Summary
The dual burden of tuberculosis and diabetes has attracted much attention in the past decade as diabetes prevalence has increased dramatically in countries already afflicted with a high burden of tuberculosis. The confluence of these two major diseases presents a serious threat to global public health; at the same time it also presents an opportunity to learn more about the key elements of human immunity to tuberculosis that may be relevant to the general population. Some effects of diabetes on innate and adaptive immunity which are potentially relevant to tuberculosis defense have been identified, but have yet to be verified in humans and are unlikely to fully explain the interaction of these two disease states. This review provides an update on the clinical and epidemiological features of tuberculosis in the diabetic population and relates them to recent advances in understanding the mechanistic basis of tuberculosis susceptibility and other complications of diabetes. Issues that merit further investigation, such as geographic host and pathogen differences in the diabetes/tuberculosis interaction, the role of hyperglycemia-induced epigenetic reprogramming in immune dysfunction and the impact of diabetes on lung injury and fibrosis caused by tuberculosis, are highlighted in this review.
Keywords: bacterial infections, diabetes, host/pathogen interaction, infectious disease, tuberculosis
Introduction
An adverse effect of diabetes mellitus (DM) on the host defense against tuberculosis (TB) has been recognized for over a century [1] but has only recently been investigated in detail as DM rates have increased dramatically since the 1980s in low and middle income countries where TB is already prevalent [2]. Insulin-resistant type 2 DM comprises 90% of the global cases of DM and a large proportion of the people afflicted with the dual TB/DM burden [1;2]; however autoimmune type 1 DM is also associated with TB susceptibility [3]. This is unsurprising since both types of DM share a similar spectrum of other complications that develop as a consequence of chronic hyperglycemia [4]. Diabetes increases the risk of developing TB disease about three-fold, as reported in a systematic review of 13 observational studies [5]. The range of risk estimates from individual studies included in that review was notably broad; from 0.99 to 7.83 [5]. Divergent results have since been reported in additional studies of DM and TB risk [6–9]. Factors contributing to this variability include almost universally retrospective study design, weak diagnostic criteria for DM in some studies, and the likelihood that host and microbial genetics as well as environmental factors in diverse geographic locations differentially influence TB defense. The moderate increase in TB risk associated with DM has a major global public health impact, owing to the large and growing population of diabetic people living in high TB burden settings [10].
The scope of the TB/DM problem in India, the country with the highest case load of both diseases, was highlighted in a 2011 study by Viswanathan et al. [11]. The authors of that study performed oral glucose tolerance tests on 827 patients newly diagnosed with TB in Chennai. In that cohort, 25.3% of TB patients were confirmed to have DM and 24.5% had pre-diabetes by WHO criteria [11]. A possible association of pre-diabetes with TB risk is surprising in light of clinical evidence linking susceptibility to poor glycemic control [6]. Pre-diabetes could develop as an effect rather than a cause of TB, or TB risk might be increased in pre-diabetics by a factor other than hyperglycemia, such as dyslipidemia. The lung is the predominant site of TB disease in the general, immunocompetent population, accounting for 70–80% of cases [12]. The study by Viswanathan [11], along with another from India [13], found that DM specifically increases the risk for pulmonary TB but not extrapulmonary disease. This contrasts with increased TB risk in the setting of HIV/AIDS or treatment with TNF inhibitors where extrapulmonary TB is disproportionately increased [14;15]. This finding suggests that the host defense mechanisms impacted by DM may be particularly relevant to TB susceptibility in the general population.
In addition to increasing the risk to develop active TB, DM increases TB disease severity. A systematic review by Baker et al. [16] of 33 published studies on TB outcomes reported summary risk ratios of 1.89 for death and 3.89 for relapse after completion of treatment in diabetic as opposed to non-diabetic TB patients. Risk estimates from individual outcomes studies were as varied as the literature on TB disease risk, presumably attributable to similar issues of study design and diverse populations among the various reports. A recent prospective study of TB outcomes in 1,262 patients in southern Mexico confirmed increased adjusted odds ratios (ORs) of greater radiographic severity (OR=1.80), delayed sputum conversion (OR=1.51), treatment failure (OR=2.93), TB recurrence (OR=1.76) and TB relapse (OR=1.83) [17] in diabetic individuals compared with non-diabetic individuals. Recurrent TB was caused by Mycobacterium tuberculosis (Mtb) with the same genotype in 81% of cases [17], indicating a predominance of relapse over exogenous reinfection. Diabetes increases the risk that standard TB treatment will fail to sterilize infection.
The true impact of DM on TB risk and disease outcome will ultimately be resolved by additional prospective studies in different geographic locales, but the available data are sufficient to conclude that it has a negative impact on global public health beyond the diabetic population per se. Given their three-fold increased risk for progression from latent TB infection (LTBI) to active disease, higher rates of sputum smear positivity at diagnosis [11], and increased risk for recurrence/relapse after TB treatment, diabetic TB patients must make a disproportionately higher contribution to TB transmission. These considerations provide a strong motivation to understand the mechanisms of TB susceptibility in DM.
Immunological mechanisms of susceptibility
Natural infection with Mtb occurs by inhalation of a small number of bacilli that invade and replicate in resident alveolar macrophages, which are the predominant myeloid cell type in the airspace of the healthy lung. Infected macrophages fail to restrict bacillary replication, resulting in cellular death by apoptosis or necrosis and horizontal spread to macrophages, myeloid DCs and neutrophils recruited from the periphery [18]. Bacilli are conveyed by mDCs from the lung to thoracic nodes, where adaptive immunity is primed [19]. Antigen-specific T cells expand and traffic to the lung where they promote an effective antimicrobial response through macrophage-activity cytokines and by cytotoxic T cell targeting of Mtb-infected macrophages. This T-cell-mediated macrophage activation limits Mtb replication and prevents TB disease for the lifetime of most infected humans, but is rarely if ever sterilizing. Latent infection progresses to active disease in roughly 5–10% of Mtb-infected people [20]. Risk progression from latent infection to active disease is considerably higher with comorbid CD4+ T-cell deficiency (HIV/AIDS) or loss of protective cytokine function (IFN-γ, IL-12 or TNF-α) through pharmacological inhibition, autoimmunity or Mendelian susceptibility. Paradoxically, established TB disease in diabetic humans is characterized by an excess of these normally protective cells and cytokines in the circulation, as discussed below.
