences of human and mouse P2X7R, respectively.
The precise mechanisms explaining the non-responsiveness of P2X7R to ATP in swine macrophages remain to be elucidated. Since the functional expression of P2X7R has been reported in swine ovary theca cells [9], this may be due to differences in tissue and/or cell types. It is also likely that the presence of alternative splice variants of P2X7R may be related to the non-functional expression of P2X7R in swine macrophages. In fact, several P2X7R splice variants have been defined in human and rodents [19,20]. Although we have confirmed the expression of full length of swine P2X7R by immunoblotting (around 75-kDa band in Figs. 1C and 4D) and DNA sequence analysis of 1785 bp amplified product obtained by RT-PCR (data not shown), future experiments will be required to determine whether other P2X7R splice variants are expressed in swine macrophages. Given the importance of P2X7R in innate immune defense in various animal species, our findings might provide new insights into the regulation of the innate immune system in swine.
Acknowledgements
The authors would like to thank the staff of the Swine Management Section of the National Institute of Livestock and Grassland Science for taking care of the pigs. This study was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomic-based Technology for Agricultural Improvement, AGB-1004) and a Grant-in-Aid for Scientific Research (Category C: Grant# 25450521) from the Japan Society for the Promotion of Science (JSPS).
Contributor Information
Takato Takenouchi, Email: ttakenou@affrc.go.jp.
Hiroshi Kitani, Email: kitani@affrc.go.jp.
References
- 1.Jarvis M.F., Khakh B.S. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. 18657557 [DOI] [PubMed] [Google Scholar]
- 2.Abbracchio M.P., Burnstock G., Boeynaems J.M., Barnard E.A., Boyer J.L., Kennedy C. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacological Reviews. 2006;58:281–341. doi: 10.1124/pr.58.3.3. 16968944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surprenant A., Rassendren F., Kawashima E., North R.A., Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. 8614837 [DOI] [PubMed] [Google Scholar]
- 4.Wiley J.S., Sluyter R., Gu B.J., Stokes L., Fuller S.J. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–332. doi: 10.1111/j.1399-0039.2011.01780.x. 21988719 [DOI] [PubMed] [Google Scholar]
- 5.Ferrari D., Pizzirani C., Adinolfi E., Lemoli R.M., Curti A., Idzko M., Panther E., Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. Journal of Immunology. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. 16547218 [DOI] [PubMed] [Google Scholar]
- 6.Rassendren F., Buell G.N., Virginio C., Collo G., North R.A., Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. Journal of Biological Chemistry. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. 9038151 [DOI] [PubMed] [Google Scholar]
- 7.Jalilian I., Peranec M., Curtis B.L., Seavers A., Spildrejorde M., Sluyter V., Sluyter R. Activation of the damage-associated molecular pattern receptor P2X7 induces interleukin-1β release from canine monocytes. Veterinary Immunology and Immunopathology. 2012;149:86–91. doi: 10.1016/j.vetimm.2012.05.004. 22652409 [DOI] [PubMed] [Google Scholar]
- 8.Smith R.A., Alvarez A.J., Estes D.M. The P2X7 purinergic receptor on bovine macrophages mediates mycobacterial death. Veterinary Immunology and Immunopathology. 2001;78:249–262. doi: 10.1016/s0165-2427(01)00245-8. 11292527 [DOI] [PubMed] [Google Scholar]
- 9.Vázquez-Cuevas F.G., Juárez B., Garay E., Arellano R.O. ATP-induced apoptotic cell death in porcine ovarian theca cells through P2X7 receptor activation. Molecular Reproduction and Development. 2006;73:745–755. doi: 10.1002/mrd.20447. 16541451 [DOI] [PubMed] [Google Scholar]
- 10.Kitani H., Yoshioka M., Takenouchi T., Sato M., Yamanaka N. Characterization of the liver-macrophages isolated from a mixed primary culture of neonatal swine hepatocytes. Results in Immunology. 2014;4:1–7. doi: 10.1016/j.rinim.2014.01.001. 24707456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takenouchi T., Iwamaru Y., Imamura M., Fukuhara S., Sugama S., Sato M. Cytochalasin D enhances the accumulation of a protease-resistant form of prion protein in ScN2a cells: involvement of PI3 kinase/Akt signalling pathway. Cell Biology International. 2012;36:1223–1231. doi: 10.1042/CBI20120329. 22985412 [DOI] [PubMed] [Google Scholar]
- 12.Takenouchi T., Ogihara K., Sato M., Kitani H. Inhibitory effects of U73122 and U73343 on Ca2+ influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochimica et Biophysica Acta. 2005;1726:177–186. doi: 10.1016/j.bbagen.2005.08.001. 16122875 [DOI] [PubMed] [Google Scholar]
- 13.Takenouchi T., Sato M., Kitani H. Lysophosphatidylcholine potentiates Ca2+ influx, pore formation and p44/42 MAP kinase phosphorylation mediated by P2X7 receptor activation in mouse microglial cells. Journal of Neurochemistry. 2007;102:1518–1532. doi: 10.1111/j.1471-4159.2007.04570.x. 17437542 [DOI] [PubMed] [Google Scholar]
- 14.Takenouchi T., Iwamaru Y., Sugama S., Tsukimoto M., Fujita M., Sekigawa A. The activation of P2X7 receptor induces cathepsin D-dependent production of a 20-kDa form of IL-1β under acidic extracellular pH in LPS-primed microglial cells. Journal of Neurochemistry. 2011;117:712–723. doi: 10.1111/j.1471-4159.2011.07240.x. 21395581 [DOI] [PubMed] [Google Scholar]
- 15.Sim J.A., Young M.T., Sung H.Y., North R.A., Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. 15254086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys B.D., Dubyak G.R. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. Journal of Leukocyte Biology. 1998;64:265–273. doi: 10.1002/jlb.64.2.265. 9715267 [DOI] [PubMed] [Google Scholar]
- 17.Virginio C., Church D., North R.A., Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. 9364483 [DOI] [PubMed] [Google Scholar]
- 18.Schachter J., Motta A.P., de Souza Zamorano A., da Silva-Souza H.A., Guimarães M.Z., Persechini P.M. ATP-induced P2X7-associated uptake of large molecules involves distinct mechanisms for cations and anions in macrophages. Journal of Cell Science. 2008;121:3261–3270. doi: 10.1242/jcs.029991. 18782864 [DOI] [PubMed] [Google Scholar]
- 19.Nicke A., Kuan Y.H., Masin M., Rettinger J., Marquez-Klaka B., Bender O. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. Journal of Biological Chemistry. 2009;284:25813–25822. doi: 10.1074/jbc.M109.033134. 19546214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheewatrakoolpong B., Gilchrest H., Anthes J.C., Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochemical and Biophysical Research Communications. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. 15896293 [DOI] [PubMed] [Google Scholar]


