Abstract
Background
Ginsenosides are the major components responsible for the biochemical and pharmacological actions of ginseng, and have been shown to have various biological activities. In this study, we investigated the antiviral activities of seven ginsenosides [protopanaxatriol (PT) type: Re, Rf, and Rg2; protopanaxadiol (PD) type: Rb1, Rb2, Rc, and Rd)] against coxsackievirus B3 (CVB3), enterovirus 71 (EV71), and human rhinovirus 3 (HRV3).
Methods
Assays of antiviral activity and cytotoxicity were evaluated by the sulforhodamine B method using the cytopathic effect (CPE) reduction assay.
Results
The antiviral assays demonstrated that, of the seven ginsenosides, the PT-type ginsenosides (Re, Rf, and Rg2) possess significant antiviral activities against CVB3 and HRV3 at a concentration of 100 μg/mL. Among the PT-type ginsenosides, only ginsenoside Rg2 showed significant anti-EV71 activity with no cytotoxicity to cells at 100 μg/mL. The PD-type ginsenosides (Rb1, Rb2, Rc, and Rd), by contrast, did not show any significant antiviral activity against CVB3, EV71, and HRV3, and exhibited cytotoxic effects to virus-infected cells. Notably, the antiviral efficacies of PT-type ginsenosides were comparable to those of ribavirin, a commonly used antiviral drug.
Conclusion
Collectively, our findings suggest that the ginsenosides Re, Rf, and Rg2 have the potential to be effective in the treatment of CVB3, EV71, and HRV3 infection.
Keywords: antiviral activity, CVB3, EV71, ginsenosides, HRV3
1. Introduction
The Picornaviridae are currently divided into nine genera, three of which (Hepatoviruses, Rhinoviruses, and Enteroviruses) are causative agents of human diseases [1]. Enteroviruses such as coxsackievirus, poliovirus, and echovirus are small, nonenveloped viruses possessing a single-stranded RNA genome in positive orientation that acts directly as mRNA in infected cells. Enteroviruses are of high clinical relevance with coxsackievirus B3 (CVB3), which can cause heart-muscle infection, being an important member. In addition, Enterovirus 71 (EV71) is a causative agent of hand, foot, and mouth disease and herpangina that can also cause severe neurological disease including brainstem encephalitis and poliomyelitis-like paralysis [2–5]. Human rhinovirus (HRV) represents one of the most important etiological agents of the common cold [6]. Although HRV-induced upper respiratory illness is usually mild and self-limiting, there is increasing evidence linking HRV infection to more serious medical complications including asthma exacerbation [7].
To date, no effective antiviral therapies have been approved for either the prevention or treatment of diseases caused by viruses classified within the Picornaviridae family, including CVB3, EV71, and HRV [8]. In this regard, many trials have been conducted to find antiviral components from plants. Such trials have specifically targeted plants with intrinsic defense mechanisms in the form of secondary metabolites against a broad range of viral infections, in contrast to adaptive immunity induced in mammals. Indeed, medicinal plants are gaining popularity as suitable alternative sources of antiviral agents because of their multiple targets, minor side effects, low potentials to cause resistance, and low costs [9–13]. Although several hundreds of plants with the potential to contain novel antiviral agents have been studied, a number of potentially useful medicinal plants still need to be evaluated and exploited for therapeutic applications against the genetically and functionally diverse virus families. Of these potential agents, we have focused on ginsenosides, which are some of the major components of the ginseng plant, Panax ginseng Meyer. The root of P. ginseng (Araliaceae) is the most well-known medicinal plant in the Asian region and is frequently used in traditional medicine [14]. Ginsenosides are triterpenoid glycosides containing dammarane [15], and are generally divided into two groups: the protopanaxadiol (PD) and protopanaxatriol (PT) ginsenoside groups. The sugar moieties in the PD group including Rb1, Rb2, Rc, Rd, Rg3, and Rh3 are attached at the 3-position of dammarane-type triterpenes, whereas the sugar moieties in the PT group including Re, Rf, Rg1, Rg2, and Rh1 are attached at the 6-position of dammarane-type triterpenes [16]. As the major components in ginseng, ginsenosides have various biological activities such as anticancer [17], antiaging [18,19], and antitumor activities [20]. Moreover, the antiviral activities of ginseng against influenza virus [15], norovirus [21], and HBV [22] have recently been reported. Although a variety of pharmacological activities associated with seven ginsenosides (PT group: Re, Rf, and Rg2; PD group: Rb1, Rb2, Rc, and Rd) have been demonstrated, antiviral activities especially against CVB3, EV71 and HRV3, which are representative viruses of the picornaviridae and have drawn a great attention in terms of potential therapeutics, have not been reported. Therefore, in the current study, we investigated the antiviral activities of seven ginsenosides against CVB3, EV71, and HRV3.