Several laboratories have described increased susceptibility to TB in animal models combining TB and DM. Saiki et al. [21] used streptozotocin (STZ) to deplete insulin-producing cells and cause hyperglycemia in imprinting control region (ICR) mice. Hyperglycemic mice and untreated controls were challenged i.v. with a high dose of Mtb Schacht. At 3 months post-infection (p.i.), >90% of hyperglycemic mice had died vs. <10% of the euglycemic controls [21]. Yamashiro et al. [22] challenged STZ-treated ICR mice with Mtb H37Rv (105 CFU i.v.) and found a ~0.5 log higher lung bacterial burden at 14 days p.i. compared with that in untreated controls, rising to ~1.5 logs higher at day 35. The IFN-γ content in lung tissues homogenates 14 days p.i. was significantly lower in the diabetic group, as was IFN-γ production by Mtb antigen (PPD)-stimulated splenocytes tested at 8 days p.i [22]. Insulin treatment starting 2 days after STZ administration resulted in a lower bacterial burden at day 35 [22]. Increased TB susceptibility have also been reported in rat models of type 1 and type 2 DM [23;24], and in sucrose-fed guinea pigs [25].
Martens et al. [26] investigated TB susceptibility in STZ-treated C57BL/6 mice that were hyperglycemic for <4 weeks (acute) or ≥ 12 weeks (chronic) before low dose aerosol challenge with Mtb Erdman. Results comparing STZ-treated mice with sham-treated controls are summarized as follows: i) chronic but not acute hyperglycemia increased TB susceptibility; ii) peak bacillary burden was ~1.5 log higher in chronically hyperglycemic mice compared with sham-treated controls and acutely hyperglycemic mice but was held to a plateau value at 8 and 16 weeks p.i.; iii) the pattern of pulmonary histopathology was similar in hyperglycemic and control healthy mice; iv) the volume of pulmonary inflammation at 4 weeks p.i. was similar in hyperglycemic and control mice but was progressively greater in the hyperglycemic group at 8 and 16 weeks p.i.; v) hyperglycemic mice had greater absolute numbers of CD4+ and CD8+ T cells, macrophages and neutrophils in the lung 16 weeks p.i. but only neutrophils were proportionately increased compared with euglycemic controls; vi) lung tissue levels of IFN-γ, IL-1β and TNF-α were higher in STZ-treated mice than euglycemic controls at 16 weeks p.i.; vii) there was no difference in the expression of inducible nitric oxide synthase (iNOS) between groups [26]. TB susceptibility, like other diabetic complications, results from the cumulative effect of chronic hyperglycemia rather than from an immediate impact of elevated blood glucose. Preserved TB defense in acutely hyperglycemic mice indicates that susceptibility is not a consequence of insulin deficiency or a confounding effect of STZ [26]. Chronic hyperglycemia does not impair the response to IFN-γ signaling reflected by iNOS expression. Key findings were replicated in spontaneously insulin-deficient Akita mice [26], providing further confidence that TB susceptibility is s not an artifact of STZ treatment.
The immunological basis of TB susceptibility after aerosol challenge under chronic hyperglycemia was uncertain given that the lungs of diabetic mice contained an excess of all major leukocyte types and cytokines normally associated with successful TB defense in control mice [26]. This apparent paradox was resolved by experiments tracking the immune response weekly from 7 to 28 days p.i, which showed lower IFN-γ levels in the lungs of diabetic mice at the critical 2-week time point when the lung Mtb load is rising logarithmically after low dose aerosol challenge [26]. This difference in early IFN-γ production between hyperglycemic and control mice was lost by 4 weeks p.i. and diabetic mice had more leukocytes and higher lung cytokine levels than controls at later time points. Taken together, data from the various animal studies cited [21–26] confirm that DM is associated with a temporally delayed but otherwise seemingly unimpaired cellular immune response to Mtb, resulting in higher plateau bacillary burden and more extensive inflammation.
The role of Innate Immunity in TB susceptibility
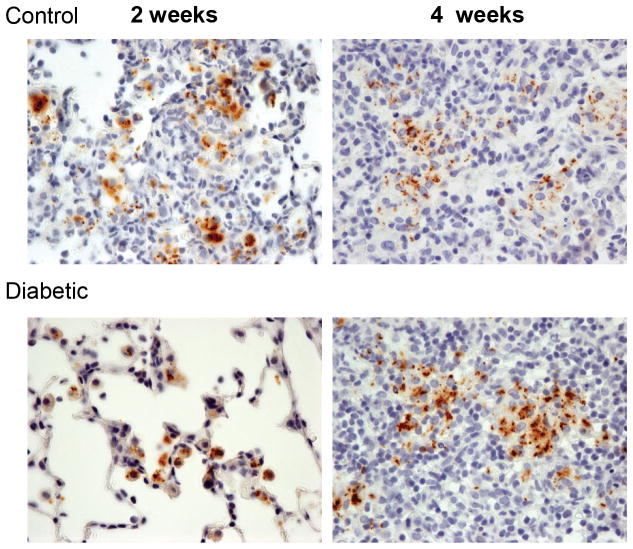
The capacity to arrest phagosome maturation in macrophages is a key Mtb virulence mechanism enabling intracellular bacterial replication and logarithmic increase in lung bacterial load until adaptive immunity has been primed [27]. Despite mounting a weak effector response against Mtb, innate immune cells such as macrophages and DCs play key roles in sensing infection, orchestrating adaptive immune priming, and expressing enhanced antimicrobial functions once activated or targeted for cytolysis by antigen-specific T cells. Perturbation of monocyte/macrophage or DC functions could therefore contribute to TB susceptibility in DM. Two weeks after aerosol challenge of mice, Mtb-infected alveolar macrophages are detectable by immunostaining lung sections with anti-PPD (Fig. 1) [28]. In euglycemic control mice, early lesions are comprised of clusters of myeloid cells filling the alveoli and surrounding Mtb-infected macrophages [28]. At the same 2-week time point in Mtb-infected hyperglycemic mice, the Mtb-containing alveolar macrophages are surrounded by few or no recruited myeloid cells. However by 4 weeks p.i., the inflammatory lung lesions in hyperglycemic and control mice are indistinguishable [28]. These results suggest two hypotheses that are not mutually exclusive: i) the sentinel function of Mtb-infected alveolar macrophages is impaired by DM, resulting in reduced expression of signals that recruit naïve macrophages, DCs, neutrophils and innate lymphocytes to the airspace; ii) DM produces a barrier to leukocyte transmigration into the airspace even if adequate recruiting signals are produced by infected alveolar macrophages. These hypotheses have not been directly tested in the context of TB/DM in vivo, but data from diabetic humans and animal models are consistent with both, as discussed below.
