Abstract
Ginseng is widely used for its promising healing and restorative properties as well as for its possible tonic effect in traditional medicine. Nowadays, many studies focus on purified individual ginsenoside, an important constituent in ginseng, and study its specific mechanism of action instead of whole-plant extracts on cardiovascular diseases (CVDs). Of the various ginsenosides, purified ginsenosides such as Rb1, Rg1, Rg3, Rh1, Re, and Rd are the most frequently studied. Although there are many reports on the molecular mechanisms and medical applications of ginsenosides in the treatment of CVDs, many concerns exist in their application. This review discusses current works on the countless pharmacological functions and the potential benefits of ginseng in the area of CVDs. Results: Both in vitro and in vivo results indicate that ginseng has potentially positive effects on heart disease through its various properties including antioxidation, reduced platelet adhesion, vasomotor regulation, improving lipid profiles, and influencing various ion channels. To date, approximately 40 ginsenosides have been identified, and each has a different mechanism of action owing to the differences in chemical structure. This review aims to present comprehensive information on the traditional uses, phytochemistry, and pharmacology of ginseng, especially in the control of hypertension and cardiovascular function. In addition, the review also provides an insight into the opportunities for future research and development on the biological activities of ginseng.
Keywords: antioxidant effect, cardiovascular diseases, lipid profile, myocardial protection, vasomotor tone
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death globally. According to the World Health Organization, CVD was responsible for 30% of all deaths in 2005. Although typically considered a disease of developed countries, its incidence is increasing in the developing world as well. CVD usually stems from vascular dysfunction, for example, as a result of atherosclerosis, thrombosis, or high blood pressure, which then compromises organ function. Most notably, the heart and brain can be affected, as in myocardial infarction and stroke, respectively. In the past few decades, major improvements have been made in treating some types of CVD. However, new treatment options are urgently needed for all types of CVD. Moreover, improving diagnosis is crucial, because by detecting the early stages of disease, the focus of therapy could be shifted from treatment to prevention [1]. CVD is the leading cause of morbidity and mortality in millions of people around the world, which include a variety of diseases such as peripheral vascular disease, coronary artery disease, heart failure, dyslipidemias, and hypertension [2]. People of all races, age, and gender suffer commonly from these diseases. Heart failure, myocardial rupture, or arrhythmia is a result of myocardial necrosis following infarction [3]. Myocardial infarction and sudden death continue to remain as one of the leading causes of morbidity and mortality in many countries, despite vast advances in the past five decades. In addition, risk factors such as cigarette smoking, elevated low-density lipoprotein cholesterol, low levels of high-density lipoprotein cholesterol, diabetes mellitus, and hypertension are the primary causes of CVD [4]. Recent studies elucidate that vascular inflammation may also manifest in atherosclerosis and coronary artery disease [5]. Endothelial dysfunction has been stimulated by risk factors involved in CVD, such as expression of adhesion molecules by these dysfunctional endothelial cells, which promote the binding and influx of T cells and mast cells [6]. An inflammatory condition within the arterial wall is created by interleukins, cytokines, and reactive oxygen species (ROS) produced by white blood cells. Low-density lipoprotein is an atherogenic lipoprotein that accesses the subendothelial space and undergoes oxidative modification when trapped in the intercellular matrix [7].
Panax ginseng is a traditional herbal medicine that has been used therapeutically for more than 2000 years. It is the most valuable of all medicinal plants, especially in Korea, China, and Japan. The name panax means “all healing,” and has possibly stemmed from traditional belief that the various properties of ginseng can heal all aspects of the illness encountered by the human body (i.e., it acts as a panacea for the human body). Among the ginseng species, Korean ginseng (P. ginseng), Chinese ginseng (Panax notoginseng), and American ginseng (Panax quinquefolius) are the most common throughout the world. Numerous studies focus on the research of individual ginsenosides instead of using whole ginseng extract against various diseases [8–13]. Of the various ginsenosides, Rb1, Rg1, Rg3, Re, and Rd are the most frequently studied [13].
This review describes the medicinal potentials of using ginseng and ginsenosides in the treatment of CVD. The review explores recent studies carried out to understand the mechanisms that lead to various diseases and discusses the implications of these advances for identifying new therapeutic targets and developing new therapeutic strategies, including the potential use of ginseng and its metabolite (i.e., ginsenosides) for treating CVD.
2. Efficacy of ginseng in improving circulation and antioxidant activity
Ginseng and ginsenosides have vasorelaxation, antioxidative, anti-inflammatory, and anticancer properties. In addition, ginsenosides have also shown to have an effect on the nervous system [14]. Moreover, ginseng has shown more benefit in individuals with diseases compared with healthy individuals [15–17]. In addition, a previous study supported its growing evidence for its indications in CVDs [12]. P. ginseng roots and extracts have been traditionally used by Koreans to renew the body and mind, and improve physical condition. Ginseng is also widely used in individuals with cardiovascular risk factors such as hypertension and hypercholesterolemia. Cardiac ischemia can cause myocardial injury that leads to the production of ROS, and in such cases, treatment with ginseng restores coronary blood flow to normal levels [18]. Alteration or loss of cellular function results in nonspecific damage to lipids, proteins, and DNA by ROS. The life span of animals bearing a tumor has gradually increased after ginseng treatment [19]. Oxidation-induced damage of erythrocyte membrane was reduced by ginsenosides Rg2 and Rh1 [20], and the energy metabolism and protection of the mitochondria have been effectively regulated by polysaccharides from P. ginseng [21]. Facilitation of antioxidant effect through Nrf2 and levels of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase were significantly increased by ginseng [22,23].