2. Materials and methods
2.1. Viruses, cell lines, and reagents
CVB3, EV71, and HRV3 were supplied by Korea Research Institute Bioscience and Biotechnology, Ochang-eup, South Korea. A human cervix epithelial cell line (HeLa, CCL-2) and African green monkey kidney cells (Vero, CCL-81) were purchased from the American Type Culture Collection (Manassas, VA, USA). HeLa and Vero cells were maintained in minimal essential medium supplemented with 10% fetal bovine serum and 0.01% antibiotic–antimycotic solution. Antibiotic–antimycotic solution, trypsin–EDTA, fetal bovine serum and minimal essential medium were supplied by Gibco BRL (Grand Island, NY, USA). Tissue culture plates were purchased from Falcon (BD Biosciences, Franklin Lakes, NJ, USA). Ribavirin and sulforhodamine B (SRB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The seven ginsenosides were obtained from Dr. Bae L (Elohim, Co., Daejeon, South Korea). Stock solutions (100 mg/mL) of the antiviral compounds were dissolved in dimethyl sulfoxide (DMSO) and were subsequently diluted in the culture medium. The final DMSO concentration in the culture medium did not exceed 0.1%, which was found to have no visible toxic effect on the cells. As a negative control, 0.1% DMSO was also added to all no-drug control samples.
2.2. SRB assays of antiviral activity
Assays of antiviral activity and cytotoxicity were evaluated by the SRB method using cytopathic effect (CPE) reduction recently reported [23]. Briefly, 1 day prior to infection, Vero cells were seeded onto a 96-well culture plate at a concentration of 2 × 104 cells/well. The following day, the culture medium was removed and cells were washed with phosphate-buffered saline (PBS). The infectivity of each virus was determined by the SRB method monitoring CPE, allowing for the percentage of cell viability to be determined. Based on the mammalian cell viability determined for each virus, 0.09 mL of diluted virus suspension of CVB3 or EV71 containing CCID50 (50% cell culture infective dose) of virus stock was added to mammalian cells. This dose was selected to produce the appropriate CPEs 48 hours after infection. For compound treatments, 0.01 mL of the medium containing the selected concentration of compound was added to the cells. The antiviral activity of each test material was determined using a 10-fold diluted concentration range of 0.1–100 μg/mL. Four wells were used as virus controls (virus-infected, nondrug-treated cells), whereas four wells were used as cell controls (noninfected, nondrug-treated cells). Culture plates were incubated at 37°C in 5% CO2 for 48 h. After washing once with PBS, 100 μL of cold (−20°C) 70% (v/v) acetone was added to each well and left for 30 min at −20°C. The acetone was removed from cells, after which 96-well plates were left to dry in oven at 60°C for 30 min. Then, 100 μL of 0.4% (w/v) SRB in 1% acetic acid (v/v) was added to each well and incubated at room temperature for 30 min. Unbound SRB was removed by washing the plates five times with 1% acetic acid (v/v), and the plates were then left to dry in an oven. After drying for 1 day, cell morphology was assessed under a microscope at 4 × 10 magnification (AXIOVERT10; Zeiss, Göttingen, Deutschland) and images were acquired. Fixed SRB in wells was solubilized with 100 μL of unbuffered Tris-base solution (10 mM), and plates were incubated at room temperature for 30 min. Absorbance in each well was read at 540 nm using a VERSAmax microplate reader (Molecular Devices, Palo Alto, CA, USA) and a reference absorbance of 620 nm. The antiviral activity of each test compound in CVB3- or EV71-infected cells was calculated as a percentage of the corresponding untreated control.
2.3. Cell Titer-Glo assays of antiviral activity
The antiviral activity of seven ginsenosides against HRV3 was determined using a Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega, Madison, Wisconsin, USA). The Cell Titer-Glo Reagent induces cell lysis and the generation of luminescence proportional to the amount of ATP present in cells. The resulting luminescence intensity is measured using a luminometer (Molecular Devices) according to the manufacturer's instructions. Briefly, HeLa cells were seeded onto a 96-well culture plate, after which 0.09 mL of diluted HRV3 suspension containing CCID50 of the virus stock, and 0.01 mL culture medium supplemented with 20 mM MgCl2 and the appropriate concentration of ginsenosides, was added to the cells. The antiviral activity of each test material was determined using a concentration series of 0.1 μg/mL, 1 μg/mL, 10 μg/mL, and 100 μg/mL. Culture plates were incubated at 37°C in 5% CO2. After 48 h, 100 μL of Cell Titer-Glo reagent was added to each well, and the plate was incubated at room temperature for 10 min. The resulting luminescence was measured and the percentage cell viability was calculated as described for the antiviral activity assays. Cell morphology was assessed as described for the SRB assay.
