Abstract
Background
Hyperhidrosis is generalised or focal excessive sweating and carries a substantial psychological and social burden. This study compares botulinum toxin versus iontophoresis with topical aluminium chloride hexahydrate in palmar hyperhidrosis.
Methods
The study included 60 cases of palmar hyperhidrosis randomly allocated to 2 groups. One group was given botulinum toxin type A 100 units per palm and the other group subjected to digital iontophoresis with topical application of aluminium chloride hexahydrate lotion for 4 weeks. They were assessed 4 weeks later and those without improvement were crossed over to the other arm for another 4 weeks. Those with improvement were followed up in the same arm for 6 months.
Results
Botulinum therapy showed significant improvement in the initial (80%) as well as cross over cases (75%) as compared to iontophoresis and aluminium chloride (47%) for initial cases and (17%) for cross over cases.
Conclusion
Better improvements were seen with botulinum therapy than with iontophoresis and topical therapy. Residual effects of relief lasted on an average for 4 months for botulinum toxin whereas it was one month with iontophoresis and topical therapy. Advantage with iontophoresis and topical therapy was that it was non invasive and did not require regional anaesthesia as with botulinum therapy.
Keywords: Hyperhidrosis, Botulinum toxin, Iontophoresis, Aluminium chloride
Introduction
Hyperhidrosis, a condition characterized by excessive sweating can be generalized or focal. Eccrine glands produce a thin, odourless solution hypotonic to plasma resulting in hyperhidrosis if secreted in excess. Generalized hyperhidrosis involves the entire body and usually part of an underlying condition, most often an infectious, endocrine or neurologic disorder. Focal hyperhidrosis is idiopathic, occurring in healthy people. It affects one or more body areas, most often the palms, armpits, soles or face. Almost 3% of the general population, largely people aged between 25 and 64 years, experience hyperhidrosis.1 Palmar and axillary hyperhidrosis have the earliest average onset at about 13 and 19 years respectively. As many as 82% of patients with palmar hyperhidrosis have reported onset in childhood.2
Primary Focal Hyperhidrosis should be diagnosed when “focal, visible, excessive sweating of at least 6 months duration” is present without apparent cause with at least two of the following six criteria:1
-
•
Bilateral and relatively symmetric distribution
-
•
Impairment of daily activities
-
•
Frequency of at least one episode per week
-
•
Age of onset <25 years
-
•
Positive family history
-
•
Cessation of focal sweating during sleep.
Hyperhidrosis Disease Severity Scale (HDSS)
It is used to assess QOL in patients with excessive sweating. It helps clinicians quickly determine the level of interference with daily activities and to formulate treatment guidelines according to disease severity (Table 1).3 Some studies have used equivalent grades signifying dry, moist, wet and dripping wet hyperhidrosis corresponding to HDSS scores.4
Table 1.
Hyperhidrosis Disease Severity Scale (HDSS).
| Patient complaints | Grades | Scores |
|---|---|---|
| My sweating is never noticeable and never interferes with my daily activities | Grade 1 (Dry) | Score 1 |
| My sweating is tolerable but sometimes interferers with my daily activities | Grade 2 (Moist) | Score 2 |
| My sweating is barely tolerable and frequently interferes with my daily activities | Grade 3 (Wet) | Score 3 |
| My sweating is intolerable and always interferes with my daily activities | Grade 4 (Dripping wet) | Score 4 |
Treatment for primary focal hyperhidrosis aims to reduce the level of sweat secreted to a level that is acceptable to the patient. Various topical antiperspirants like topical anticholinergics, boric acid, 2–5% tannic acid solutions, resorcinol, potassium permanganate, formaldehyde and aluminium chloride have been used.
Iontophoresis involves using direct current to cause a reversible disruption of the ion channel in the secretory glomeruli of the sweat glands thereby reducing the sweat production.
Aluminium chloride has the ability to temporarily close the pores of sweat glands in lower and mid epidermis for several days until it is exfoliated by the physiological regeneration of skin. Application is at night when the exocrine sweat glands are largely inactive; thereby allowing active ingredients to penetrate the skin. Long term treatment results in atrophy of the sweat gland acini.5
The sweat glands are anatomically sympathetic and functionally cholinergic. By preventing the exocytosis of acetylcholine, botulinum toxin exerts an inhibitory effect on the cholinergically innervated eccrine secretory cells and reduces sweat production. The injections are painful and topical anaesthetic cream, ethyl chloride liquid spray, cold packs or regional anaesthesia can be used.