Ginsenosides protect from myocardial reperfusion injury by increasing 6-keto-prostaglandin F1α production and decreasing lipid peroxidation [24]. Rabbit pulmonary endothelium was protected from ROS toxicity by ginsenosides [8]. In addition, ginseng prevented ROS toxicity by stimulating nitric oxide (NO) production. Endothelial dysfunction was induced by homocysteine and human immunodeficiency virus protease inhibitors; however, these were successfully blocked by ginsenoside Rb1 and other ginsenosides by inhibiting the production of ROS [25,26]. Ginsenoside Re is a potent antioxidant that protects cardiomyocytes against oxidant-mediated injury. Such protection is, at least in part, mediated by its radical scavenging properties, especially for H2O2 and hydroxyl radicals. As a major constituent in ginseng extract, ginsenoside Re may play an important role in antioxidant actions to increase cardiomyocyte survival and contractile function during ischemia and reperfusion [27,28]. These results suggest that ginsenoside Re functions as an antioxidant, protecting cardiomyocytes from oxidant injury induced by both exogenous and endogenous oxidants, and that its protective effects may be mostly attributed to scavenging H2O2 and hydroxyl radicals.
3. Efficacy of ginseng in modulating vascular function
Interestingly, when glucose is attached to the 20th carbon of dammarane triterpene, such as ginsenosides Re, Rd, and R1, the ginsenosides acted as an antioxidant. By contrast, if no sugar moieties were attached to the 20th carbon of the ginsenosides such as Rg3, Rh2, and Rg2, the ginsenosides acted as a prooxidant. In ginsenosides such as Rh1, glucose is attached to the sixth carbon instead of the 20th, and in this case, the ginsenoside acts as an antioxidant only [29]. All these aggregated reports revealed that the prevention of ROS generation by ginseng may be an important milestone in the prevention of oxidative damage. Ginsenoside Rb1 has protective effects on human umbilical vein endothelial cells in vitro [30]. Water extract of Korean red ginseng stimulates angiogenesis by activating the phosphoinositol-3-kinase (PI3K)/Akt-dependent extracellular signal-regulated kinase 1/2 and endothelial nitric oxide synthase (eNOS) pathways in human umbilical vein endothelial cells [31]. Angiomodulatory and neurological effects are also shown by ginsenosides [32]. One study shows that potassium channels of vascular smooth muscle cells have been activated by ginsensoside Re through the PI3K/Akt and NO pathways [33]. Another study shows that ginsenoside Re has nongenomic effects in endothelial cells through the glucocorticoid receptor (GR) [34]. Capillary morphogenesis was attenuated by ginsenoside Rb1 [35]. Another in vitro study revealed the enhancement of vascular endothelial cell proliferation and migration by extracts of P. ginseng and P. notoginseng [36]. Saponin from P. notoginseng shows angiogenic effects on both human umbilical vein endothelial cells and in zebrafish models [37]. It is also reported that atherosclerotic lesions in apolipoprotein E (ApoE)-deficient mice and tumor necrosis factor-alpha-induced endothelial adhesion molecule expression have been reduced by P. notoginseng [38]. Production of NO was increased by ginsenoside Rg3 by increasing phosphorylation and expression of eNOS [39]. In human umbilical vein endothelial cells, fibroblast growth factor-induced angiogenesis was inhibited by compound K through the modulation of p38 mitogen-activated protein kinase (PK) and Akt [40]. The aforementioned reports propose that the saponin extracted from ginseng protects vascular endothelial cells through the NO-, Akt-, and GR-mediated signaling pathways. Effects of ginseng and ginsenosides have been sufficiently studied on the cardiovascular system. Through the production and release of NO, endothelium regulates blood vessel tone [41–43]. Production of NO has been stimulated by ginsenosides by a number of ways. It is reported that NO production in human aortic endothelial cells was induced by purified ginsenoside Rb1 [44]. Ginsenoside stimulates NO release in human umbilical vein endothelial cells by phosphorylation of GR, PI3K, Akt/PKB, and eNOS [45]. In isolated canine corpus cavernosum model, ginsenoside Rg3 induced vasodilation [46], which shows that arterial stiffness has been improved by Korean red ginseng and ginsenosides [47].
4. Efficacy of ginseng in adjusting vasomotor functions
Vascular smooth muscle dysfunction and the inhibition of angiotensin II-induced proliferation were stimulated by ginsenoside Rg3 [48,49]. In addition, Korean red ginseng improves arterial stiffness in hypertension [50]. Overall, these results show the improvement in vasomotor function by ginseng. It has initially been thought that ginseng may increase blood pressure to harmful levels. However, previous studies have shown that ginseng cures patients with low blood pressure, restoring it to normal levels. In addition, ginseng also reduces blood pressure in patients with high blood pressure [51]. The blood pressure lowering activity of Korean ginseng is attributed to the production of vascular endothelial cell-derived NO [52]. Recent studies have shown that ginseng has biochemical and pharmacological activities beneficial for blood pressure control, where lower doses have greater antihypertensive effects than higher doses [53], and improve blood circulation through vasodilation [52]. The antihypertensive effect of ginseng is mediated by the inhibition of myogenic responses on the blood vessels [54]. In addition, ginseng protects against tissue damage and is also a novel therapy for heart failure [55].