Figure 1.
Diabetes affects early events in the host-pathogen interaction of TB. Control C57BL/6 mice (top) and mice with 12 weeks of STZ-induced diabetes (bottom) were challenged with ~100 CFU of Mtb Erdman by aerosol [28]. Lung sections prepared from mice after 2 weeks (left) or 4 weeks (right) of TB disease were stained with anti-PPD Ab to detect infecting bacilli (brown). At the 2-week time point, sites of Mtb infection in control mice were characterized by aggregates of recruited myeloid cells. In contrast, the lungs of diabetic mice contained Mtb-infected alveolar macrophages with few or no recruited myeloid cells in their vicinity. After 4 weeks of TB disease, the lesions of control and diabetic mice were indistinguishable. This is interpreted as reflecting a delay in initiating an innate response by alveolar macrophages initially infected with inhaled Mtb [28]. Original magnification ×400.
The defective sentinel hypothesis is indirectly supported by a report that peripheral blood monocytes from patients with type 2 DM have a reduced capacity to bind or ingest Mtb bacilli compared to monocytes from euglycemic controls [29]. This phenotype was associated with poor glycemic control and attributable to alterations in the complement pathway of opsonization rather than monocyte phagocytic machinery per se [29]. An altered route of Mtb entry could therefore also influence the timely activation of alveolar macrophages following inhalation of bacilli. Alveolar macrophages from hyperglycemic rats have been shown to produce less nitric oxide (NO) after overnight incubation with Mtb than macrophages from euglycemic controls [23]. That result, obtained in the absence of IFN-γ-producing lymphocytes, implies a direct effect of the diabetic milieu on an intrinsic macrophage response to Mtb. Data from diabetic humans with or without TB disease are consistent with that hypothesis. Wang et al. [30] reported that alveolar macrophages from diabetic TB patients have a surface phenotype consistent with a reduced activation state compared with that of macrophages from non-diabetic TB patients or uninfected hosts. They also showed that diabetic alveolar macrophages produce less H2O2 than cells from euglycemic subjects in response to phorbol 12-myristate 13-acetate. However more detailed investigation of basal and Mtb-stimulated responses by primary alveolar macrophages is required to strengthen the argument that impaired sentinel function contributes to diabetic TB susceptibility.
There is a considerable body of literature describing adverse effects of DM on innate immunity in contexts other than TB. Several studies report functional changes in macrophages, including reduced phagocytic activity for sheep red blood cells and various microbes, decreased adhesion and chemotactic activity, skewing to an M2 phenotype, and reduced cytokine expression in response to diverse stimuli including lipopolysaccharide (LPS) and IFN-γ [31–35]. In contrast, other studies report enhanced monocyte/macrophage responses with hyperglycemia. Mo et al. [36] described increased IL-1β, CCL2/MCP-1 and TNF-α expression by alveolar macrophages from rabbits with alloxan-induced DM in response to particulate stimulation. Moreover, Deveraj et al. [37] reported increased basal production of IL-6 by THP-1 cells cultured in media with high (15 mmol/L) but not low (5.5 mmol/L) glucose content. These inconsistent results likely reflect differences in approach including human samples vs. animal models, cell lines vs. primary cells, acute vs. chronic hyperglycemia, and the potential that DM could simultaneously depress certain monocyte/macrophage functions while amplifying others.
Myeloid DCs convey Mtb from the site of initial macrophage infection in the airspace to the lung-draining lymph nodes [19]. Vallerskog et al. [28] showed that this process is delayed in hyperglycemic mice but mDC migration from lung to lymph node (and co-stimulatory molecule expression) in response to LPS was no different in hyperglycemic mice than that in control mice. This suggests that DM impairs mDC trafficking to the site of infection rather than an intrinsic effect on APC function or migration from lung to lymph nodes. There are as yet no other published studies of mDC function in TB/DM but the effects of DM on DCs have been investigated in other contexts. Musilli et al. [38] reported increased numbers of circulating DCs in patients with type 2 DM. Surendar et al. [39] found that circulating mDCs and plasmacytoid DCs from diabetic subjects have increased expression of HLA-DR and CD123 which correlates with poor glycemic control. This activation was attributed to higher plasma levels of GM-CSF in the diabetic cohort [39]. Together, these data suggest that DM is more likely to increase rather than repress mDC activation, in contrast to a repressive impact on macrophage function.
Neutrophils may play a host-protective role in TB, accelerating the kinetics of immune priming and Th1-cell recruitment to the lung in Mtb-challenged mice [40;41]. However, at later time points in TB disease neutrophils are associated with poorly controlled infection and tissue injury [42]. The impact of hyperglycemia on neutrophils in TB has not been investigated beyond the trend for neutrophilic inflammation in mice [26] but neutrophils have been a focus of DM research in other contexts. Key findings for neutrophils in hyperglycemic subjects include increased adhesion and integrin expression [43], reduced chemotaxis [44], a phagocytic defect [45;46], and reduced microbicidal activity for certain bacteria and fungi as compared with neutrophils from euglycemic controls [47–49]. Not all published studies are in agreement and whether or not any of these findings relate to TB susceptibility in diabetic people remains to be seen. One potentially relevant observation is that glycated collagen impedes neutrophil migration compared with non-glycated collagen [50]. This effect depends on the receptor for advanced glycation end-products (RAGE), which is expressed on neutrophils and other leukocytes [51;52]. Lung matrix proteins have slow turnover and accumulate glycation over time with DM [53], suggesting a mechanism for the barrier hypothesis of delayed innate inflammation in the lungs of diabetic mice.
The role of innate lymphocytes in TB defense has not been fully defined and might be more significant in the human host than in rodent models [54]. Zhang et al. [55] reported that TB patients with DM had a higher proportion of NKT cells in peripheral blood and bronchoalveolar lavage (BAL) fluid than non-diabetic TB patients, whereas basal peripheral blood NKT-cell counts were not different. The functional significance of increased numbers of NKT cells, and whether it is cause or effect of diabetic TB susceptibility, is not presently known. There are as yet no reports on NK cells in the context of TB and DM.
