Abstract
Background
Panax ginseng has distinct and impressive health benefits, such as improved blood pressure and immune system functioning. Rg3-enriched Korean Red Ginseng (REKRG) isolated from Korean Red Ginseng contains a high percentage of Rg3.
Methods
In this study, we examined the effects of REKRG on endothelial cell nitric oxide synthase (eNOS) activation and adhesion molecules in endothelial cells and vascular function in rats.
Results
REKRG dose-dependently increased eNOS phosphorylation and nitric oxide (NO) production in endothelial cells. In addition, REKRG markedly inhibited the tumor necrosis factor-α (TNF-α)-mediated induction of intercellular adhesion molecule (ICAM)-1 and cyclooxygenase (COX)-2 expressions in endothelial cells. REKRG improved endothelium-dependent vasorelaxation in the Wistar-Kyoto (WKY) rat and spontaneously hypertensive rats (SHRs) compared with controls. Furthermore, REKRG treatment for 6 weeks increased serum NO levels and reduced the mean aortic intima-media thickness compared with controls.
Conclusion
Taken together, these results suggest that REKRG increased vascular function and improved immune system functioning. Therefore, REKRG is a very useful food for preventing or improving various cardiovascular diseases.
Keywords: eNOS, NO, Panax ginseng, REKRG, SHR
1. Introduction
Hypertension is one of the major risk factors for the development of cardiovascular disease and modulation of the immune system [1,2] and is characterized by impaired vascular endothelial function [2–4]. Vascular endothelial cells are located in the intima, which is the inner lining of the vasculature, and they play an important role in the regulation of vascular tone by various vasoactive factors, such as nitric oxide (NO) [5]. Disruption of endothelial cell function is characterized by impaired bioavailability of NO [2,6] and induces vascular disease, which in turn contributes to smooth muscle cell proliferation [7] and stimulation of inflammatory molecules, such as intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and cyclooxygenase (COX)-2. NO is a major endothelium-dependent relaxing factor. It is produced from l-arginine by the activity of endothelial cell nitric oxide synthase (eNOS) [8] and induces vascular smooth muscle relaxation by activation of guanylate cyclase [9]. Some studies have shown that blood pressure was enhanced in eNOS knockout mice [10,11] as well as in rats in which eNOS was inhibited with Nω-nitro-l-arginine methyl ester (L-NAME) [12]. It was also reported that the bioavailability of NO was reduced in patients with established hypertension compared with the control group [2,6].
For thousands of years, Panax ginseng has been used as a traditional tonic medicine. The protective effects of P. ginseng related to cardiovascular functions are reportedly associated with vasorelaxation and stimulation of NO produced by eNOS [13,14]. Ginsenosides consist of two major groups according to the chemical structure of the fraction. The first is the panaxadiol group, which includes Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, and Rs1. The second is the panaxatriol group, which includes Re, Rf, Rg1, Rg2, and Rh1. Individual ginsenosides exert different effects via different mechanisms in various tissues. The combination of ginsenosides in ginseng extracts may be important for providing more powerful therapeutic and pharmacological effects [15–17]. Notably, ginsenoside Rg3 provides various protective effects, including anti-inflammatory [18] and antitumor effects [19], and it also enhances NO production and eNOS activity [20].
The aim of this study was to investigate whether Rg3-enriched Korean Red Ginseng (REKRG), a ginsenoside fraction enriched in Rg3, increases eNOS activity and NO production and exhibits anti-inflammatory effects.