2.4. Cytotoxicity
To measure cytotoxicity, cells were seeded onto a 96-well culture plate at a density of 2 × 104 cells/well. The following day, the culture medium containing serially diluted compounds was added to the cells and incubated for 48 h, after which the culture medium was removed and cells were washed with PBS. The next step was conducted as described above for the antiviral activity assay. To calculate the CC50 values, the data were expressed as percentages relative to controls, and CC50 values were obtained from the resulting dose–response curves.
2.5. Statistical analyses
Differences across more than three groups were analyzed using one-way analysis of variance (Graphpad PRISM, version 5.01, San Diego, CA, USA). All results were expressed as mean ± standard deviation. Significant differences in direct comparisons were determined using a Tukey's post hoc test. Differences with p < 0.05, p < 0.01, and p < 0.001 were considered statistically significant.
3. Results
3.1. Antiviral activity of ginsenosides against CVB3
The antiviral activities of ginsenosides against CVB3 were assessed using the SRB method, which monitors the alteration of CPE induced by virus infection. As a positive control, ribavirin, a commonly used antiviral drug, was included. Of the seven ginsenosides tested, ginsenosides Re, Rf, and Rg2, which are classified as PT-type ginsenosides, significantly inhibited CVB3-induced CPE, and increased the cell viability of Vero cells (Fig. 1). CVB3 infection induced approximately 60% cell death in Vero cells (40% of cell viability), and the treatment of cells with 100 μg/mL of Re, Rf, and Rg2 increased the cell viability to 75%, 60%, and 50%, respectively. Furthermore, 10 μg/mL of ginsenosides Re and Rg2 also significantly reduced the CPE of CVB3 infection in Vero cells, albeit a weaker protective effect than that of ribavirin at the same concentration. By contrast, the PD-type ginsenosides Rb1, Rb2, Rc, and Rd did not exhibit any antiviral activity against CVB3, and 100 μg/mL of Rd, Rc, and Rb2 even significantly increased CVB3 infection-induced cytotoxicity (Fig. 1).
Fig. 1.
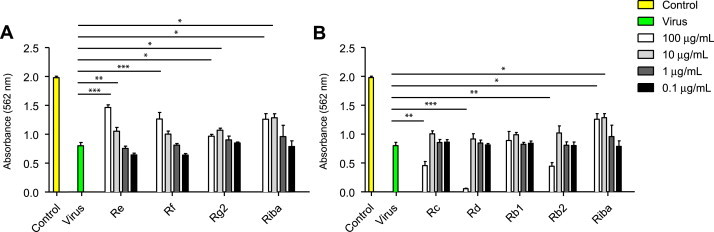
Antiviral activity of ginsenosides against CVB3 in Vero cells. Vero cells were infected with EV71, after which they were treated with the indicated concentrations (0.1–100 μg/mL) of ginsenosides for 48 h. Ribavirin was used as a positive control for antiviral activity. Antiviral activity was investigated using a CPE reduction assay. Data are presented as mean ± SD from three independent experiments each carried out in triplicate. CPE, cytopathic effect; CVB3, coxsackievirus B3; EV71, enterovirus 71.
In Vero cells treated with ribavirin after CVB3 infection, the drug exhibited significant antiviral activity at 100 μg/mL and 10 μg/mL (Fig. 1), and the maximal efficacy of ribavirin was comparable to those of PT-type ginsenosides. Ribavirin itself was slightly toxic to Vero cells (cell viability of approximately 81% at 100 μg/mL), whereas none of the seven ginsenosides alone was toxic to Vero cells at the same concentration (Table 1). Collectively, these results suggest that ginsenosides Re, Rf, and Rg2 have significant antiviral activity against CVB3 without inducing cytotoxicity in Vero cells.
Table 1.