The role of Adaptive immunity in TB susceptibility
A timely Th1-biased adaptive immune response is the major determinant of outcomes in human TB and in animal models of TB [54]. With the recognition that DM increases susceptibility to TB it was anticipated that impaired expression of adaptive immunity would be identified as the culprit mechanism; this hypothesis was suggested by early data from a mouse model reporting IFN-γ expression only at 2 weeks p.i. [22]. It was therefore surprising when Restrepo et al. [56] reported that PPD restimulation of whole blood in a cohort of diabetic TB patients from southern Texas and northern Mexico resulted in higher production of IFN-γ, IL-2, TNF-α and GM-CSF than matched non-diabetic TB patients. This finding was confirmed and extended by Kumar et al. [57] who compared T-cell frequencies in pulmonary TB patients with or without DM from a cohort in Chennai, India. The authors of that study employed intracellular cytokine staining to interrogate CD4+ T cells from whole blood at baseline and after Mtb antigen-specific (CFP-10 and ESAT-6) stimulation or non-specific stimulation with anti-CD3 plus anti-CD28 mAb. Diabetic TB patients had higher frequencies of mono- and dual-functional Th1 cells and higher frequencies of Th17 cells at baseline and with antigen stimulation, but lower frequencies of Treg cells [57]. There was no difference in the frequencies of central or effector memory T-cells between the diabetic and non-diabetic groups. If confirmed, a relative deficiency in Treg cells could be a factor contributing to the increased inflammation characteristic of pulmonary TB in diabetics. A subsequent study of plasma cytokine levels in diabetic patients by Kumar et al. [58] confirmed the downstream effects of increased Th-cell frequencies, with increased levels of Th1 (IFN-γ, IL-2), Th2 (IL-5) and Th17 (IL-17A) cytokines in diabetic TB patients compared with the non-diabetic group. In contrast, diabetics had lower plasma levels of IL-22 than non-diabetic TB patients. The potential significance of reduced IL-22 in TB is unknown but lower IL-22 has been linked to impaired pulmonary epithelial barrier integrity with Klebsiella pneumoniae infection [59].
Cytokine over-expression by T cells in the context of TB/DM has not been seen in all clinical studies. Stalenhoef et al. [60], in measuring cytokine production from whole blood of Indonesian TB patients with or without DM stimulated with LPS, phytohemagglutinin or Mtb sonicate, found no difference in the expression of proinflammatory cytokines, including IFN-γ. The only significant difference found in this study was reduced IL-10 production in the diabetic group. This contrasts with data from Restrepo and Kumar (and the mouse model) [26;56–58], possibly reflecting differences in the populations studied or in methods such as the source of Mtb antigen. The preponderance of evidence suggests that the antigen-specific T-cell response in TB/DM is quantitatively greater but functionally not more effective than the response of non-diabetic TB patients. Successful TB defense reflects a balance between protective vs. damaging immune activation. The immunological complications of DM may shift this equilibrium towards damage.
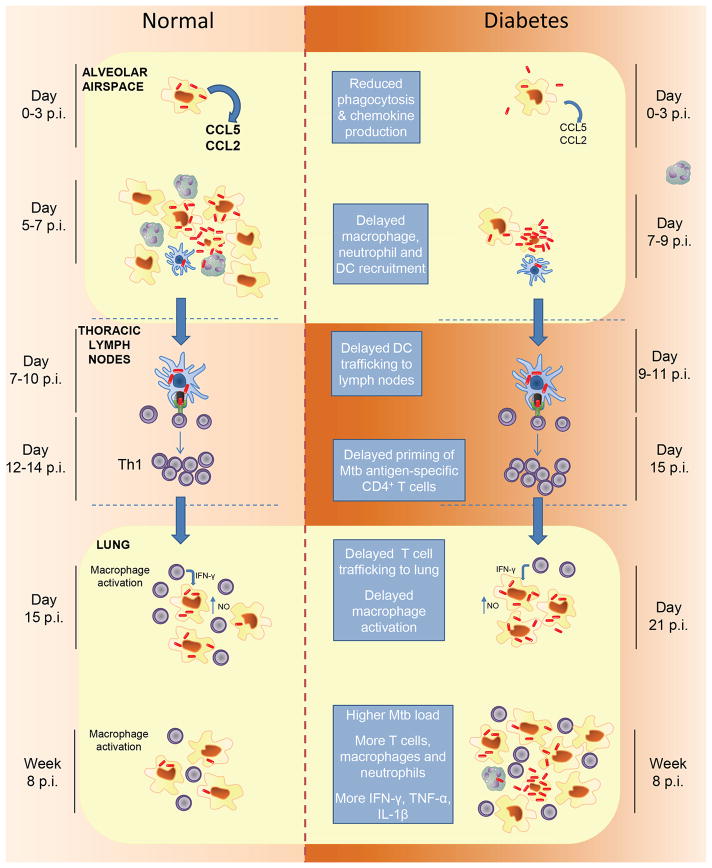
Delayed priming of adaptive immunity is a major cause of TB susceptibility in hyperglycemic mice (Fig. 2) [28]; however the exact relationship between delayed priming and post-primary TB in adult humans is unknown. While the age of type 2 DM onset has declined in the past decade [61] it is still likely that initial LTBI precedes DM in most cases. However, repeated exposure to Mtb is common in high burden settings where it is estimated that >70% of newly diagnosed TB cases arise from infection acquired within the previous 18 months [62]. In diabetic people, progression of recently acquired infection to TB disease could result from impaired sentinel function of alveolar macrophages regardless of prior exposures. While this question has not yet been studied directly, evidence that 10–20% of LTBI patients have a peripheral blood transcriptional signature of active TB disease [63] supports the concept that a subgroup of people with LTBI may have clinically unapparent but biologically active foci of infection at high risk for progression to clinically evident TB. It will be interesting to see if DM increases the prevalence of active TB peripheral blood transcriptional signatures in LTBI.
Figure 2.
A proposed model for TB susceptibility in DM. A defective innate response to inhaled Mtb by diabetic hosts results in a critical delay in priming adaptive immunity. Resident alveolar macrophages are the first cells to become infected with inhaled Mtb. They fail to restrict Mtb replication but in non-diabetic hosts (left) produce signals that recruit na macrophages, neutrophils and mDCs to the alveolus. Infection spreads to recruited cells, including mDCs [18], which then transport bacilli to lung-draining lymph nodes where the adaptive response is primed (Day 7–10 p.i.). Antigen-specific T cells traffic to the lungs and mediate an effector response capable of restricting Mtb replication (Day 15 p.i.). Diabetic hosts (right) are slow to mount an innate response to the alveolar macrophages initially infected with Mtb. The delay in recruiting mDCs to the alveolar airspace to acquire bacilli leads to downstream delays in their delivery of antigen to thoracic lymph nodes and the subsequent expression of adaptive immunity in the lung during the period of logarithmic Mtb replication. This results in a higher plateau lung bacterial load once effective control is exerted, associated with increased severity of immune pathology and worse outcomes in TB/DM. Additional factors contributing to excessive inflammation may be a relative deficiency of Treg cells [57], accelerated death of Mtb-infected macrophages due to elevated intracellular methylglyoxal [73], and intrinsic proinflammatory effects of DM resulting from increased mitochondrial generation of superoxide [65]. The timeline of events following inhalation of Mtb are based on data published by Vallerskog et al. [28].