The condition carries a substantial psychological and social burden, since it interferes with daily activities. Early detection and management of hyperhidrosis can significantly improve a patient’s quality of life. The aim of this study was to compare the efficacy and results of botulinum toxin injections versus digital iontophoresis with topical 20% aluminium chloride hexahydrate lotion therapy in palmar hyperhidrosis.
Material and methods
A total of 60 cases of primary focal palmar hyperhidrosis with HDSS scores 3 & 4 were enrolled for the study. They were randomized into 2 groups of 30 each such that each group had equal number of HDSS 4 and HDSS 3 cases (i.e.13 and 17 respectively). Alternate HDSS 3/4 case was allotted either arm of therapy. One group was treated with digital iontophoresis along with topical 20% aluminium chloride hexahydrate lotion overnight application to dry palms and the other group with botulinum toxin.
Inclusion criteria
-
•
Patients with focal primary hyperhidrosis meeting diagnostic criteria
-
•
Ages between 10 yrs and 50 yrs
-
•
Both sexes
-
•
Patients with degree of sweating either HDSS 3 or 4 (Table 1)
Exclusion criteria
-
•
Pregnancy and lactation
-
•
Motor neuron disease
-
•
Amino glycoside antibiotics or with known hypersensitivity to botulinum toxin
-
•
Cases of HDSS 1,2 and cases with generalised and other focal hyperhidrosis
Investigations
-
•
Thyroid function tests
-
•
Blood glucose levels
-
•
Uric acid levels
-
•
Psychiatric assessment if necessary
A Minor's starch iodine test is done for qualitative identification of the areas of excessive sweating over the palms. Iodine solution (1–5%) is applied to a dry surface and after a few seconds starch is sprinkled over this area. The starch and iodine interact in the presence of sweat, leaving purplish sediment, recorded by photographs.1
Iontophoresis arm
Iontophoresis using Digital iontophoresis machine was exhibited on a thrice weekly basis using direct current (5–20 mA) both in the forward and reverse direction. Starting at zero, the current was increased slowly till the patient experienced a mild pricking sensation. After 10 min, the current is slowly reduced to zero and the polarity reversed since the anode may be more effective.5 The procedure was repeated for another 10 min after which the current was gradually lowered to zero and the patient freed.
Botulinum arm
According to the areas identified by the photograph, 1 cm by 1 cm grids are marked on the palm by a skin pencil to identify the grids within which botulinum toxin is to be injected.
Regional block anaesthesia to median and ulnar nerves is done 30 min before injection, after ensuring no hypersensitivity to lignocaine by skin test for 20 min (Fig. 1). For median nerve block, the tendons of Palmaris longus and flexor carpi radialis are identified by flexing the wrist. A 30 gauge needle is inserted between the tendons 1 cm proximal to the crease of the wrist perpendicularly to all the planes of skin into the deep fascia. Local anaesthetic 3–5 ml is slowly injected as the needle is withdrawn. The ulnar nerve is anesthetized by palpating the ulnar artery and introducing a needle between it and flexor carpi ulnaris directed toward the ulnar styloid process 1 cm proximal to the crease of the wrist. The bone should be contacted above the process and 3–5 ml of local anaesthetic is injected.
Fig. 1.
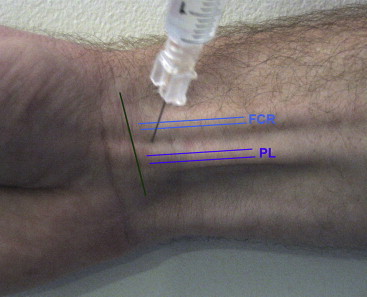
Surface anatomy of various structures at the wrist for median nerve block.
Botulinum toxin vial of 100 units is diluted with 2.5 ml saline so that each mark on the insulin syringe corresponds to 1 unit. In each grid 2 units are injected at the junction of the dermis and subcutaneous tissue where the sweat glands are located. One hand was treated at one session with 2 week interval between one hand and the other to avoid discomfort in both hands together.6 HDSS scores are recorded at baseline and after 4 weeks. If no improvement within 4 weeks the case is crossed over to the other arm.