Cytotoxicity of Ginsenosides in Vero Cells
| Ginsenosides | Concentration (μg/mL) |
|||
|---|---|---|---|---|
| 1 | 10 | 100 | ||
| PT type | Re | 109 ± 1.02 | 109 ± 2.53 | 106.9 ± 1.03 |
| Rf | 117 ± 1.92 | 121 ± 1.63 | 105 ± 3.41 | |
| Rg2 | 102 ± 1.15 | 109 ± 1.50 | 106 ± 2.35 | |
| Ribavirin | 101 ± 2.90 | 105 ± 0.13 | 80.7 ± 1.22 | |
| PD type | Rb1 | 110 ± 0.62 | 117 ± 2.16 | 106 ± 0.40 |
| Rb2 | 110 ± 4.49 | 106 ± 1.04 | 107 ± 1.08 | |
| Rc | 104 ± 1.04 | 111 ± 1.02 | 106 ± 1.01 | |
| Rd | 101 ± 1.41 | 101 ± 2.35 | 104 ± 1.72 | |
| Ribavirin | 101 ± 1.86 | 105 ± 1.22 | 80.7 ± 0.13 | |
Results are presented as the mean percentage values obtained from three independent experiments carried out in triplicate ± SD
PD, protopanaxadiol; PT, protopanaxatriol
3.2. Antiviral activity of ginsenosides against EV71 infection
Together with coxsackievirus A16, EV71 is one of the two major causative agents of hand, foot, and mouth disease, and thus we sought to investigate whether ginsenosides have antiviral activity against EV71 infection in Vero cells. Most ginsenosides assessed using the SRB method did not have significant antiviral activity against EV71, and only ginsenoside Rg slightly inhibited EV71 infection-induced cytotoxicity (Fig. 2). Infection with EV71 induced substantial cell death in Vero cells, resulting in approximately 25% cell viability. The antiviral effect of Rg2 (10 μg/mL and 100 μg/mL) in EV71-infected cells improved cell viability by 40%. The antiviral effect of Rg2 was shown to be dose-dependent, and the maximal antiviral efficacy of the compound is comparable to that of ribavirin. By contrast, other ginsenosides tested did not have significant antiviral activity against EV71 infection (Fig. 2).
Fig. 2.
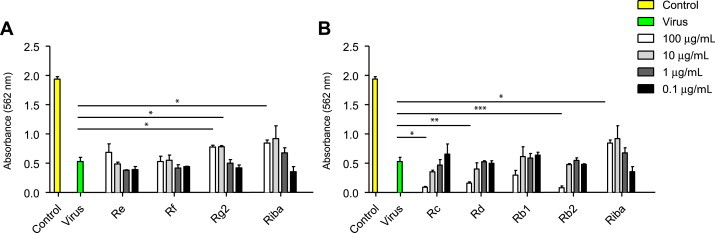
Antiviral activity of ginsenosides against EV71 in Vero cells. Vero cells were infected with EV71, after which they were treated with the indicated concentrations (0.1–100 μg/mL) of ginsenosides for 48 h. Ribavirin was used as a positive control for antiviral activity. Antiviral activity was assessed using a CPE reduction assay. Data are presented as mean ± SD from three independent experiments each carried out in triplicate. CPE, cytopathic effect; EV71, enterovirus 71.
3.3. Antiviral activity of ginsenosides against HRV3 infection
To assess the antiviral activity of ginsenosides against HRV, HeLa cells were infected with HRV3 and treated with each of the seven ginsenosides of interest at the indicated concentrations. HRV3 infection itself induced cell death in HeLa cells and resulted in 50% cell viability (Fig. 3). Similar to the antiviral effect against CVB3, two PT-type ginsenosides (Rf and Rg2) significantly increased cell viability to 80% (Fig. 3) as shown using the luminescent cell viability assay described in the “Materials and methods” section. The ginsenoside Re, however, had little protective effect in HRV3-infected HeLa cells. Furthermore, none of the PD-type ginsenosides (Rd, Rc, Rb1, and Rb2) had a protective on cell viability, but instead the compounds (100 μg/mL) significantly increased HRV3 infection-induced cell death in HeLa cells (Fig. 3), despite not inducing cytotoxicity in uninfected HeLa cells (Table 1). Collectively, these results suggest that the PT-type ginsenosides Rf and Rg2 have antiviral activity against HRV3.
Fig. 3.
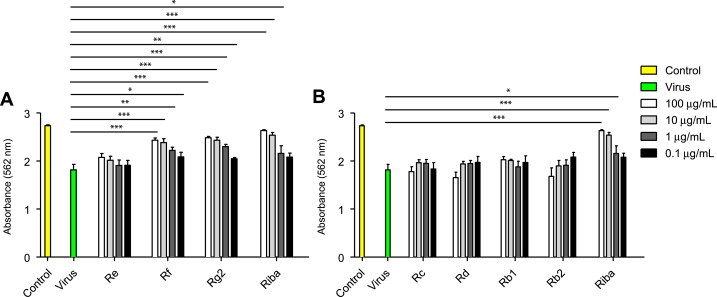
Antiviral activity of ginsenosides against HRV3 in HeLa cells. HeLa cells were infected with HRV3, after which they were treated with the indicated concentrations of ginsenosides for 48 h. Antiviral activity was assessed using a Cell Titer-Glo Luminescent cell viability assay. Data are presented as mean ± SD from three independent experiments each carried out in triplicate. HRV3, human rhinovirus 3.