TB Immune pathology
Evidence from mouse and man indicates that DM exacerbates TB immune pathology. The pattern of histopathology in diabetic and non-diabetic mice is indistinguishable but it is quantitatively greater in diabetic mice at 8 weeks p.i. and beyond [26]. Greater severity of immune pathology in human TB/DM is inferred from the increased mortality hazard and worse radiographic severity of disease. Amplified lung injury and fibrosis might result from higher bacillary load and/or result from the intrinsic proinflammatory microenvironment characteristic of DM [64;65].
Nagareddy et al. [66] reported that hyperglycemia increases neutrophil secretion of S100A6/A9, which in turn stimulates macrophages to secrete GM-CSF and common myeloid progenitor cells to secrete M-CSF. These growth factors promote expansion of myeloid progenitors in diabetic mice, increasing numbers of circulating neutrophils and Ly6-Chi monocytes that populate atherosclerotic plaques in STZ-treated, hyperglycemic low density lipoprotein receptor (LDLR)−/− mice. The expansion of monocytes and neutrophils caused by hyperglycemia could be another factor contributing to excessive inflammation in TB/DM.
Higher plateau lung bacterial load has been documented in hyperglycemic mice [22;26] but there are presently no methods to accurately quantify bacillary load or inflammation in humans. Higher bacillary burden is inferred from reports that TB/DM patients are more likely to have positive sputum smear on presentation and delayed culture conversion with treatment [11;17]. Chest x-ray results suggest increased TB inflammation in humans but this lacks sensitivity and is hard to quantify. Chest CT scan is superior in this regard but has not been used for most TB/DM studies to date. The best available evidence for increased inflammation in human TB/DM is the increased level of proinflammatory cytokines in peripheral blood [58]. This association could be validated by future studies using more sophisticated lung imaging methods including CT and positron emission tomography, by peripheral blood transcriptomics [63], or with biomarkers such as exhaled nitric oxide [67;68].
Diabetes is not only associated with increased inflammation but also with its persistence and impaired resolution. A comparative proteomic study of skin wounds in people with and without DM revealed a pattern of differences reflecting increased inflammation, impaired angiogenesis and accelerated cell death in DM [69]. Diabetic nephropathy is one of the most common serious complications of DM, culminating in irreversible fibrosis. This problem has stimulated interest in anti-fibrotic therapies for diabetic kidney disease [70]. If DM is shown to accelerate the damage and fibrosis that are responsible for most TB-related morbidity and mortality, diabetic individuals might be an attractive target population to test host-directed antifibrotic or antiprotease therapies with the goal of reducing mortality and preventing pulmonary impairment after TB.
Biochemical mechanisms of TB susceptibility
It is reasonable to postulate that TB susceptibility in DM results from mechanisms related to those responsible for microvascular and macrovascular complications that are shared by type 1 and type 2 DM. Brownlee and Giacco. [4;65] identified hyperglycemia-dependent mitochondrial overproduction of superoxide as the key upstream event activating complication pathways that include: increased polyol and hexomsamine flux; increased protein kinase C (PKC) activation; increased formation of advanced glycation end products (AGEs); and increased expression of RAGE and its endogenous ligands. These pathways have not been extensively studied in hematopoietic cells but they have obvious potential to disrupt immune function. Polyol pathway flux consumes NADPH, increasing oxidative stress by impeding regeneration of the reactive oxygen species (ROS) scavenger reduced glutathione [71]. Increased hexosamine pathway flux leads to the overproduction of uridine diphosphate N-acetylglucosamine, providing excess substrate for enzymatic O-GlcNAcylation that can modify the function of nuclear and cytoplasmic proteins including transcription factors such as NF-κB [72]. Non-enzymatic glycation can similarly cause functional alterations of intracellular and extracellular proteins. Moreover, the AGE intermediate methylglyoxal was shown to promote macrophage apoptosis following Mtb infection [73]. Hyperglycemia-induced ROS production increases RAGE expression and the expression of HMGB1 and S100A8/9, which are more physiologically significant as RAGE ligands than AGE-modified proteins [74]. Finally, hyperglycemia-induced ROS inhibit GAPDH activity, thereby raising intracellular levels of triose phosphate, which is a precursor for diacylglycerol. This results in pathologically increased activation of the β and δ isoforms of PKC [75], which participate in immune regulation at many levels. The potential for each of these pathways to influence protective immunity is evident but has not been directly investigated in TB or any other DM-related infection
Epigenetic reprogramming is a more recently recognized diabetic complication mechanism that could be a contributing factor in TB susceptibility. The term “metabolic memory” covers a range of phenomena in DM in which a prolonged period of tight glycemic control leads to reduced vascular complications even years after glycohemoglobin returns to a pre-intervention baseline [76]. Conversely, tissue damage can progress in some diabetic people even after correction of hyperglycemia. In both cases these responses may reflect posttranslational chromatin modification that influences gene expression. Epigenetic effects of DM have not been studied in the context of TB but immune cells are certainly vulnerable to this complication mechanism. Miao et al. [77] reported that peripheral blood T cells from type 1 DM patients have a distinct profile of chromatin histone H3 lysine 9 dimethylation. Epigenetic changes in blood monocytes from type 2 DM patients have also been identified [78]. Diabetes is associated with ROS-mediated DNA damage [65]. One consequence of DNA damage is telomere shortening in peripheral blood monocytes, which could accelerate their senescence following transmigration into tissues [79].
Parsing the roles of so many candidate mechanisms in diabetic TB susceptibility will be a daunting task but selecting a treatable pathway might already be feasible. Selective blockade of individual complication pathways such as polyol flux, protein glycation and PKC activation has had modest success in animal models and human studies [80–82]. A more promising approach might be targeting the mitochondrial ROS over-production that appears to be the common upstream event activating all of these pathways [83].