Scoring
Subjective scores (self reported by patient)
No improvement
Mild improvement: ≥25%
Good improvement: ≥50%
Excellent improvement: ≥75%
Objective scores
Score of HDSS
The validity and reliability of the HDSS was analysed and confirmed in other studies that found correlations with the more complex HHIQ, Dermatology Life Quality Index, and mostly with the gravimetric sweat production for the followup, highlighting that a 1-point improvement of the HDSS score corresponds to 50% of reduction in sweat production and 2-point improvement corresponds to 80% of reduction in sweat production.7–10
Based on this results were objectively scored as
- –Excellent: improvement in HDSS score by ≥2 points (i.e. 80% reduction)
-
•(4–1) or (4–2, 3–1)
-
•
- –Good: improvement in HDSS score by 1 point (i.e. 50% reduction)
-
•(4–3)(3–2)
-
•
–No improvement: No improvement in HDSS score
Both groups observed for 4 wks and if no improvement seen, they were crossed over to other arm after 4 wks.
Statistical tests
Statistical tests used to record the significance were Chi-square test with Yates correction and Fisher's exact test.
Results
Age distribution
The youngest case in the botulinum arm was of 13 years and eldest was 35 years. In the iontophoresis arm the youngest was 10 years and eldest 43 years. Maximum incidence of cases was in the age group of 11–20 yrs with a total of 27 cases, followed by 25 cases in the 21–30 yrs age group (Fig. 2).
Fig. 2.
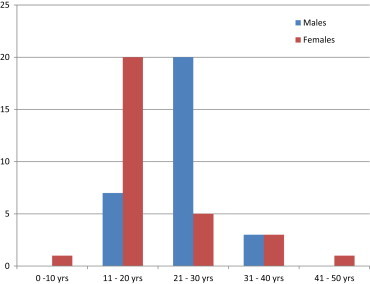
Age distribution of males and female cases of hyperhidrosis.
Sex distribution
There were 9 female patients in the botulinum arm and 12 in the iontophoresis group leading to a total of 21 patients. Rest 39 were male patients, i.e. 21 cases in the botulinum arm and 18 cases in the iontophoresis group.
Botulinum toxin arm
All patients received 100 units per palm of botulinum toxin under wrist block analgesia to both the palms two weeks apart. Response in terms of improvement was recorded in 24 of 30 cases (80%) while 6 cases did not show any significant improvement. After 4 weeks these 6 cases were crossed over to iontophoresis arm.
Iontophoresis and aluminium chloride lotion arm
Out of 30 cases, 14 showed significant improvement (46%) while 16 cases did not improve. After 4 weeks these 16 cases were crossed over to botulinum toxin therapy.
Improvement was statistically significant with botulinum toxin therapy than with iontophoresis and aluminium chloride therapy; P value: 0.007 (Table 2).
Table 2.
Statistical comparison of improvements in both arms.
| Result | Botulinum toxin | Iontophoresis |
|---|---|---|
| Improved | 24 (80.0%) | 14 (46.7%) |
| Not improved | 6 (20.0%) | 16 (53.3%) |
| Total | 30 (100.0%) | 30 (100.0%) |
| Inference | Improved is significantly more associated with botulinum toxin with P = 0.007** | |
Statistical comparison of excellent and good improvements in both arms
The number of cases showing excellent and good improvements were significantly more in botulinum arm than iontophoresis and aluminium chloride arm; 17 (56.7%) excellent and 7 (23.3%) good in botulinum arm versus 8 (26.6%) and 6 (20%) in iontophoresis arm. Statistically significant for botulinum arm (p value = 0.037) (Table 3, Fig. 3).
Table 3.
Statistical comparison of excellent and good improvements in both arms.
| Improvement | Botulinum toxin | Iontophoresis |
|---|---|---|
| Excellent | 17 (56.7%) | 8 (26.7%) |
| Good | 7 (23.3%) | 6 (20.0%) |
| Not improved | 6 (20.0%) | 16 (53.3%) |
Fig. 3.
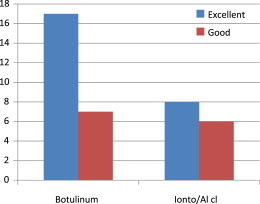
Degree of excellent/good improvement in both arms.
Cross over cases in both arms
Of the 16 cases of iontophoresis arm with no improvements, which were crossed over to botulinum arm after 4 wks, 12 showed excellent improvement (75%) and 4 cases still did not show any improvement.
Out of the 6 cases that did not improve with botulinum toxin after 4 weeks, only one case showed excellent improvement (16%) with iontophoresis and aluminium chloride lotion while 5 cases still did not show any improvement.
When the excellent improvements among the cross over cases was compared between the two arms, the botulinum arm was statistically significant; p value = 0.077 (Table 4).
Table 4.