2. Materials and methods
2.1. Preparation of ginsenoside Rg3-enriched Korean Red Ginseng
Dried Korean Red Ginseng (P. ginseng) root was purchased from Gumsan Nonghyup (Gumsan, Korea). Korean ginseng was extracted two times with 10 volumes of ethanol at 50°C for 7 hours (1st 50%, 2nd 85%), and then concentrated under vacuum at 50°C. The crude extract was dissolved in water and enzyme-acid hydrolysis to maximize ginsenoside Rg3 was performed (raw ginsenoside was hydrolyzed to Rg3) in acidic (pH 2.5∼3.5) and thermophilic (65∼80°C) condition. The enzyme, which has β-glycosidase activity including cellulase, hemicellulose, and glucosidase activity, was produced by Aspergillus niger. To remove acid solution and concentrate Rg3, the reactant was passed through DIAION HP20 resin (Mitsubishi Chemical Industries, Tokyo, Japan) packed column. The ginsenoside Rg3 was concentrated to powder under vacuum conditions. It was kindly provided by BTGin Corporation (Occheon, Korea).
2.2. High-performance liquid chromatography analysis
The powder was dissolved in 70% methanol, and ginsenosides including Rg3 was analyzed by high-performance liquid chromatography (HPLC). HPLC was carried out on an Liquid chromatography (LC) system equipped with a quaternary gradient pump (Spectra 4000) and UV detector (Spectra 2000; Thermo Scientific, San Jose, CA, USA). A reversed-phase column (Hypersil gold C18, 100 mm 4.6 mm, internal diameter 5 μm; Thermo Scientific) was used for quantitative determination of ginsenosides Rg3. The mobile phase consisted of acetonitrile and water with a flow rate at 1.6–2.5 mL/min, and the column was kept at room temperature. The detection wavelength was set at 203 nm.
2.3. Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA, USA) and cultured in Endothelial Growth Medium-2 from Lonza (Walkersville, MD, USA). Subconfluent, proliferating HUVECs were used between passages 2 and 8.
2.4. Animals and experimental protocols
The Animal Care Committee of Chungnam National University approved the animal care and all experimental procedures conducted in this study. All instrumentation was used under aseptic conditions. Male Wistar rats and spontaneously hypertensive rats (SHRs; 3 months old) were each divided into two groups (n = 5) randomly: a normal saline group and a REKRG group. REKRG (10 mg/kg) was orally administered to animals for 6 weeks.
2.5. Antibodies and Western blotting
Anti-ICAM-1, anti-eNOS, and anti-COX-2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antiphospho-eNOS, antiphospho-Akt, and anti-Akt antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Western blot analysis of whole cell lysates (30 μg) was performed using the appropriate primary and secondary antibodies. Blots were imaged using a chemiluminescence assay kit from Pharmacia-Amersham (Freiburg, Germany), and band densities were quantified using a Gel Doc 2000 ChemiDoc system and Quantity One software from Bio-Rad (Hercules, CA, USA). Values were normalized to a β-actin loading control.
2.6. Real-time polymerase chain reaction
Total RNA was isolated from cells using the acid guanidinium thiocyanate–phenol–chloroform method. Real-time polymerase chain reaction (PCR) was performed using the Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with the Super Script III Platinum SYBR Green One-Step qRT-PCR Kit (Invitrogen, Carlsbad, CA, USA). Primers used to amplify human ICAM-1 were as follows: 5′-CAGTGACCATCTACAGCTTTCCG-3′ and 5′-GCTGCTACCACAGTGATGATGACAA-3′. Primers used for human COX-2 were as follows: 5′-GGTCTGGTGCCTGGTCTGATGATG-3′ and 5′-GTCCTTTCAAGGAGAATGGTGC-3′. Primers used for human glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which was used as an internal control, were as follows: 5′-ATGACATCAAGAAGGTGGTG-3′ and 5′-CATACCAGG AAAATGAGCTTG-3′. Dissociation curves were monitored to check for aberrant formation of primer-dimers.
2.7. Nitrite and nitrate measurements
The NO metabolites nitrite (NO2) and nitrate (NO3), the stable breakdown products of NO, were quantified using a commercially available kit (Nitrate/Nitrite Fluorometric Assay Kit, Cayman Chemicals, Lexington, KY, USA), as per the manufacturer's instructions. Medium and blood plasma were deproteinized using a 10-kDa cutoff filter (Microcon YM10, Millipore, Billerica, MA, USA). After subtraction of background fluorescence, the total protein amounts were determined from the normalized values.