Diabetic Comorbidities
Cigarette smoking, dyslipidemia and vitamin D deficiency act synergistically with hyperglycemia to promote vascular and renal complications of DM. Smoking and vitamin D deficiency have been linked individually to human TB susceptibility [84;85]. The magnitude of risk imposed by either condition alone is modest but when combined with DM could identify a subset of people with LTBI having particularly high risk for progression, and/or a subset of people with TB disease at much higher risk for adverse outcomes. The latter was shown in a cohort of Korean TB patients where DM or smoking independently increased 12-month mortality ~two-fold compared with that of non-diabetic non-smokers while the mortality hazard was raised >fourfold in diabetic TB patients who also smoked [86]. The mechanism of increased TB susceptibility in smokers is unknown, but cigarette smoke exposure was shown to inhibit the pulmonary T-cell response to Mtb in mice [87]. The potential combined effect of DM and vitamin D deficiency on TB susceptibility has not been studied but the prevalence of vitamin D deficiency is increased in type 2 diabetics and has been linked to DM pathogenesis [88].
Type 2 DM is associated with elevated total and LDL cholesterol, reduced levels of HDL cholesterol, and hypertriglyceridemia. Hyperlipidemia has not been a focus of any clinical TB studies perhaps since these conditions were until recently rare in high TB burden countries. Apolipoprotein E (ApoE)−/− and LDLR−/− mice are susceptible to TB when fed high but not low cholesterol chow [89;90]. In common with diabetic mice, both hypercholesterolemic models exhibit neutrophilic pathology which is extreme in ApoE−/− mice that develop massive necrotic lung lesions and Mtb-studded neutrophil extracellular traps in BAL [18;89]. Like diabetic mice, adaptive immune priming is delayed in Mtb-infected ApoE−/− mice but is more extreme such that the Mtb burden reaches a lethal level and these mice die within 4–6 weeks of aerosol challenge. The neutrophilic inflammatory response of LDLR−/− is intermediate between diabetic and in ApoE−/− mice, and LDLR−/− mice mount a timely adaptive immune response that controls Mtb replication. The difference between ApoE−/− and LDLR−/− mice might reflect their different cholesterol profiles. With equivalent elevation of total serum cholesterol, LDL cholesterol is higher in LDLR−/− mice while VLDL cholesterol is higher in ApoE−/− mice. Intercellular free cholesterol, which is cytotoxic and could promote neutrophilic inflammation, was not measured in those reports. Dyslipidemia might synergize with DM to further exacerbate TB pathology in human hosts. Such a mechanism, which might account for potential differences in TB susceptibility between type 2 and type 1 diabetics, has yet to be investigated.
Conclusions
By increasing the risk for and severity of TB disease, DM exerts a significant negative impact on public health, particularly in countries where both conditions are prevalent. Given the complexity of diabetic complication mechanisms and the numerous pathways involved, it is likely that the immune response to Mtb infection is affected at multiple levels. Data from mice suggest that an impaired innate response to initial infection with a resulting delay in the adaptive immune effector response is a key mechanism of susceptibility. A refined understanding of the immunological and biochemical basis of TB susceptibility in DM will inform the rational development of implementation and therapeutic strategies to mitigate the dual burden of these diseases. Such investigation can also take advantage of the perturbation in protective immunity caused by DM to reveal critical determinants of the host-pathogen interaction in TB relevant to the general population.
Acknowledgments
This work was supported in part by National Institutes of Health Grant HL081149 (to H.K.).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Gauld WR, Lyall A. Tuberculosis as a complication of diabetes mellitus. Brit Med J. 1947;1:677–679. doi: 10.1136/bmj.1.4506.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Magliano DJ, Zimmet PZL. The worldwide epidemiology of type 2 diabetes mellitus – present and future perspectives. Nat Rev Endocrinol. 2011;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 3.Webb EA, Hesseling AC, Schaaf HS, Gie RP, Lombard CJ, Spitaels A, Delport S, et al. High prevalence of Mycobacterium tuberculosis infection and disease in children and adolescents with type 1 diabetes mellitus. Int J Tuberc Lung Dis. 2009;13:868–874. [PubMed] [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 5.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker MA, Lin HH, Chang HY, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Dis. 2012;54:818–825. doi: 10.1093/cid/cir939. [DOI] [PubMed] [Google Scholar]
- 7.Leegaard A, Riis A, Kornum JB, Prahl JB, Thomsen VO, Sorensen HT, Horsburgh CR, Thomsen RW. Diabetes, glycemic control, and risk of tuberculosis: a population-based case-control study. Diabetes Care. 2011;34:2530–2535. doi: 10.2337/dc11-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Shu W, Wang M, Hou Y, Xia Y, Xu W, Bai L, et al. Pulmonary tuberculosis incidence and risk factors in rural areas of China: a cohort study. PLoS One. 2013;8:e58171. doi: 10.1371/journal.pone.0058171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhaber-Fiebert JD, Jeon CY, Cohen T, Murray MB. Diabetes mellitus and tuberculosis in countries with high tuberculosis burdens: individual risks and social determinants. Int J Epidemiol. 2011;40:417–428. doi: 10.1093/ije/dyq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapur A, Harries AD. The double burden of diabetes and tuberculosis - Public health implications. Diabetes Res Clin Pract. 2013 doi: 10.1016/j.diabres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan V, Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, Srinivasan R, Selvam JM, Kapur A. Prevalence of Diabetes and Pre-Diabetes and Associated Risk Factors among Tuberculosis Patients in India. PLoS One. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis. 2011;91:497–509. doi: 10.1016/j.tube.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Shenoy VP, Bairy I, Srinivasa H, Mukhopadhyay C. Diabetes mellitus and HIV as co-morbidities in tuberculosis patients of rural south India. J Infect Public Health. 2011;4:140–144. doi: 10.1016/j.jiph.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Gray JM, Coh DL. Tuberculosis and HIV coinfection. Semin Respir Crit CareMed. 2013;34:32–43. doi: 10.1055/s-0032-1333469. [DOI] [PubMed] [Google Scholar]