Statistical comparison of excellent and good improvements in cross over cases in both arms.
| Improvement | Iontophoresis to Botox | Botox to iontophoresis |
|---|---|---|
| Excellent | 12 (75.0%) | 1 (16.7%) |
| Good | 0 | 0 |
| Not improved | 4 (25.0%) | 5 (83.3%) |
| Total | 16 (100.0%) | 6 (100.0%) |
| Inference | Excellent improvement is statistically associated with botulinum toxin with P = 0.077+ | |
Improvements by HDSS severity in both arms
There were 26 cases of HDSS 4 and 34 cases of HDSS 3 distributed equally in both arms leading to the total sample size of 60 cases.
HDSS 4 cases
Botulinum arm
All the 13 initial cases as well as 12 cross over cases of HDSS 4 improved with botulinum giving an improvement of 96%.
Iontophoresis arm
Only one of the initial 13 cases of HDSS 4 improved with iontophoresis and aluminium chloride lotion therapy giving an improvement of 4%.
No failure of therapy was recorded among the HDSS 4 category.
HDSS 3 cases
Botulinum arm
Eleven of the initial 17 cases improved while none of the four crossed over cases improved leading to an improvement of 32%.
Iontophoresis arm
Thirteen of the initial 17 cases improved while one of the crossed over 6 cases improved leading to an improvement of 41%.
A total of 9 HDSS 3 cases (27%) failed to respond to either arm of treatment.
Botulinum toxin therapy proved effective for HDSS 4, i.e. severe cases of hyperhidrosis while iontophoresis and aluminium chloride lotion therapy was not helpful in HDSS 4 cases. Statistically significant (p = 0.003) (Table 5).
Table 5.
Statistical comparison of severity score and improvement in each arm (initial cases).
| Result | Botulinum toxin (n = 30) | Iontophoresis (n = 30) | P value |
|---|---|---|---|
| Improved | |||
| HDSS 4 | 13 (43.3%) | 1 (3.3%) | 0.005 |
| HDSS 3 | 11 (36.7%) | 13 (43.3%) | |
| Not improved | |||
| HDSS 4 | 0 | 12 (40.0%) | 0.003 |
| HDSS 3 | 6 (20.0%) | 4 (13.3%) | |
Mean duration to achieve improvement in both arms
Botulinum toxin
Of the 24 cases which showed improvement, the response started 2 wks after injections and was maintained till 4 months after the injections. Thereafter there was recurrence though not as much as pre-therapy levels. Repeat injections were given at six months after initial injections.
Iontophoresis/aluminium chloride arm
Of the 11 cases that showed improvement, response was seen three weeks into the treatment and persisted as long as the medicine was applied. Once stopped the relapse was observed 1 month after stopping treatment.
Effect of wrist block analgesia
Botulinum toxin was given under wrist block for 30 patients allotted to this group as well as 16 cross over cases from the other group. So a total of 46 patients were given wrist block analgesia.
Of these 46, 38 patients had complete analgesia and no pain during therapy while 8 patients had partial analgesia of whom 6 patients experienced mild pain and 2 patients had moderate amount of pain during therapy which was minimized by using ice packs prior to injections.
Adverse effects: One patient who was given wrist block had mild motor weakness involving opponens action for about 2 weeks following treatment which recovered later. No other side effects were observed.
Discussion
Age and sex distribution: Maximum incidence (45%) was in the younger age groups (11–20 yrs) followed by 21–30 yrs age group (42%) as in the literature.1 The higher incidence can also be attributed to the service population in this study.
The lesser incidence of female cases in this study could be attributed to the less work exposure for females in a service population. Otherwise hyperhidrosis is known to occur equally in both genders.1 Females were more reluctant to take up botulinum toxin injections than males which could explain the lower incidence of females in botulinum group.
Improvements on treatment: Botulinum toxin therapy proved to be a more effective modality of treating hyperhidrosis than iontophoresis with aluminium chloride as shown by the percentage improvement (80% vs. 47%) and statistical significance (p = 0.007). No comparative studies exist in literature between these two arms to best of knowledge of the author. Botulinum toxin proved effective in both HDSS 4 (96%) cases as well as HDSS 3 (32%) cases while iontophoresis and topical aluminium chloride lotion was marginally more effective for HDSS 3 cases (41%) than the HDSS 4 cases (4%).
Excellent and good degree of improvement was statistically associated with botulinum toxin while iontophoresis and aluminium chloride lotion therapy was associated with good or mild improvement.
Acceptance of therapy: Iontophoresis being non invasive was easily accepted by patients. Severe cases of hyperhidrosis on the other hand were eager for botulinum injection therapy to get rid of their ailment.