2.8. Vascular reactivity
Wistar-Kyoto (WKY) rats and SHRs were sacrificed via sodium pentobarbital overdose. A mid-sternal split was performed quickly, and the descending thoracic aorta excised carefully and placed in ice-cold Krebs buffer (118.3mM NaCl, 4.7mM KCl, 2.5mM CaCl2, 1.2mM KH2PO4, 25mM NaHCO3, 1.2mM MgSO4, 11mM glucose, 0.0026mM CaNa2 EDTA). The aorta was cleaned of excess fat and cut transversely into 5–10 rings (2.0–3.0 mm). Endothelium-dependent vasorelaxation was measured by the aortic rings as described previously [21].
2.9. Histology
A 1.5-cm section of the ascending thoracic aorta was dissected from the heart. Paraffin sections were cut (5 μm) and stained with hematoxylin and eosin. The mean values of the vessel wall thickness and cross sectional area from the endothelial surface to the adventitia were determined from digitalized microphotographs using commercial imaging analysis software (Axio Scope software, Thornwood, NY, USA).
2.10. Statistical analysis
All experiments were performed at least three times. Statistical analysis was performed according to the SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation. Statistical significance was determined using analysis of variance (ANOVA) followed by a multiple comparison test with a Bonferroni adjustment and by p < 0.05.
3. Results
3.1. Comparison of HPLC chromatograms REKRG and KRG
11 ginsenosides (Rg1, Re, Rf, Rh1, Rg2, Rb1, Rc, Rb2, Rg3, Rk1, and Rg5) were analyzed by HPLC. HPLC chromatograms of REKRG and KRG are shown in Fig. 1. The amount of Rg1, Re, Rf, Rh1, Rg2, Rb1, Rc, Rb2, Rg3, Rk1, and Rg5 was 0.6, 1.9, 12.3, 5, 4.2, 3.8, 1.2, 1, 100, 12, and 21 in REKRG and 2.9, 4.2, 0.3, 0.1, 0.2, 5.9, 2.2, 2.1, 0.3, 0.05, and 0.12 in KRG. These results show that the concentration of ginsenoside Rg3 in REKRG is ∼300 times greater than in KRG (Table 1).
Fig. 1.
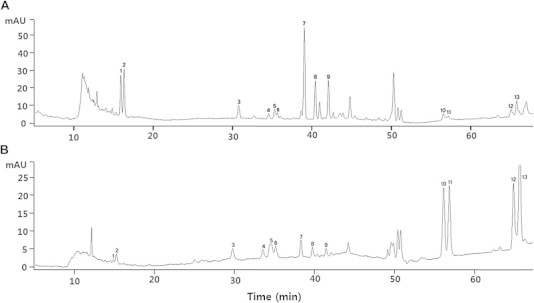
High-performance liquid chromatography (HPLC) chromatograms of Korean Red Ginseng and Rg3 enriched Korean Red Ginseng. (1) Rg1. (2) Re. (3) Rf. (4) Rh1(S). (5) Rh1(R)+Rg2(S). (6) Rg2(R). (7) Rb1. (8) Rc. (9) Rb2. (10) Rg3(S). (11) Rg3(R). (12) Rk1. (13) Rg5.
Table 1.