5. Efficacy of ginseng in improving cardiac functions
Saponins from P. notoginseng protected the heart against doxorubicin-induced cardiotoxicity [56] and blocked the cardiac hypertrophy induced by monocrotaline in rats [57]. Left ventricular hypertrophy produced by aortic coarctation was protected by ginsenoside Rg1 through NO functions [58]. Electromechanical alternans was suppressed by ginsenoside Re in cardiomyocytes [59], and myocardial infarction after ischemia and reperfusion was preconditionally protected by ginsenoside Rb1 [60]. Another study showed that ginsenoside Rg1 inhibits left ventricular hypertrophy [61]. P. ginseng also suppresses apoptosis in neonatal cardiocytes by modulating Bcl-2 and caspase-3 activities during hypoxia and reperfusion [62]. Furthermore, cardiomyocytes have been protected by ginsenoside Rg1 from oxidative injury through antioxidation and intracellular calcium modulation [63]. Total saponin, panaxadiol, and panaxatriol from ginseng have been able to protect cardiomyocytes from ischemia and reperfusion injuries [64]. Cardiac injury in diabetes induced by streptozotocin has been prevented by ginsenoside Rb1 [65] and unfavorable postmyocardial remodeling was reduced by ginseng [66]. Some studies suggest that cardiac hypertrophy and heart failure are prevented by ginseng through Nhe-1 modulation and reduction of calcineurin activation [67]. Recent studies also show that cardiac protection by NO was facilitated by compound K through the Akt/PI3K pathway [68]. Acute cardiac injury from ischemia and reperfusion has been protected through the GR and estrogen receptor-activated risk pathway by the eNOS-dependent mechanism in rats [69]. Thus, these studies suggest that ginseng preserves heart function after myocardial tissue deterioration.
6. Efficacy of ginseng in inhibiting platelet aggregation
Increasing evidence proves the ability of ginseng to inhibit platelet aggregation. The red ginseng has a direct inhibitory effect on platelet aggregation in in vivo antithrombotic and ex vivo antiplatelet models, and this could correlate with its ability to increase NO production. It has been reported that the saponin fraction of Korean red ginseng enhances the formation of citrulline from exogenously added arginine, which activates NOS, and purified ginsenosides from ginseng enhanced the release of NO from endothelial cells of the rat aorta. Korean red ginseng also shows a significant protective effect on arterial thrombosis in vivo, which may be due to antiplatelet activity rather than anticoagulation activity, and this result suggests that red ginseng intake may be beneficial for individuals with high risks of thrombosis and CVDs [70–72]. Dihydroginsenoside Rg3 potently inhibited platelet aggregation through the modulation of downstream signaling components such as cyclic adenosine monophosphate and extracellular signal-regulated kinase 2 [73]. Protopanaxadiol or protopanaxatriol-type ginsenosides have a complicated effect on hemin-induced hemolysis, which depends on the interaction between the sugar moieties at different positions [74].
Post-treatment with P. notoginseng significantly reduced the lipopolysaccharide-mediated microcirculatory disturbance by inhibiting adherence of leukocytes to the venular wall, degranulation of mast cells, and the release of cytokines [75]. A total of seven ginsenosides, namely Rg6, F4, Rk3, Rh4, Rs3, Rs4, and Rs5, isolated from processed ginseng were evaluated for their effects on platelet aggregation induced by adenosine diphosphate (ADP), collagen, arachidonic acid, and U46619 (thromboxane A2 mimetic drug). The acetylated ginsenosides such as Rs3, Rs4, and Rs5 only had mild effects on aggregation induced by four stimulators. Some of the ginsenosides including Rg6, F4, Rh4, Rs3, and Rs5 showed negligible effects on ADP and collagen-induced platelet aggregation [76]. There are some synergistic interactions between Korean red ginseng and warfarin in patients with cardiac valve replacement. Korean red ginseng could be used with close monitoring and under appropriate instruction in patients who take warfarin during cardiac valve replacement. Because such patients could take higher amounts of Korean red ginseng along with warfarin, this combination can also be applied in cardiac valve treatment [77]. Coronary perfusion flow of isolated heart can be increased by total ginsenosides, which also protected heart tissues from ischemia/reperfusion injury. This effect of total ginsenosides is mediated by activation of PI3K/Akt-eNOS signaling and NO production [78]. These results suggest that ginseng has a potent antithrombotic effect in vivo, which may be due to the antiplatelet activity rather than the anticoagulation activity, and that ginseng intake may be beneficial for individuals with high risks of thrombosis and CVDs.
7. Efficacy of ginseng in adjusting lipid profile
Patients with myocardial ischemia showed an improvement in coronary flow following treatment with red ginseng extract [79]. Thus, this result shows that blood circulation was significantly improved by the anticoagulant properties of ginseng. It is well-known that the hypolipidemic and hypoglycemic effects of red ginseng were dramatically increased by the bifidus fermentation process [80]. Although hypercholesterolemia increases platelet aggregation, using Korean red ginseng reduces the aggregation through the suppression of diacylglycerol liberation in a high-cholesterol diet [81]. Saponins from P. notoginseng decrease atherosclerosis by regulating the lipid with its anti-inflammatory effects [82], and total Panax notoginsenosides was reported to inhibit atherosclerosis in ApoE-knockout mice [83]. In foam cells, cholesterol ester can be reduced by saponins from P. notoginseng by modulating the adenosine triphosphate-binding cassette transporter A1 [84]; in addition, acidic polysaccharides from Korean red ginseng were also reported to possess antihyperlipidemic activity [85]. Atherosclerosis in ApoE-knockout mice was inhibited by the action of ginsenoside Rd [86] as well. These findings suggest the antihyperlipidemic effect of P. ginseng.