3.4. Effect of ginsenosides on CVB3- and HRV3-induced cytotoxicity
In order to examine the potential morphological alteration of Vero cells by ginsenosides, cells were treated with the compounds for 48 h and assessed by microscopy. In the absence of infection with CVB3, cells treated with DMSO or 100 μg/mL ginsenosides showed no obvious signs of cytotoxicity, exhibiting the typical spread-out shape associated with the normal morphology of Vero cells (Fig. 4). Infection of Vero cells with CVB3 resulted in a severe CPE, whereas CVB3-infected Vero cells treated with ginsenosides Re, Rf, and Rg2, exhibited noticeably reduced CPE compared with untreated CVB3-infected cells. Treatment of CVB3-infected Vero cells with ribavirin significantly reduced CPE. These results indicate that the CPE of CVB3 infection is prevented by ginsenosides Re, Rf, and Rg2.
Fig. 4.
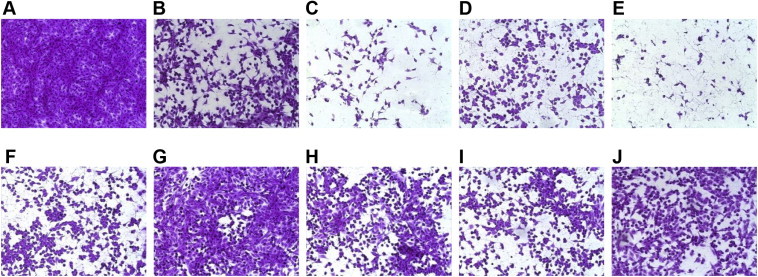
Morphological assessment of coxsackievirus B3 (CVB3)-infected Vero cells following ginsenoside treatment. CVB3-infected and uninfected Vero cells were treated with either ribavirin or 100 μg/mL ginsenosides. After staining of viable cells with sulforhodamine B (SRB), cell morphology was assessed by microscopy. (A) Untreated, uninfected cells; (B–I) uninfected cells treated with ginsenosides (B) Rb1, (C) Rb2, (D) Rd, (E) Rc, (F) Re, (G) Rf, and (H) Rg2; and with ribavirin; (J) untreated CVB3-infected cells; (K–R) CVB3-infected cells treated with ginsenosides (K) Rb1, (L) Rb2, (M) Rd, (N) Rc, (O) Re, (P) Rf, (Q) Rg2; and with (R) ribavirin.
The viability of HeLa cells following HRV3 infection was also monitored. In the absence of HRV3 infection, the treatment of HeLa cells with ginsenosides for 48 h altered neither the viability nor the morphology of the cells compared with vehicle-treated cells (Fig. 5). HRV3 infection reduced the viability of cells, and as assessed using the SRB assay, ribavirin was found to significantly inhibit HRV3 infection-induced cell death. Likewise, ginsenosides Re, Rf, and Rg2 reduced HRV3 infection-induced cell death, whereas ginsenosides Rd, Rc, and Rb2 induced severe cytotoxicity in HeLa cells infected with HRV3. The CPE of HRV3 infection is thus prevented by treatment with ginsenosides Re, Rf, and Rg2.
Fig. 5.
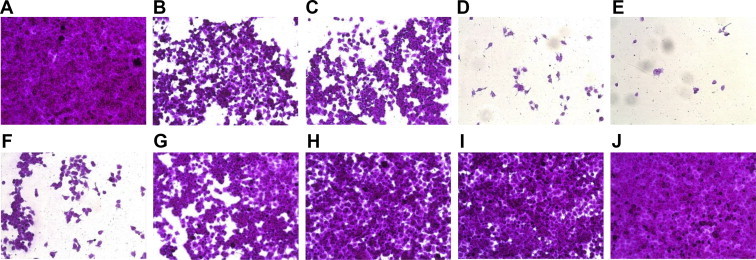
Morphological assessment of human rhinovirus 3 (HRV3)-infected Hela cells following ginsenosides treatment. HRV3-infected and uninfected HeLa cells were treated with either ribavirin or 100 μg/mL ginsenoside. After staining of viable cells with sulforhodamine B (SRB), cell morphology was assessed by microscopy. (A) Untreated, uninfected cells; (B–I) uninfected cells treated with ginsenosides (B) Rb1, (C) Rb2, (D) Rd, (E) Rc, (F) Re, (G) Rf, (H) Rg2; and with (I) ribavirin; (J) untreated HRV3-infected cells; (K—R) HRV3-infected cells treated with ginsenosides (K) Rb1, (L) Rb2, (M) Rd, (N) Rc, (O) Re, (P) Rf, (Q) Rg2; and with (R) ribavirin.