The saponin contents in Rg3-enriched Korean Red Ginseng and Korean Red Ginseng (mg/g)
| Rg1 | Re | Rf | Rg2 | Rh1 | Rb1 | Rc | Rb2 | Rg3(S) | Rg3(R) | Rk1 | Rg5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REKRG | 0.6 | 1.9 | 12.3 | 5 | 4.2 | 3.87 | 1.2 | 1 | 45 | 55 | 12 | 21 |
| KRG | 2.9 | 4.2 | 0.3 | 0.1 | 0.2 | 5.9 | 2.2 | 2.1 | 0.2 | 0.1 | 0.05 | 0.12 |
3.2. REKRG stimulates the phosphorylation of Akt and eNOS and increases NO production in HUVECs
Because Rg3 enhances eNOS phosphorylation and NO production [20], we next examined whether REKRG has an effect on Akt and eNOS activation in endothelial cells. HUVECs were incubated with 0.1–1 μg/mL REKRG for 24 hours. Cells were then harvested and processed for Western blot analysis. REKRG concentration-dependently stimulated Ser-437 phosphorylation of Akt and Ser-1177 phosphorylation of eNOS (Fig. 2A, 2B). We also examined NO levels in the culture medium after HUVECs were exposed to 0.1–1 μg/mL REKRG for 24 hours. NO levels were increased compared with control (Fig. 2C). These results show that REKRG stimulates the Akt/eNOS signaling pathway, leading to increased NO production in endothelial cells.
Fig. 2.
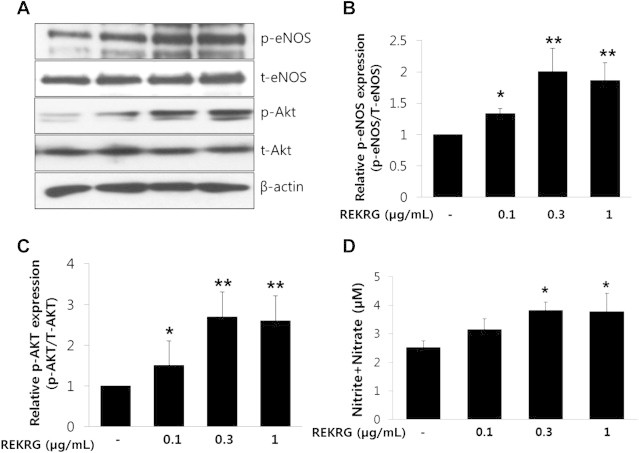
The effect of Rg3-enriched Korean Red Ginseng (REKRG) on endothelial nitric oxide synthase (eNOS) activation in human umbilical vein endothelial cells (HUVECs). (A,B) The phosphorylation of Akt and eNOS was increased dose-dependently in HUVECs treated with various concentrations (0.1–1 μg/mL) of REKRG for 24 hours. The cells were harvested and subjected to Western blot analysis for p-eNOS (upper panels). β-actin is shown as a loading control. p-eNOS expression levels were quantified by densitometric analysis (lower panels of A and B). Western blots are representative of three independent experiments. *p < 0.05, **p < 0.01 compared with untreated cells. (C) REKRG treatment dose-dependently increased nitric oxide (NO) production. Metabolites of NO (nitrite and nitrate) were measured in the media of HUVECs treated with 0.1–1 μg/mL REKRG. *p < 0.05 compared with untreated cells. Data are presented as mean ± standard deviation (n = 3).
3.3. REKRG inhibits ICAM-1 and COX-2 expression induced by TNF-α in HUVECs
It is well known that Rg3 has an anti-inflammatory effect [18]. Therefore, we next examined the effect of REKRG on TNF-α-induced increases in ICAM-1 and COX-2 expression in HUVECs. TNF-α increased ICAM-1 and COX-2 expression at both the protein and messenger RNA (mRNA) levels in HUVECs (Fig. 3A, 3B). However, the TNF-α-induced increases in VCAM-1 and COX-2 expression at the protein and mRNA levels in HUVECs were blunted by REKRG in a concentration-dependent manner (Fig. 3A, 3B), suggesting that REKRG can inhibit inflammatory proteins and possibly the early stage of atherosclerosis.
Fig. 3.