8. Efficacy of ginseng in regulating Ca2+ channels
Previous studies have shown that ginsenoside Rd treatment attenuates basilar hypertrophic inward remodeling in 2k2c hypertensive rats without affecting systemic blood pressure. Ginsenoside Rd reversed the increase in store-operated Ca2+ channel (SOCC) or receptor-operated Ca2+ channel (ROCC) but not in voltage-dependent Ca2+ channel–mediated Ca2+ entry. In vitro, ginsenoside Rd concentration dependently inhibited endothelin-1-induced basilar arterial vascular smooth muscle cells (BAVSMCs) proliferation and Mn2+ quenching rate within the same concentration range as required for inhibition of increased SOCC- or ROCC-mediated Ca2+ entries during hypertension. These results provide in vivo evidence for attenuation of hypertensive cerebrovascular remodeling after ginsenoside Rd treatment. The underlying mechanism might be associated with inhibitory effects of ginsenoside Rd on voltage-independent Ca2+ entry and BAVSMC proliferation [87]. Intracellular Ca2+ is the central regulator of cardiac contractility. Moreover, it is becoming increasingly apparent that alterations in myocyte Ca2+ regulation may be critically important in both the mechanical dysfunction and arrhythmogenesis associated with congestive heart failure. Thus, it is imperative to have a clear and relatively quantitative understanding of how cellular Ca2+ levels are regulated during the normal contraction–relaxation cycle. During the cardiac action potential, L-type Ca2+ channels are activated and Ca2+ enters the cell through Ca2+ current (ICa); a much smaller amount of Ca2+ also enters by Na+–Ca2+ exchange (NCX). The Ca2+ influx triggers Ca2+ release from the sarcoplasmic reticulum (SR) and, to some extent, can also directly contribute to activation of the myofilaments. The Ca2+ entry along with the amount released from the SR through the Ca2+-induced Ca2+ release increases cytosolic-free [Ca2+] ([Ca2+]i), resulting in the binding of Ca2+ to multiple cytosolic Ca2+ buffers. One of the most functionally important cytosolic Ca2+ buffers is the thin-filament protein troponin C (TnC). When Ca2+ binds to TnC, it switches on the myofilaments in a cooperative manner, thereby activating contraction. For relaxation and diastolic filling to occur, [Ca2+]i must decline such that Ca2+ dissociates from TnC, thereby turning off the contractile machinery. The following four Ca2+ transporters remove Ca2+ from the cytosol: (1) SR Ca2+–adenosine triphosphatase (Ca2+–ATPase), (2) sarcolemmal NCX, (3) sarcolemmal Ca2+–ATPase, and (4) mitochondrial Ca2+ uniporter. The SR Ca2+–ATPase and NCX are the most important quantitatively [88]. Because cardiac functions are carried out by the calcium ions [Ca2+], these are crucial for the modulation of intracellular calcium signaling. Intracellular Ca2+ levels are tightly regulated by the Ca2+-activated signaling pathways (Fig. 1).
Fig. 1.
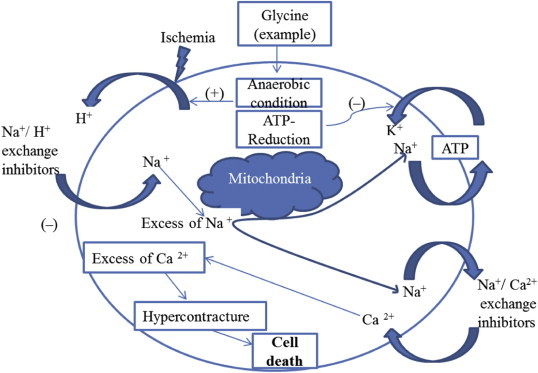
Pathogenesis of hypercontracture after cardiac ischemia/reperfusion during the overload of sodium and calcium. ATP, adenosine triphosphate.
Ginsenosides with sugar moieties attached only to the C-3 position of the steroid-like structure, equivalent to the sugar position in cardiac glycosides, have an inhibitory effect on Na+/K+–ATPase activity. However, their inhibitory potency was significantly reduced when a monosaccharide was linked to the C-6 or C-20 position of the steroid-like structure; replacement of the monosaccharide with a disaccharide molecule at either position caused the disappearance of the inhibitory potency. Molecular modeling and docking confirmed that the difference in Na+/K+–ATPase inhibitory potency among ginsenosides was due to the steric hindrance of sugar attachment at the C-6 and C-20 positions of the steroid-like structure. The cardiac therapeutic effects of ginseng and San Qi should be at least partly attributed to the effective inhibition of Na+/K+–ATPase by their metabolized ginsenosides with sugar moieties attached only to the C-3 position of the steroid-like structure [89].