- 15.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kaszinca J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tuberculosis factor α-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 16.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, Ottmani SE, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L, Ferreyra-Reyes L, Delgado-Sanchez G, Bobadilla-Del-Valle M, Canizales-Quintero S, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68:214–220. doi: 10.1136/thoraxjnl-2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Repasy T, Lee J, Marino S, Martinez N, Kirschner DE, Hendricks G, Baker S, et al. Intracellular Bacillary Burden Reflects a Burst Size for Mycobacterium tuberculosis In Vivo. PloS Pathog. 2013;9:e1003190. doi: 10.1371/journal.ppat.1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 20.Horsburgh RC., Jr Priorities for the treatmetn of latent tuberculosis infection in the United States. N Engl J Med. 2004;350:2060–2070. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 21.Saiki O, Negoro S, Tsuyuguchi I, Yamamura Y. Depressed immunological defence mechanisms in mice with experimentally induced diabetes. Infect Immun. 1980;28:127–131. doi: 10.1128/iai.28.1.127-131.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, Saito A. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2005;139:57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugawara I, Mizuno S. Higher susceptibility of type 1 diabetic rats to Mycobacterium tuberculosis infection. Tohoku J Exp Med. 2008;216:363–370. doi: 10.1620/tjem.216.363. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara I, Yamada H, Mizuno S. Pulmonary tuberculosis in spontaneously diabetic go to kakizaki rats. Tohoku J Exp Med. 2004;204:135–145. doi: 10.1620/tjem.204.135. [DOI] [PubMed] [Google Scholar]
- 25.Podell BK, Ackart DF, Kirk NM, Eck SP, Bell C, Basaraba RJ. Non-diabetic hyperglycemia exacerbates disease severity in Mycobacterium tuberculosis infected guinea pigs. PLoS One. 2012;7:e46824. doi: 10.1371/journal.pone.0046824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallerskog T, Martens GW, Kornfeld H. Diabetic Mice Display a Delayed Adaptive Immune Response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez DI, Twahirwa M, Schlesinger LS, Restrepo BI. Reduced Mycobacterium tuberculosis association with monocytes from diabetes patients that have poor glucose control. Tuberculosis (Edinb) 2013;93:192–197. doi: 10.1016/j.tube.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CH, Yu CT, Lin HC, Liu CY, Kuo HP. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis. 1999;79:235–242. doi: 10.1054/tuld.1998.0167. [DOI] [PubMed] [Google Scholar]
- 31.Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 2007;76:44–50. doi: 10.1016/j.diabres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Ferracini M, Martins JO, Campos MR, Anger DB, Jancar S. Impaired phagocytosis by alveolar macrophages from diabetic rats is related to the deficient coupling of LTs to the Fc gamma R signaling cascade. Mol Immunol. 2010;47:1974–1980. doi: 10.1016/j.molimm.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Lecube A, Pachon G, Petriz J, Hernandez C, Simo R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One. 2011;6:e23366. doi: 10.1371/journal.pone.0023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu HF, Zhang HJ, Hu QX, Liu XY, Wang ZQ, Fan JY, Zhan M, Chen FL. Altered polarization, morphology, and impaired innate immunity germane to resident peritoneal macrophages in mice with long-term type 2 diabetes. J Biomed Biotechnol. 2012;2012:867023. doi: 10.1155/2012/867023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun C, Sun L, Ma H, Peng J, Zhen Y, Duan K, Liu G, Ding W, Zhao Y. The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J Cell Physiol. 2012;227:1670–1679. doi: 10.1002/jcp.22891. [DOI] [PubMed] [Google Scholar]
- 36.Mo Y, Wan R, Wang J, Chien S, Tollerud DJ, Zhang Q. Diabetes is associated with increased sensitivity of alveolar macrophages to urban particulate matter exposure. Toxicology. 2009;262:130–137. doi: 10.1016/j.tox.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta} Diabetes. 2005;54:85–91. doi: 10.2337/diabetes.54.1.85. [DOI] [PubMed] [Google Scholar]
- 38.Musilli C, Paccosi S, Pala L, Gerlini G, Ledda F, Mugelli A, Rotella CM, Parenti A. Characterization of circulating and monocyte-derived dendritic cells in obese and diabetic patients. Mol Immunol. 2011;49:234–238. doi: 10.1016/j.molimm.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Surendar J, Mohan V, Pavankumar N, Babu S, Aravindhan V. Increased levels of serum granulocyte-macrophage colony-stimulating factor is associated with activated peripheral dendritic cells in type 2 diabetes subjects (CURES-99) Diabetes Technol Ther. 2012;14:344–349. doi: 10.1089/dia.2011.0182. [DOI] [PubMed] [Google Scholar]
- 40.Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–7119. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang DD, Lin Y, Moreno JR, Randall TD, Khader SA. Profiling early lung immune responses in the mouse model of tuberculosis. PLoSOne. 2011;6:e16161. doi: 10.1371/journal.pone.0016161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, Via LE, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191:773–784. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritter L, Davidson L, Henry M, Davis-Gorman G, Morrison H, Frye JB, Cohen Z, et al. Exaggerated neutrophil-mediated reperfusion injury after ischemic stroke in a rodent model of type 2 diabetes. Microcirculation. 2011;18:552–561. doi: 10.1111/j.1549-8719.2011.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tater D, Tepaut B, Bercovici JP, Youinou P. Polymorphonuclear cell derangements in type I diabetes. Horm Metab Res. 1987;19:642–647. doi: 10.1055/s-2007-1011899. [DOI] [PubMed] [Google Scholar]
- 45.Marhoffer W, Stein M, Schleinkofer L, Federlin K. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res Clin Pract. 1993;19:183–188. doi: 10.1016/0168-8227(93)90112-i. [DOI] [PubMed] [Google Scholar]
- 46.Chanchamroen S, Kewcharoenwong C, Susaengrat W, Ato M, Lertmemongkolchai G. Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect Immun. 2009;77:456–463. doi: 10.1128/IAI.00503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wykretowicz A, Wierusz-Wysocka B, Wysocki J, Szczepanik A, Wysocki H. Impairment of the oxygen-dependent microbicidal mechanisms of polymorphonuclear neutrophils in patients with type 2 diabetes is not associated with increased susceptibility to infection. Diabetes Res Clin Pract. 1993;19:195–201. doi: 10.1016/0168-8227(93)90114-k. [DOI] [PubMed] [Google Scholar]
- 48.Boland OM, Blackwell CC, Clarke BF, Ewing DJ. Effects of ponalrestat, an aldose reductase inhibitor, on neutrophil killing of Escherichia coli and autonomic function in patients with diabetes mellitus. Diabetes. 1993;42:336–340. doi: 10.2337/diab.42.2.336. [DOI] [PubMed] [Google Scholar]