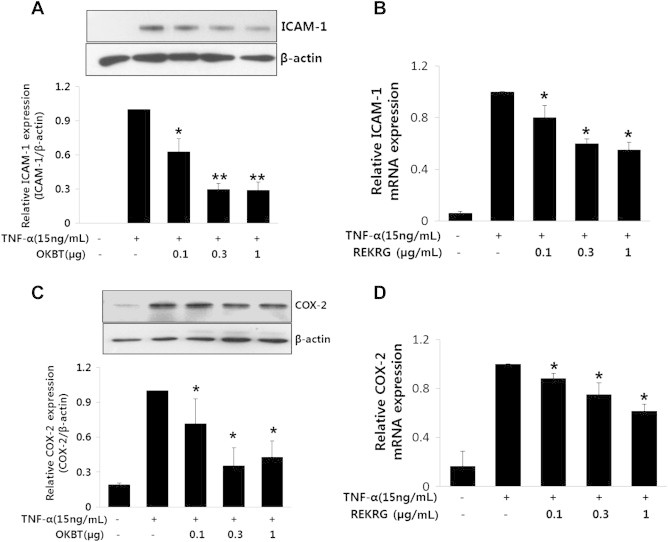
The effect of Rg3-enriched Korean Red Ginseng (REKRG) on the expression of intercellular adhesion molecule (ICAM)-1 and cyclooxygenase (COX)-2 induced by tumor necrosis factor-α (TNF-α) in human umbilical vein endothelial cells (HUVECs). (A) and (C) REKRG dose-dependently inhibited TNF-α-induced ICAM-1 and COX-2 protein expression in HUVECs. Cells were pretreated with various concentrations (0.1–1 μg/mL) of REKRG for 30 minutes and then treated with 15 ng/mL TNF-α for 8 hours in the presence of REKRG. The cells were harvested and subjected to Western blot analysis for ICAM-1 (upper panel of A) and COX-2 (upper panel of C). β-actin is shown as a loading control. The levels of ICAM-1 and COX-2 were quantified by densitometric analysis (lower panel of A and C). All Western blots shown are representative of three independent experiments. *p < 0.05 compared with control cells. (B,D) REKRG dose-dependently inhibited TNF-α-induced ICAM-1 and COX-2 mRNA expression in HUVECs. *p < 0.05 compared with untreated cells. Data are presented as mean ± standard deviation (n = 3).
3.4. REKRG improves impaired endothelial-dependent vascular relaxation in the aorta in SHRs
Many studies have shown that various ginsenosides, including Rg3, have a beneficial effect on vascular function [20]. Therefore, we investigated whether REKRG affects acetylcholine-induced relaxation in rat aortic rings. Acetylcholine-induced relaxation was measured in the presence of REKRG in an organ bath. In WKY rat aortic rings, endothelium-dependent vasorelaxation was not affected by 1 μg/mL REKRG treatment (Fig. 4A). However, compared with control rings, 1 μg/mL REKRG treatment improved impaired endothelium-dependent vasorelaxation in SHR aortic rings (Fig. 4B).
Fig. 4.
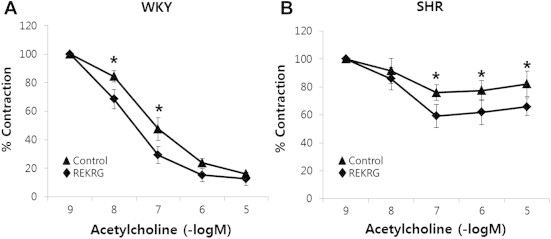
The effects of Rg3-enriched Korean Red Ginseng (REKRG) on vascular tone. (A) REKRG treatment did not affect endothelium-dependent vasorelaxation in Wistar-Kyoto (WKY) rats. (B) REKRG treatment improved impaired endothelium-dependent vasorelaxation in rats. Endothelium-dependent vasorelaxation was assessed after pre-treatment of aortic rings with REKRG (1 μg/mL) for 30 min. *p < 0.05 compared with untreated aortic rings. Data are presented as mean ± standard deviation (n = 4–5).
3.5. REKRG increases serum NO levels and reduces aortic intima-media thickness in SHRs
REKRG (10 mg/kg) was administered to rats for 6 weeks by gastric gavage. We next examined the effect of REKRG on serum NO levels. Compared with controls, 10 mg/kg REKRG increased serum NO levels in SHRs (Fig. 5A).