9. Concluding remarks
This review summarized current information about the efficacy of ginseng on major cardiovascular risk factors such as hypertension, cardiac disease, hyperlipidemia, oxidative stress, and ion regulation. Ginseng is a traditional herbal remedy whose antiquity stretches back to ancient times. The active constituent ginsenosides play a vital role in the medicinal effects of ginseng. Ginsenosides exhibit their vast range of activities on CVD through the inhibition of ROS production, stimulation of NO production, improvement in blood circulation, enhancement of vasomotor tone, and regulation of the lipid profile. However, the exact mechanisms of action of ginsenosides are still unidentified. In the future, each ginsenoside must be studied on its specific mechanism of action on CVD. The common use of ginseng as an herbal remedy requires strict investigations to assess both its efficacy and its safety.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Gielen S., Landmesser U. The Year in Cardiology 2013: cardiovascular disease prevention. Eur Heart J. 2014;35:307–312. doi: 10.1093/eurheartj/eht551. [DOI] [PubMed] [Google Scholar]
- 2.Lim K.H., Ko D., Kim J.H. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. J Ginseng Res. 2013;37:273–282. doi: 10.5142/jgr.2013.37.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunstall-Pedoe H., Vanuzzo D., Hobbs M., Mähönen M., Cepaitis Z., Kuulasmaa K., Keil U. Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet. 2000;355:688–700. doi: 10.1016/s0140-6736(99)11181-4. [DOI] [PubMed] [Google Scholar]
- 4.Toth P.P. Making a case for quantitative assessment of cardiovascular risk. J Clin Lipidol. 2007;1:234–241. doi: 10.1016/j.jacl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Act local, act global: inflammation and the multiplicity of “vulnerable” coronary plaques. J Am Coll Cardiol. 2005;45:1600–1602. doi: 10.1016/j.jacc.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 6.Davies M.J., Gordon J.L., Gearing A.J., Pigott R., Woolf N., Katz D., Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I., Williams K.J., Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 8.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 9.Buettner C., Yeh G.Y., Phillips R.S., Mittleman M.A., Kaptchuk T.J. Systematic review of the effects of ginseng on cardiovascular risk factors. Ann Pharmacother. 2006;40:83–95. doi: 10.1345/aph.1G216. [DOI] [PubMed] [Google Scholar]
- 10.Lim K.H., Lim D.J., Kim J.H. Ginsenoside-Re ameliorates ischemia and reperfusion injury in the heart: a hemodynamics approach. J Ginseng Res. 2013;37:283–292. doi: 10.5142/jgr.2013.37.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W., Chai H., Lin P.H., Lumsden A.B., Yao Q., Chen C.J. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit. 2004;10:187–192. [PubMed] [Google Scholar]
- 13.Cheng Y., Shen L.H., Zhang J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 14.Nah S.Y., Kim D.H., Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brekhman I.I., Dardymov I.V. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9:419–430. doi: 10.1146/annurev.pa.09.040169.002223. [DOI] [PubMed] [Google Scholar]
- 16.Bittles A.H., Fulder S.J., Grant E.C., Nicholls M.R. The effect of ginseng on lifespan and stress responses in mice. Gerontology. 1979;25:125–131. doi: 10.1159/000212330. [DOI] [PubMed] [Google Scholar]
- 17.Zhou D.H. Preventive geriatrics: an overview from traditional Chinese medicine. Am J Chin Med. 1982;10:32–39. doi: 10.1142/S0192415X82000063. [DOI] [PubMed] [Google Scholar]
- 18.Bolli R. Superoxide dismutase 10 years later: a drug in search of a use. J Am Coll Cardiol. 1991;18:231–233. doi: 10.1016/s0735-1097(10)80244-x. [DOI] [PubMed] [Google Scholar]
- 19.Miller S.C., Delorme D., Shan J.J. CVT-E002 stimulates the immune system and extends the life span of mice bearing a tumor of viral origin. J Soc Integr Oncol. 2009;7:127–136. [PubMed] [Google Scholar]
- 20.Samukawa K., Suzuki Y., Ohkubo N., Aoto M., Sakanaka M., Mitsuda N. Protective effect of ginsenosides Rg(2) and Rh(1) on oxidation-induced impairment of erythrocyte membrane properties. Biorheology. 2008;45:689–700. [PubMed] [Google Scholar]
- 21.Li X.T., Chen R., Jin L.M., Chen H.Y. Regulation on energy metabolism and protection on mitochondria of Panax ginseng polysaccharide. Am J Chin Med. 2009;37:1139–1152. doi: 10.1142/S0192415X09007454. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Ichikawa T., Jin Y., Hofseth L.J., Nagarkatti P., Nagarkatti M., Windust A., Cui T. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J Ethnopharmacol. 2010;130:222–230. doi: 10.1016/j.jep.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn S.H., Kim S.K., Kim Y.O., Kim H.D., Shin Y.S., Yang S.O., Kim S.Y., Lee S.W. A comparison of antioxidant activity of Korean White and Red Ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J Ginseng Res. 2013;37:442–450. doi: 10.5142/jgr.2013.37.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996;23:728–732. doi: 10.1111/j.1440-1681.1996.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhou W., Chai H., Lin P.H., Lumsden A.B., Yao Q., Chen C. Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2005;41:861–868. doi: 10.1016/j.jvs.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Chai H., Yao Q., Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–499. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 27.Xie J.T., Shao Z.H., Vanden Hoek T.L., Chang W.T., Li J., Mehendale S., Wang C.Z., Hsu C.W., Becker L.B., Yin J.J. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Deng H.L., Zhang J.T. Anti-lipid peroxilative effect of ginsenoside Rb1 and Rg1. Chin Med J (Engl) 1991;104:395–398. [PubMed] [Google Scholar]