4. Discussion
P. ginseng is a traditional medicine that has been used in Korea and China for more than 5000 years [24]. Steaming and fermentation of skinned ginseng resulted in red ginseng having a somewhat different chemical composition compared with the original ginseng. Many saponins including ginsenosides found in ginseng and red ginseng have been shown to have various beneficial effects including adjuvant properties and antiviral activity. Some ginsenosides elicited adjuvant effects when used in combination with several vaccines including influenza and porcine parvovirus vaccines [15,25]. Ginsenosides Rg1, Rg2, Rg3, Rb1, and Re, in particular, exhibit potent adjuvant activity, and ginsenoside Re increased the immune response against an inactivated H3N2 influenza vaccine [15]. Furthermore, the oral administration of ginsenoside Rb2 prior to infection of mice with hemagglutinating virus of Japan protected the infected mice from severe acute lung infection. This effect was shown to be due to antiviral activity of Rb2 as well as an enhancement of mucosal immunity by the compound [26]. Interestingly, a recent study showed that ginsenosides Rg1 and Rb1, as well as red ginseng extract, exhibited antiviral activity against hepatitis A virus, which is classified in the Picornaviridae family together with Enteroviruses [27]. However, there have been no previous reports on the antiviral activity of ginsenosides against other viruses included in Picornaviridae. In the current study, we show that ginsenosides Re, Rf, and Rg2 have significant antiviral activity against CVB3 and HRV3 infection, and thus, considering their potential adjuvanticity, these compounds may be effective in eliminating CVB3 and HRV3 in infected hosts.
It is believed that CVB3 is an etiological agent causing myocarditis and dilated cardiomyopathy, and outbreaks of CVB3 infection occur worldwide annually [28]. Currently, there are no effective therapeutic agents against CVB3, and only ribavirin has been shown to have weak antiviral activity against CVB3 infection [29–31]. Similarly, no therapeutics are available for the treatment of HRV infection, and most associated treatments function only to reduce the symptoms of the infection. Because of the challenges associated with the development of appropriate vaccines as a means of controlling rhinovirus infection, mainly due to the genetic variability of rhinoviruses, most research efforts toward combating rhinovirus infection have been focused on the development of effective antiviral drugs. A great variety of compounds and compound classes have been shown to exhibit antirhinovirus activity in vitro, but few have been found to be effective at the clinical level. The antiviral activities of whole extracts produced from Uncaria tomentosa, Guettarda platypoda [32], rhizome of Tamus communis [33], Calendula arvensis [34], root of Allium sativum [35], Zingiber officinale [36], and Eleutherococcus senticosus [37] have been reported; however, antiviral activities of ginsenosides and even of ginseng against HRV have not yet been reported.
Pleconaril is an orally administrable small-molecule inhibitor of human picornavirus replication. The compound is known to be integrated into a hydrophobic pocket within the major coat protein of viruses including human Picornaviridae, and to inhibit the correct functioning of this protein. Consequently, pleconaril inhibits the attachment of some viruses to their cellular receptors and blocks the viral uncoating process [38,39]. Recently, however, the US Food and Drug Administration did not approve pleconaril for clinical use owing to concerns of emergence of viral resistance and side effects in patients [40]. Ribavirin has also been used in attempts to treat various DNA and RNA virus infections, although acquired resistance to the drug has been demonstrated in various virus populations and in some patients [29]. The development of antiviral drugs targeting viruses classified in the Picornaviridae family is therefore urgently required. In the current study, the antiviral activities of ginsenosides against CVB3, EV71, and HRV3 have been evaluated and compared with the currently used antiviral drug ribavirin, which exhibits some antiviral activity. The results of our study demonstrating the antiviral activities of ginsenosides suggest that the compounds may provide a therapeutic option for the treatment of CVB3, EV71, and HRV3 infection; furthermore, the compounds could potentially be effective against Picornaviridae viruses in general.