Fig. 5.
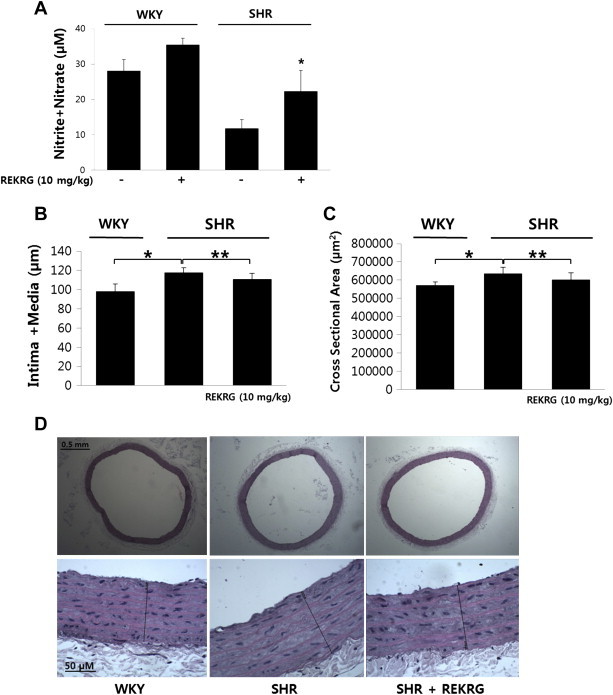
The effect of Rg3-enriched Korean Red Ginseng (REKRG) on aortic structure. (A) REKRG treatment increased nitric oxide (NO) production. Metabolites of NO (nitrite and nitrate) were measured in the serum. *p < 0.05 compared with control. Data represent the mean ± standard deviation (SD; n = 4–5). (B,C) The intima-media thickness and cross sectional area were quantified using a computer-assisted imaging device. The mean value of the vessel wall thickness from the endothelial surface to the adventitia was determined from five different locations spanning the entire cross-section. *p < 0.05 compared with Wistar-Kyoto rats. **p < 0.05 compared with SHR. Data represent the mean ± SD (n = 5–6). (D) Representative images of hematoxylin and eosin-stained aortic rings.
NO inhibits smooth muscle cell migration and proliferation [7]; therefore, we next examined the vascular structure is changed by REKRG in SHR. Digitalized microphotographs of histological sections were used to measure vessel wall thickness and cross sectional area (Fig. 5B, 5C). The aortic intima-media thickness and cross-sectional area in SHR were significantly thicker than it in WKY. Interestingly, REKRG administration for 6 weeks resulted in decreased aortic intima-media thickness and cross sectional area in SHRs, suggesting that chronic administration of REKRG may change vascular tone and structure.
4. Discussion
High blood pressure produces chronic stress in the body and is a major risk factor for vascular disease. It is associated with morphological alteration and dysfunction of vascular endothelial cells, which can lead to atherosclerosis. The protective effects of ginseng and ginsenosides have been widely studied and shown to have new beneficial effects on hypertension [14] and various diseases, such as atherosclerosis, cancer, and thrombosis [19,22–24]. In this study, we showed that REKRG increases NO production and induces endothelium-dependent vasorelaxation in aortic rings from SHRs. Furthermore, REKRG administration via gastric gavage increased serum NO levels and reduced blood pressure and aortic intima-media thickness.
It is unclear whether absorption of intact ginsenosides can take place in the human gastrointestinal tract and whether their hydrolysis products, protopanaxadiol (PPD) and protopanaxatriol (PPT), reach the systemic circulation. Pharmacokinetic analysis of Rg3 showed that the time to reach the peak plasma concentration after oral administration was 150.0 ± 73.5 h [25]. The data showed that the oral bioavailability of Rg3 was 2.63, which limits its beneficial effect. Furthermore, the amount of Rg3 in Korean Red Ginseng is usually less than 0.5%, even when steam heat treatment of ginseng roots, which strongly increases the amount of Rg3, is used. Therefore, in order to improve the biodistribution of Rg3 in vivo, we used REKRG, a ginsenoside fraction containing a high percentage of Rg3 isolated from P. ginseng, in this study.