- 29.Lü J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He F., Guo R., Wu S.L., Sun M., Li M. Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol. 2007;50:314–320. doi: 10.1097/FJC.0b013e3180cab12e. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y.M., Namkoong S., Yun Y.G., Hong H.D., Lee Y.C., Ha K.S., Lee H., Kwon H.J., Kwon Y.G., Kim Y.M. Water extract of Korean red ginseng stimulates angiogenesis by activating the Pi3k/Akt-dependent Erk1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull. 2007;30:1674–1679. doi: 10.1248/bpb.30.1674. [DOI] [PubMed] [Google Scholar]
- 32.Leung K.W., Yung K.K., Mak N.K., Yue P.Y., Luo H.B., Cheng Y.K., Fan T.P., Yeung H.W., Ng T.B., Wong R.N. Angiomodulatory and neurological effects of ginsenosides. Curr Med Chem. 2007;14:1371–1380. doi: 10.2174/092986707780597916. [DOI] [PubMed] [Google Scholar]
- 33.Nakaya Y., Mawatari K., Takahashi A., Harada N., Hata A., Yasui S. The phytoestrogen ginsensoside Re activates potassium channels of vascular smooth muscle cells through Pi3k/Akt and nitric oxide pathways. J Med Invest. 2007;54:381–384. doi: 10.2152/jmi.54.381. [DOI] [PubMed] [Google Scholar]
- 34.Leung K.W., Leung F.P., Huang Y., Mak N.K., Wong R.N. Non-genomic effects of ginsenoside-Re in endothelial cells via glucocorticoid receptor. FEBS Lett. 2007;581:2423–2428. doi: 10.1016/j.febslet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 35.Papapetropoulos A. A ginseng-derived oestrogen receptor beta (Erbeta) agonist, Rb1 ginsenoside, attenuates capillary morphogenesis. Br J Pharmacol. 2007;152:172–174. doi: 10.1038/sj.bjp.0707360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei Y., Gao Q., Chen K.J. Effects of extracts from Panax notoginseng and Panax ginseng fruit on vascular endothelial cell proliferation and migration in vitro. Chin J Integr Med. 2008;14:37–41. doi: 10.1007/s11655-008-0037-0. [DOI] [PubMed] [Google Scholar]
- 37.Hong S.J., Wan J.B., Zhang Y., Hu G., Lin H.C., Seto S.W., Kwan Y.W., Lin Z.X., Wang Y.T., Lee S.M. Angiogenic effect of saponin extract from Panax notoginseng on HUVECs in vitro and zebrafish in vivo. Phytother Res. 2009;23:677–686. doi: 10.1002/ptr.2705. [DOI] [PubMed] [Google Scholar]
- 38.Wan J.B., Lee S.M., Wang J.D., Wang N., He C.W., Wang Y.T., Kang J.X. Panax notoginseng reduces atherosclerotic lesions in ApoE-deficient mice and inhibits TNF-alpha-induced endothelial adhesion molecule expression and monocyte adhesion. J Agric Food Chem. 2009;57:6692–6697. doi: 10.1021/jf900529w. [DOI] [PubMed] [Google Scholar]
- 39.Hien T.T., Kim N.D., Pokharel Y.R., Oh S.J., Lee M.Y., Kang K.W. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: essential roles of estrogen receptor-dependent Pi3-kinase and Amp-activated protein kinase. Toxicol Appl Pharmacol. 2010;246:171–183. doi: 10.1016/j.taap.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Jeong A., Lee H.J., Jeong S.J., Lee H.J., Lee E.O., Bae H., Kim S.H. Compound K inhibits basic fibroblast growth factor-induced angiogenesis via regulation of P38 mitogen activated protein kinase and Akt in human umbilical vein endothelial cells. Biol Pharm Bull. 2010;33:945–950. doi: 10.1248/bpb.33.945. [DOI] [PubMed] [Google Scholar]
- 41.Furchgott R.F., Vanhoutte P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;53:557–573. [PubMed] [Google Scholar]
- 42.Ignarro L.J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 44.Yu J., Eto M., Akishita M., Kaneko A., Ouchi Y., Okabe T. Signaling pathway of nitric oxide production induced by ginsenoside Rb1 in human aortic endothelial cells: a possible involvement of androgen receptor. Biochem Biophys Res Commun. 2007;353:764–769. doi: 10.1016/j.bbrc.2006.12.119. [DOI] [PubMed] [Google Scholar]
- 45.Leung K.W., Cheng Y.K., Mak N.K., Chan K.K., Fan T.P., Wong R.N. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–3216. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 46.Kang Y.J., Sohn J.T., Chang K.C. Relaxation of canine corporal smooth muscle relaxation by ginsenoside saponin Rg3 is independent from eNOS activation. Life Sci. 2005;77:74–84. doi: 10.1016/j.lfs.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Jovanovski E., Jenkins A., Dias A.G., Peeva V., Sievenpiper J., Arnason J.T., Rahelic D., Josse R.G., Vuksan V. Effects of Korean red ginseng (Panax Ginseng C.A. Mayer) and its isolated ginsenosides and polysaccharides on arterial stiffness in healthy individuals. Am J Hypertens. 2010;23:469–472. doi: 10.1038/ajh.2010.5. [DOI] [PubMed] [Google Scholar]
- 48.Lee J.Y., Lim K.M., Kim S.Y., Bae O.N., Noh J.Y., Chung S.M., Kim K., Shin Y.S., Lee M.Y., Chung J.H. Vascular smooth muscle dysfunction and remodeling induced by ginsenoside Rg3, a bioactive component of ginseng. Toxicol Sci. 2010;117:505–514. doi: 10.1093/toxsci/kfq201. [DOI] [PubMed] [Google Scholar]