- 49.Ueta E, Osaki T, Yoneda K, Yamamoto T. Prevalence of diabetes mellitus in odontogenic infections and oral candidiasis: an analysis of neutrophil suppression. J Oral Pathol Med. 1993;22:168–174. doi: 10.1111/j.1600-0714.1993.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 50.Toure F, Zahm JM, Garnotel R, Lambert E, Bonnet N, Schmidt AM, Vitry F, et al. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem J. 2008;416:255–261. doi: 10.1042/BJ20080054. [DOI] [PubMed] [Google Scholar]
- 51.Collison KS, Parhar RS, Saleh SS, Meyer BF, Kwaasi AA, Hammami MM, Schmidt AM, et al. RAGE-mediated neutrophil dysfunction is evokded by advanced glycation end products (AGEs) J Leukoc Biol. 2002;71:433–444. [PubMed] [Google Scholar]
- 52.Kierdorf K, Fritz G. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol. 2013;94:55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 53.Myint T, Hoshi S, Ookawara T, Miyazawa N, Suzuki K, Taniguchi N. Immunological detection of glycated proteins in normal and streptozotocin-induced diabetic rats using anti hexitol-lysine IgG. Biochim Biophys Acta. 1995;1272:73–79. doi: 10.1016/0925-4439(95)00067-e. [DOI] [PubMed] [Google Scholar]
- 54.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q, Xiao HP, Cui HY, Sugawara I. Significant increase in natural-killer T cells in patients with tuberculosis complicated by type 2 diabetes mellitus. J Int Med Res. 2011;39:105–111. doi: 10.1177/147323001103900113. [DOI] [PubMed] [Google Scholar]
- 56.Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, Cortes-Penfield N, McCormick JB. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific Th1 and Th17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208:739–748. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Fay MP, Nutman TB, Babu S. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17 and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10:441–449. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van der Ven-Jongekrijg J, Ottenhoff THM, van der Meer JW, Nelwan RH, et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2008;27:97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- 61.Mohan V, Jaydip R, Deepa R. Type 2 diabetes in Asian Indian youth. Pediatr Diabetes. 2007;8 (Suppl 9):28–34. doi: 10.1111/j.1399-5448.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 62.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–286. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 63.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, et al. An Interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol(Lausanne) 2012;3:170. doi: 10.3389/fendo.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birrell MA, McCluskie K, Hardaker E, Knowles R, Belvisi MG. Utility of exhaled nitric oxide as a noninvasive biomarker of lung inflammation in a disease model. Eur Respir J. 2006;28:1236–1244. doi: 10.1183/09031936.00048506. [DOI] [PubMed] [Google Scholar]
- 68.Van Beek SC, Nhung NV, Sy DN, Sterk PJ, Tiemersma EW, Cobelens FG. Measurement of exhaled nitric oxide as a potential screening tool for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2011;15:185–192. [PubMed] [Google Scholar]
- 69.Krisp C, Jacobsen F, McKay MJ, Molloy MP, Steinstraesser L, Wolters DA. Proteome analysis reveals anti-angiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics. 2013 doi: 10.1002/pmic.201200502. [DOI] [PubMed] [Google Scholar]
- 70.Karihaloo A. Anti-fibrosis therapy and diabetic nephropathy. Curr Diab Rep. 2012;12:414–422. doi: 10.1007/s11892-012-0290-7. [DOI] [PubMed] [Google Scholar]
- 71.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 72.Xing D, Gong K, Feng W, Nozell SE, Chen YF, Chatham JC, Oparil S. O-GlcNAc modification of NFkappaB p65 inhibits TNF-alpha-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS One. 2011;6:e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rachman H, Kim N, Ulrichs T, Baumann S, Pradl L, Nasser EA, Bild M, et al. Critical role of methylglyoxal and AGE in mycobacteria-induced macrophage apoptosis and activation. PLoS One. 2006;1:e29. doi: 10.1371/journal.pone.0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 76.Intine RV, Sarras MP., Jr Metabolic memory and chronic diabetes complications: potential role for epigenetic mechansisms. Curr Diab Rep. 2012;12:551–559. doi: 10.1007/s11892-012-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation. Diabetes. 2008;57:3189–3198. doi: 10.2337/db08-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miao F, Wu X, Zhang L, Yuan YC, Riggs AD, Natarajan R. Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J Biol Chem. 2007;282:13854–13863. doi: 10.1074/jbc.M609446200. [DOI] [PubMed] [Google Scholar]
- 79.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 80.Sorbinil Retinopathy Trial Research Group. A randomized trial of sorbinil an aldose reductase inhibitor in diabetic retinopathy. Arch Opthalmol. 1990;108:1234–1244. doi: 10.1001/archopht.1990.01070110050024. [DOI] [PubMed] [Google Scholar]
- 81.Campochirao PA. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Opthalmol Vis Sci. 2004;45:922–931. doi: 10.1167/iovs.03-0955. [DOI] [PubMed] [Google Scholar]
- 82.Cameron NE, Gibson TM, Nangle TR, Cotter MA. Inhibitors of advance glycation end product formation and neurovascular dysfunction in experimental diabetes. Ann NYAcad Sci. 2005;1043:784–792. doi: 10.1196/annals.1333.091. [DOI] [PubMed] [Google Scholar]
- 83.Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, Perego C, Muscogiuri G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7:313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 84.Jee SH, Golub JE, Jo J, Park IS, Ohrr H, Samet JM. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol. 2009;170:1478–1485. doi: 10.1093/aje/kwp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin d deficiency and tuberculosis progression. Emerg Infect Dis. 2010;16:853–855. doi: 10.3201/eid1605.091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reed GW, Choi H, Lee SY, Lee M, Kim Y, Park H, Lee J, et al. Impact of diabetes and smoking on mortality in tuberculosis. PLoS One. 2013;8:e58044. doi: 10.1371/journal.pone.0058044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng Y, Kong Y, Barnes PF, Huang FF, Klucar P, Wang X, Samten B, et al. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun. 2011;79:229–237. doi: 10.1128/IAI.00709-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim S, Kim MJ, Choi SH, Shin CS, Park KS, Jang HC, Billings LK, Meigs JB. Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. Am J Clin Nutr. 2013;97:524–530. doi: 10.3945/ajcn.112.048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martens GW, Vallerskog T, Kornfeld H. Hypercholesterolemic LDL receptor-deficient mice mount a neutrophilic response to tuberculosis despite the timely expression of protective immunity. J Leukoc Biol. 2012 doi: 10.1189/jlb.0311164. [DOI] [PMC free article] [PubMed] [Google Scholar]