Strong anti-CVB3 and anti-HRV3 activity was demonstrated for PT-type ginsenosides (Re, Rf, and Rg2), and ginsenoside Rg2 of the PT type showed anti-EV71 activity, despite its relatively weak activity. By contrast, PD-type ginsenosides (Rb1, Rb2, Rc, and Rd) did not show any antiviral activity against CVB3, EV71 and HRV3, and even increased the cytotoxicity induced by virus infection. Taken together, these results indicate that the antiviral activities of ginsenosides against CVB3, EV71, and HRV3 appear to be selectively dependent on the type of ginsenosides. Ginsenoside is divided into PD saponin and PT saponin by its chemical structure. The other study group investigated and compared the antiobesity activity of PD-type and PT-type saponins in rats fed a high fat diet. In conclusion, PD- and PT-type saponins have been shown to exert antiobesity effects in the rats fed with a high fat diet by reducing their body weight, their food consumption, and their fat storage. However, PD-type saponins have more potent antiobesity properties than PT-type saponins [41]. We think our data also demonstrate that antiviral activities are related to the chemical structures. Therefore, further studies are required to explore the detailed antiviral mechanisms of ginsenosides of the PT type as well as to assess in vivo antiviral activity.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
CVB3 and EV71 were provided by Chungcheongnam-Do Health and Environment Research Institute, Daejeon, South Korea. We also thank Dr Kwi-Sung Park for providing CB3 and EV71. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean Ministry of Education, Science and Technology (NRF-2012R1A1A2003182). This study was technically supported by Korea National Institute of Health. This research was supported by 2013 Research Grant from Kangwon National University (No. 120131474/C1009934-01-01) and by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No.2011-0009018).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Sungchan Cho, Email: sungchan@kribb.re.kr.
Hyun-Jeong Ko, Email: hjko@kangwon.ac.kr.
References
- 1.Whitton J.L., Cornell C.T., Feuer R. Host and virus determinants of picornavirus pathogenesis and tropism. Nat Rev Microbiol. 2005;3:765–776. doi: 10.1038/nrmicro1284. [DOI] [PubMed] [Google Scholar]
- 2.Chumakov M., Voroshilova M., Shindarov L., Lavrova I., Gracheva L., Koroleva G., Vasilenko S., Brodvarova I., Nikolova M., Gyurova S. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol. 1979;60:329–340. doi: 10.1007/BF01317504. [DOI] [PubMed] [Google Scholar]
- 3.McMinn P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;6:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang S.M., Lei H.Y., Huang K.J., Wu J.M., Wang J.R., Yu C.K., Su I.J., Liu C.C. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564–570. doi: 10.1086/376998. [DOI] [PubMed] [Google Scholar]
- 5.Song J., Yeo S.G., Hong E.H., Lee B.R., Kim J.W., Kim J., Jeong H., Kwon Y., Kim H., Lee S. Antiviral activity of Hederasaponin B from Hedera helix against Enterovirus 71 subgenotypes C3 and C4a. Biomol Ther. 2014;22:41–46. doi: 10.4062/biomolther.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makela M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimaki M., Blomqvist S., Hyypia T., Arstila P. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J., Message S.D., Qiu Y., Mallia P., Kebadze T., Contoli M., Ward C.K., Barnathan E.S., Mascelli M.A., Kon O.M. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest. 2014;156:1219–1229. doi: 10.1378/chest.13-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner R.B. The treatment of rhinovirus infections: progress and potential. Antiviral Res. 2001;49:1–14. doi: 10.1016/S0166-3542(00)00135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlietinck A.J., Vanden Berghe D.A. Can ethnopharmacology contribute to the development of antiviral drugs? J Ethnopharmacol. 1991;32:141–153. doi: 10.1016/0378-8741(91)90112-q. [DOI] [PubMed] [Google Scholar]
- 10.Cowan M.M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briskin D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000;124:507–514. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams J.E. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on Una de Gato and Sangre de Grado. Altern Med Rev. 2001;6:567–579. [PubMed] [Google Scholar]
- 13.Jassim S.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 14.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 15.Song X., Chen J., Sakwiwatkul K., Li R., Hu S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int Immunopharmacol. 2010;10:351–356. doi: 10.1016/j.intimp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Peng D., Wang H., Qu C., Xie L., Wicks S.M., Xie J. Ginsenoside Re: its chemistry, metabolism and pharmacokinetics. Chin Med. 2012 Feb 7;7:2. doi: 10.1186/1749-8546-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chae S., Kang K.A., Chang W.Y., Kim M.J., Lee S.J., Lee Y.S., Kim H.S., Kim D.H., Hyun J.W. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem. 2009;57:5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]