NO from vascular endothelial cells plays an important role in the regulation of vascular function, as well as in inhibition of platelet aggregation and adhesion to the endothelium [26]. In addition, endothelium-derived NO inhibits not only smooth muscle cell proliferation but also migration to form the neointima. It is well known that the reduction in blood pressure by Korean Red Ginseng may be mediated by vascular endothelial cell-derived NO, and that Korean Red Ginseng promotes NO production in vascular endothelial cells [13,14]. Korean Red Ginseng induces angiogenesis by activating PI3K/Akt-dependent extracellular signal-regulated kinase 1/2 and eNOS pathways in HUVECs [27]. The ginsenoside Re activates potassium channels of vascular smooth muscle cells through PI3k/Akt and NO pathways [28]. Moreover, the ginsenoside Rg3 increases NO production through the PI3K/Akt pathway [20]. This can lead to stimulation of eNOS phosphorylation and expression in ECV-304 endothelial cells [20] and inhibition of TNF-α-induced expression of cell adhesion molecules, including VCAM-1 and ICAM-1, in human endothelial cells [18]. Our results also showed that REKRG not only stimulates eNOS phosphorylation and NO production but also decreases VCAM-1 and COX-2 expression. These findings suggest an important role for Rg3-enriched ginseng extract in vascular protection.
In conclusion, this study showed that the stimulatory effect of REKRG administration on vascular endothelial NO production through phosphorylation of eNOS is likely to have relevance for not only inhibition of VCAM-1 and COX-2 expression but also decreased aortic intima-media thickness, which improves cardiovascular function and prevents atherosclerosis.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST; no. 2011-0023858). The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/H2CZjI.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Hee-Jung Song, Email: nrsono@naver.com.
Cuk-Seong Kim, Email: cskim@cnu.ac.kr.
References
- 1.Harrison D.G., Guzik T.J., Lob H.E., Madhur M.S., Marvar P.J., Thabet S.R., Vinh A., Weyand C.M. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panza J.A., Quyyumi A.A., Brush J.E., Jr., Epstein S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 3.Panza J.A., Casino P.R., Kilcoyne C.M., Quyyumi A.A. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 4.Walsh T., Donnelly T., Lyons D. Impaired endothelial nitric oxide bioavailability: a common link between aging, hypertension, and atherogenesis? J Am Geriatr Soc. 2009;57:140–145. doi: 10.1111/j.1532-5415.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 5.Conger J.D. Endothelial regulation of vascular tone. Hosp Pract (Off Ed) 1994;29:117–122. doi: 10.1080/21548331.1994.11443095. 125–6. [DOI] [PubMed] [Google Scholar]
- 6.Hermann M., Flammer A., Luscher T.F. Nitric oxide in hypertension. J Clin Hypertens (Greenwich) 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luscher T.F., Noll G. The pathogenesis of cardiovascular disease: role of the endothelium as a target and mediator. Atherosclerosis. 1995;118(Suppl.):S81–S90. [PubMed] [Google Scholar]
- 8.Palmer R.M., Ashton D.S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 9.Palmer R.M., Ferrige A.G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 10.Stauss H.M., Godecke A., Mrowka R., Schrader J., Persson P.B. Enhanced blood pressure variability in eNOS knockout mice. Hypertension. 1999;33:1359–1363. doi: 10.1161/01.hyp.33.6.1359. [DOI] [PubMed] [Google Scholar]