- 49.Wang T., Yu X.F., Qu S.C., Xu H.L., Sui D.Y. Ginsenoside Rb3 inhibits angiotensin II-induced vascular smooth muscle cells proliferation. Basic Clin Pharmacol Toxicol. 2010;107:685–689. doi: 10.1111/j.1742-7843.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- 50.Rhee M.Y., Kim Y.S., Bae J.H., Nah D.Y., Kim Y.K., Lee M.M., Kim H.Y. Effect of Korean red ginseng on arterial stiffness in subjects with hypertension. J Altern Complement Med. 2011;17:45–49. doi: 10.1089/acm.2010.0065. [DOI] [PubMed] [Google Scholar]
- 51.Jeon B.H., Kim C.S., Park K.S., Lee J.W., Park J.B., Kim K.J., Kim S.H., Chang S.J., Nam K.Y. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen Pharmacol. 2000;35:135–141. doi: 10.1016/s0306-3623(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 52.Shin W., Yoon J., Oh G.T., Ryoo S. Korean red ginseng inhibits arginase and contributes to endothelium-dependent vasorelaxation through endothelial nitric oxide synthase coupling. J Ginseng Res. 2013;37:64–73. doi: 10.5142/jgr.2013.37.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuksan V., Stavro M., Woo M., Leiter L.A., Sung M.K., Sievenpiper J.L. Proceedings of the 9th International Ginseng Symposium. Korean Society of Ginseng; Geumsan: 2006. Korean red ginseng (Panax ginseng) can lower blood pressure in individuals with hypertension: a randomized controlled trial; pp. 35–36. [Google Scholar]
- 54.Baek E.B., Yoo H.Y., Park S.J., Chung Y.S., Hong E.K., Kim S.J. Inhibition of arterial myogenic responses by a mixed aqueous extract of Salvia miltiorrhiza and Panax notoginseng (PASEL) showing antihypertensive effects. Korean J Physiol Pharmacol. 2009;13:287–293. doi: 10.4196/kjpp.2009.13.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner H.N., Liu X. Pergamon Press; New York: 1987. The international textbook of cardiology. [Google Scholar]
- 56.Liu L., Shi R., Shi Q., Cheng Y., Huo Y. Protective effect of saponins from Panax notoginseng against doxorubicin-induced cardiotoxicity in mice. Planta Med. 2008;74:203–209. doi: 10.1055/s-2008-1034303. [DOI] [PubMed] [Google Scholar]
- 57.Qin N., Gong Q.H., Wei L.W., Wu Q., Huang X.N. Total ginsenosides inhibit the right ventricular hypertrophy induced by monocrotaline in rats. Biol Pharm Bull. 2008;31:1530–1535. doi: 10.1248/bpb.31.1530. [DOI] [PubMed] [Google Scholar]
- 58.Deng J., Wang Y.W., Chen W.M., Wu Q., Huang X.N. Role of nitric oxide in ginsenoside Rg(1)-induced protection against left ventricular hypertrophy produced by abdominal aorta coarctation in rats. Biol Pharm Bull. 2010;33:631–635. doi: 10.1248/bpb.33.631. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y.G., Zima A.V., Ji X., Pabbidi R., Blatter L.A., Lipsius S.L. Ginsenoside Re suppresses electromechanical alternans in cat and human cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H851–H859. doi: 10.1152/ajpheart.01242.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z., Li M., Wu W.K., Tan H.M., Geng D.F. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther. 2008;22:443–452. doi: 10.1007/s10557-008-6129-4. [DOI] [PubMed] [Google Scholar]
- 61.Deng J., Lv X.T., Wu Q., Huang X.N. Ginsenoside Rg(1) inhibits rat left ventricular hypertrophy induced by abdominal aorta coarctation: involvement of calcineurin and mitogen-activated protein kinase signalings. Eur J Pharmacol. 2009;608:42–47. doi: 10.1016/j.ejphar.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y.L., Wang C.Y., Zhang B.J., Zhang Z.Z. Shenfu injection suppresses apoptosis by regulation of Bcl-2 and caspase-3 during hypoxia/reoxygenation in neonatal rat cardiomyocytes in vitro. Mol Biol Rep. 2009;36:365–370. doi: 10.1007/s11033-007-9188-x. [DOI] [PubMed] [Google Scholar]
- 63.Zhu D., Wu L., Li C.R., Wang X.W., Ma Y.J., Zhong Z.Y., Zhao H.B., Cui J., Xun S.F., Huang X.L. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem. 2009;108:117–124. doi: 10.1002/jcb.22233. [DOI] [PubMed] [Google Scholar]
- 64.Kim T.H., Lee S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48:1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y., Xia Z.Y., Dou J., Zhang L., Xu J.J., Zhao B., Lei S., Liu H.M. Protective effect of ginsenoside Rb1 against myocardial ischemia/reperfusion injury in streptozotocin-induced diabetic rats. Mol Biol Rep. 2011;38:4327–4335. doi: 10.1007/s11033-010-0558-4. [DOI] [PubMed] [Google Scholar]
- 66.Bodiga S., Wang W., Oudit G.Y. Use of ginseng to reduce post-myocardial adverse myocardial remodeling: applying scientific principles to the use of herbal therapies. J Mol Med (Berl) 2011;89:317–320. doi: 10.1007/s00109-011-0736-4. [DOI] [PubMed] [Google Scholar]
- 67.Guo J., Gan X.T., Haist J.V., Rajapurohitam V., Zeidan A., Faruq N.S., Karmazyn M. Ginseng inhibits cardiomyocyte hypertrophy and heart failure via NHE-1 inhibition and attenuation of calcineurin activation. Circ Heart Fail. 2011;4:79–88. doi: 10.1161/CIRCHEARTFAILURE.110.957969. [DOI] [PubMed] [Google Scholar]