- 18.Cho W.C., Chung W.S., Lee S.K., Leung A.W., Cheng C.H., Yue K.K. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550:173–179. doi: 10.1016/j.ejphar.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 19.Kim M.S. Korean red ginseng tonic extends lifespan in D. melanogaster. Biomol Ther. 2013;21:241–245. doi: 10.4062/biomolther.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W., Rayburn E.R., Hao M., Zhao Y., Hill D.L., Zhang R., Wang H. Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate. 2008;68:809–819. doi: 10.1002/pros.20742. [DOI] [PubMed] [Google Scholar]
- 21.Lee M.H., Lee B.H., Jung J.Y., Cheon D.S., Kim K.T., Choi C. Antiviral effect of Korean red ginseng extract and ginsenosides on murine norovirus and feline calicivirus as surrogates for human norovirus. J Ginseng Res. 2011;35:429–435. doi: 10.5142/jgr.2011.35.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang L.J., Choi Y.J., Lee S.G. Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1 signalling by ginsenoside Rg3 attenuates hepatitis B virus replication. Int J Biochem Cell Biol. 2013;45:2612–2621. doi: 10.1016/j.biocel.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Choi H.J., Kim J.H., Lee C.H., Ahn Y.J., Song J.H., Baek S.H., Kwon D.H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun T.K. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera E., Ekholm Pettersson F., Inganas M., Paulie S., Gronvik K.O. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine. 2005;23:5411–5419. doi: 10.1016/j.vaccine.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Yoo Y.C., Lee J., Park S.R., Nam K.Y., Cho Y.H., Choi J.E. Protective effect of ginsenoside-Rb2 from Korean red ginseng on the lethal infection of haemagglutinating virus of Japan in mice. J Ginseng Res. 2013;37:80–86. doi: 10.5142/jgr.2013.37.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M.H., Lee B.H., Lee S., Choi C. Reduction of hepatitis A virus on FRhK-4 cells treated with Korean red ginseng extract and ginsenosides. J Food Sci. 2013;78:M1412–M1415. doi: 10.1111/1750-3841.12205. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama T., Urano T., Osano M., Hayashi Y., Sekine S., Ando T., Makinom S. Outbreak of herpangina associated with Coxsackievirus B3 infection. Pediatr Infect Dis J. 1989;8:495–498. doi: 10.1097/00006454-198908000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi H.J., Lim C.H., Song J.H., Baek S.H., Kwon D.H. Antiviral activity of raoulic acid from Raoulia australis against Picornaviruses. Phytomedicine. 2009;16:35–39. doi: 10.1016/j.phymed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Choi H.J., Song J.H., Lim C.H., Baek S.H., Kwon D.H. Anti-human rhinovirus activity of raoulic acid from Raoulia australis. J Med Food. 2010;13:326–328. doi: 10.1089/jmf.2009.1149. [DOI] [PubMed] [Google Scholar]
- 32.Aquino R., De Simone F., Pizza C., Conti C., Stein M.L. Plant metabolites. Structure and in vitro antiviral activity of quinovic acid glycosides from Uncaria tomentosa and Guettarda platypoda. J Nat Prod. 1989;52:679–685. doi: 10.1021/np50064a002. [DOI] [PubMed] [Google Scholar]
- 33.Aquino R., Conti C., De Simone F., Orsi N., Pizza C., Stein M.L. Antiviral activity of constituents of Tamus communis. J Chemother. 1991;3:305–309. doi: 10.1080/1120009x.1991.11739110. [DOI] [PubMed] [Google Scholar]
- 34.De Tommasi N., Conti C., Stein M.L., Pizza C. Structure and in vitro antiviral activity of triterpenoid saponins from Calendula arvensis. Planta Med. 1991;57:250–253. doi: 10.1055/s-2006-960084. [DOI] [PubMed] [Google Scholar]
- 35.Weber N.D., Andersen D.O., North J.A., Murray B.K., Lawson L.D., Hughes B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58:417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- 36.Denyer C.V., Jackson P., Loakes D.M., Ellis M.R., Young D.A. Isolation of antirhinoviral sesquiterpenes from ginger (Zingiber officinale) J Nat Prod. 1994;57:658–662. doi: 10.1021/np50107a017. [DOI] [PubMed] [Google Scholar]
- 37.Glatthaar-Saalmuller B., Sacher F., Esperester A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antiviral Res. 2001;50:223–228. doi: 10.1016/s0166-3542(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 38.Pevear D.C., Fancher M.J., Felock P.J., Rossmann M.G., Miller M.S., Diana G., Treasurywala A.M., McKinlay M.A., Dutko F.J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989;63:2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeichhardt H., Otto M.J., McKinlay M.A., Willingmann P., Habermehl K.O. Inhibition of poliovirus uncoating by disoxaril (WIN 51711) Virology. 1987;160:281–285. doi: 10.1016/0042-6822(87)90075-4. [DOI] [PubMed] [Google Scholar]
- 40.Fleischer R., Laessig K. Safety and efficacy evaluation of pleconaril for treatment of the common cold. Clin Infect Dis. 2003;37:1722. doi: 10.1086/379830. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.H., Kang S.A., Han S.M., Shim I. Comparison of the antiobesity effects of the protopanaxadiol- and protopanaxatriol-type saponins of red ginseng. Phytother Res. 2009;23:78–85. doi: 10.1002/ptr.2561. [DOI] [PubMed] [Google Scholar]