- 11.Shesely E.G., Maeda N., Kim H.S., Desai K.M., Krege J.H., Laubach V.E., Sherman P.A., Sessa S.C., Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobadilla N.A., Gamba G., Tapia E., Garcia-Torres R., Bolio A., López-Zetina P., Herrera-Acosta J. Role of NO in cyclosporin nephrotoxicity: effects of chronic NO inhibition and NO synthases gene expression. Am J Physiol. 1998;274:F791–F798. doi: 10.1152/ajprenal.1998.274.4.F791. [DOI] [PubMed] [Google Scholar]
- 13.Jeon B.H., Kim C.S., Kim H.S., Park J.B., Nam K.Y., Chiang S.J. Effect of Korean Red Ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000;21:1095–1100. [PubMed] [Google Scholar]
- 14.Jeon B.H., Kim C.S., Park K.S., Lee J.W., Park J.B., Kim K.J., Kim S.H., Chang S.J., Nam K.Y. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen Pharmacol. 2000;35:135–141. doi: 10.1016/s0306-3623(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 15.Huu Tung N., Uto T., Morinaga O., Kim Y.H., Shoyama Y. Pharmacological effects of ginseng on liver functions and diseases: a minireview. Evid Based Complement Alternat Med. 2012;2012:173297. doi: 10.1155/2012/173297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 17.Qi L.W., Wang C.Z., Yuan C.S. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hien T.T., Kim N.D., Kim H.S., Kang K.W. Ginsenoside Rg3 inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules in human endothelial cells. Pharmazie. 2010;65:699–701. [PubMed] [Google Scholar]
- 19.Zhang C., Liu L., Yu Y., Chen B., Tang C., Li X. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol Med Rep. 2012;5:1295–1298. doi: 10.3892/mmr.2012.808. [DOI] [PubMed] [Google Scholar]
- 20.Hien T.T., Kim N.D., Pokharel Y.R., Oh S.J., Lee M.Y., Kang K.W. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: essential roles of estrogen receptor-dependent PI3-kinase and AMP-activated protein kinase. Toxicol Appl Pharmacol. 2010;246:171–183. doi: 10.1016/j.taap.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Jung S.B., Kim C.S., Kim Y.R., Naqvi A., Yamamori T., Kumar S., Kumar A., Irani I. Redox factor-1 activates endothelial SIRTUIN1 through reduction of conserved cysteine sulfhydryls in its deacetylase domain. PLoS One. 2013;8:e65415. doi: 10.1371/journal.pone.0065415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y.G., Zhang H.G., Zhang G.Y., Fan J.S., Li X.H., Liu Y.H., Li S.H., Lian X.M., Tang Z. Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin Exp Pharmacol Physiol. 2008;35:1238–1244. doi: 10.1111/j.1440-1681.2008.04997.x. [DOI] [PubMed] [Google Scholar]
- 23.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y., Im J.H., Han X.H., Kim T.J., Shin K.S. Antithrombotic and antiplatelet activities of Korean Red Ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 24.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 25.Ren H.C., Sun J.G., Wang G.J., A J.Y., Xie H.T., Zha W.B., Yan B., Sun F.Z., Hao H.P., Gu S.H. Sensitive determination of 20(S)-protopanaxadiol in rat plasma using HPLC-APCI-MS: application of pharmacokinetic study in rats. J Pharm Biomed Anal. 2008;48:1476–1480. doi: 10.1016/j.jpba.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Radomski M.W., Palmer R.M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.M., Namkoong S., Yun Y.G., Hong H.D., Lee Y.C., Ha K.S., Lee H., Kwon H.J., Kwon Y.G., Kim Y.M. Water extract of Korean Red Ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull. 2007;30:1674–1679. doi: 10.1248/bpb.30.1674. [DOI] [PubMed] [Google Scholar]
- 28.Nakaya Y., Mawatari K., Takahashi A., Harada N., Hata A., Yasui S. The phytoestrogen ginsensoside Re activates potassium channels of vascular smooth muscle cells through PI3K/Akt and nitric oxide pathways. J Med Invest. 2007;54:381–384. doi: 10.2152/jmi.54.381. [DOI] [PubMed] [Google Scholar]