- 68.Tsutsumi Y.M., Tsutsumi R., Mawatari K., Nakaya Y., Kinoshita M., Tanaka K., Oshita S., Compound K. a metabolite of ginsenosides, induces cardiac protection mediated nitric oxide via Akt/Pi3k pathway. Life Sci. 2011;88:725–729. doi: 10.1016/j.lfs.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Zhou H., Hou S.Z., Luo P., Zeng B., Wang J.R., Wong Y.F., Jiang Z.H., Liu L. Ginseng protects rodent hearts from acute myocardial ischemia-reperfusion injury through GR/ER-activated risk pathway in an endothelial NOS-dependent mechanism. J Ethnopharmacol. 2011;135:287–298. doi: 10.1016/j.jep.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 70.Tamura Y. Proceedings of the 6th International Ginseng Symposium. Korean Society of Ginseng; Seoul: 1993. Effects of Korean red ginseng on eicosanoid biosynthesis in platelets and vascular smooth muscle cells; pp. 28–29. [Google Scholar]
- 71.Dong-Ha Lee D.H., Cho H.J., Kim H.H., Rhee M.H., Ryu J.H., Park J.H. Inhibitory effects of total saponin from Korean red ginseng via vasodilator-stimulated phosphoprotein-Ser157 phosphorylation on thrombin-induced platelet aggregation. J Ginseng Res. 2013;37:176–186. doi: 10.5142/jgr.2013.37.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y., Im J.H., Han X.H., Kim T.J., Shin K.S. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 73.Lee W.M., Kim S.D., Park M.H., Cho J.Y., Park H.J., Seo G.S., Rhee M.H. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: critical roles of ERK2 and cAMP. J Pharm Pharmacol. 2008;60:1531–1536. doi: 10.1211/jpp/60.11.0015. [DOI] [PubMed] [Google Scholar]
- 74.Li G.X., Liu Z.Q. The protective effects of ginsenosides on human erythrocytes against hemin-induced hemolysis. Food Chem Toxicol. 2008;46:886–892. doi: 10.1016/j.fct.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Yang J.Y., Sun K., Wang C.S., Guo J., Xue X., Liu Y.Y., Zheng J., Fan J.Y., Liao F.L., Han J.Y. Improving effect of post-treatment with Panax notoginseng saponins on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Clin Hemorheol Microcirc. 2008;40:119–131. [PubMed] [Google Scholar]
- 76.Lee J.G., Lee Y.Y., Wu B., Kim S.Y., Lee Y.J., Yun-Choi H.S., Park J.H. Inhibitory activity of ginsenosides isolated from processed ginseng on platelet aggregation. Pharmazie. 2010;65:520–522. [PubMed] [Google Scholar]
- 77.Lee Y.H., Lee B.K., Choi Y.J., Yoon I.K., Chang B.C., Gwak H.S. Interaction between warfarin and Korean red ginseng in patients with cardiac valve replacement. Int J Cardiol. 2010;145:275–276. doi: 10.1016/j.ijcard.2009.09.553. [DOI] [PubMed] [Google Scholar]
- 78.Yi X.Q., Li T., Wang J.R., Wong V.K., Luo P., Wong I.Y., Jiang Z.H., Liu L., Zhou H. Total ginsenosides increase coronary perfusion flow in isolated rat hearts through activation of PI3K/Akt-eNOS signaling. Phytomedicine. 2010;17:1006–1015. doi: 10.1016/j.phymed.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 79.Ahn C.M., Hong S.J., Choi S.C., Park J.H., Kim J.S., Lim D.S. Red ginseng extract improves coronary flow reserve and increases absolute numbers of various circulating angiogenic cells in patients with first ST-segment elevation acute myocardial infarction. Phytother Res. 2011;25:239–249. doi: 10.1002/ptr.3250. [DOI] [PubMed] [Google Scholar]
- 80.Trinh H.T., Han S.J., Kim S.W., Lee Y.C., Kim D.H. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbiol Biotechnol. 2007;17:1127–1133. [PubMed] [Google Scholar]
- 81.Hwang S.Y., Son D.J., Kim I.W., Kim D.M., Sohn S.H., Lee J.J., Kim S.K. Korean red ginseng attenuates hypercholesterolemia-enhanced platelet aggregation through suppression of diacylglycerol liberation in high-cholesterol-diet-fed rabbits. Phytother Res. 2008;22:778–783. doi: 10.1002/ptr.2363. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y.G., Zhang H.G., Zhang G.Y., Fan J.S., Li X.H., Liu Y.H., Li S.H., Lian X.M., Tang Z. Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin Exp Pharmacol Physiol. 2008;35:1238–1244. doi: 10.1111/j.1440-1681.2008.04997.x. [DOI] [PubMed] [Google Scholar]
- 83.Liu G., Wang B., Zhang J., Jiang H., Liu F. Total Panax notoginsenosides prevent atherosclerosis in apolipoprotein E-knockout mice: role of downregulation of CD40 and MMP-9 expression. J Ethnopharmacol. 2009;126:350–354. doi: 10.1016/j.jep.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 84.Jia Y., Li Z.Y., Zhang H.G., Li H.B., Liu Y., Li X.H. Panax notoginseng saponins decrease cholesterol ester via up-regulating ATP-binding cassette transporter A1 in foam cells. J Ethnopharmacol. 2010;132:297–302. doi: 10.1016/j.jep.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 85.Kwak Y.S., Kyung J.S., Kim J.S., Cho J.Y., Rhee M.H. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010;33:468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 86.Li J., Xie Z.Z., Tang Y.B., Zhou J.G., Guan Y.Y. Ginsenoside-Rd, a purified component from Panax notoginseng saponins, prevents atherosclerosis in apoE knockout mice. Eur J Pharmacol. 2011;652:104–110. doi: 10.1016/j.ejphar.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 87.Cai B.X., Li X.Y., Chen J.H., Tang Y.B., Wang G.L., Zhou J.G., Qui Q.Y., Guan Y.Y. Ginsenoside-Rd, a new voltage-independent Ca2+ entry blocker, reverses basilar hypertrophic remodeling in stroke-prone renovascular hypertensive rats. Eur J Pharmacol. 2009;606:142–149. doi: 10.1016/j.ejphar.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 88.Bers D.M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 89.Chen R.J., Chung T.Y., Li F.Y., Lin N.H., Tzen J.T. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 2009;30:61–69. doi: 10.1038/aps.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]