Time for improvement: Average time for improvement in botulinum arm was 2 wks after injections as quoted in other studies.1,11 In present study, the persistence of improvement was for an average of 4 months though in literature, the relief ranges from 4 to 13 months.2,11 This may be due to brand differences of the injections or the dose of botulinum used per palm. High total doses were thought to be associated with long periods of anhidrosis in one study.12 However higher doses may be associated with increased incidence of subjective hand weakness and reduced finger pinch strength compared with lower dose.
In the iontophoresis arm effective improvement took 3 wks–4 wks to manifest as the therapy was based on compliance of the patient reporting to the department for iontophoresis as well as regular use of aluminium chloride lotion as instructed. Improvements lasted as long as the lotion was used and relapsed 4 wks after stopping therapy. In literature the average times to improvement by iontophoresis alone and aluminium chloride lotion alone are about 2 wks (6–10 treatment sessions over 2 wks) and last as long as the treatment is given.1 For those who improve on iontophoresis maintenance treatment needs to be given every 1–3 months.
Adverse effects: There were no adverse effects of iontophoresis and aluminium chloride therapy. None reported any irritation with aluminium chloride lotion as is mentioned at places in the literature.1 However one case did not tolerate iontophoresis due to feeling of shock sensations on palms.
One case in botulinum arm reported motor weakness of opponens activity of the thumb for 2 wks after injections, which later improved. No permanent motor weakness was observed in botulinum arm nor was any hypersensitivity to local anaesthetic observed.
Efficacy of wrist block: Wrist block analgesia was complete in 38 cases (83%) which shows this procedure to be an effective method of administering analgesia as quoted in a study.12 Since it is a blind procedure, one should be careful not to puncture any arteries or directly injecting into a nerve. In present study, none had any vascular accident in terms of puncturing any arteries during the procedure.
Botulinum toxin also needs to be injected in correct depth for efficacy as injecting too superficially or too deeply may not fruitful.
Grid markings in accordance with the starch iodine test also serve as a good yardstick to give botulinum as sweating is not uniform all over the palm in all cases.
To draw definite conclusions larger sample sizes with varying brands and higher doses of botulinum toxin can be tried to determine persistence of relief for longer periods.
Recommendations
First line therapy to all cases of palmar hyperhidrosis should be iontophoresis and/or aluminium chloride lotion since it is non invasive, but if there is no relief after 4 wks from first line therapy or if the patient is not subjectively satisfied with the degree of improvement, botulinum toxin injections should be administered under regional block in sufficient doses. In literature also most studies favour exhibition of iontophoresis and aluminium chloride therapy as first line to treat milder HDSS 3 cases and botulinum therapy as second line for cases of more severity of HDSS 4 type or failed improvement with iontophoresis arm.5,13
Botulinum toxin should be administered under regional wrist block analgesia for one hand at a time, preferably non-dominant hand at first followed by the dominant hand after a gap of about a week. The injections should be so timed to allow rest for a period of 3–4 days after the injections.
Regional nerve blocks to ulnar nerve should be given carefully keeping the anatomy of ulnar artery in mind and looking for its pulsation. The plunger should be first withdrawn to ensure that the needle is not in the vessel before injecting the local anaesthetic.
Botulinum toxin injections should be administered at the correct depth for maximal effect and also to avoid muscle weakness due to deeper injections. The bevel of the needle should just pierce the skin and at the first sign of ‘give’ of resistance the injection should be delivered.
Combination therapy of both arms can also be tried to prevent relapse of sweating towards the end of the 4–6 month period. If relapse after six months is severe, repeat botulinum toxin injections can be given in the motivated patients.
Conflicts of interest
All authors have none to declare.
Acknowledgement
a. This paper is based on Armed Forces Medical Research Committee Project No 3772/2008 granted by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organisation, Government of India.
b. The authors acknowledge Air Cmde A K Patra, Lt Col Aradhana Sood for study concept and Dr Basheer Ahmed, Resident (Dermatology) for assistance and support in analysis and preparation of the manuscript.
References
- 1.Haider A., Solish N. Focal hyperhidrosis: diagnosis and management. CMAJ. 2005;172(1):1–7. doi: 10.1503/cmaj.1040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornberger J., Grimes K., Naumann M. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. 2004;51:274–286. doi: 10.1016/j.jaad.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Grunfield A., Murray C.A., Solish N. Botulinum toxin for hyperhidrosis a review. Am J Clin Dermatol. 2009;10(2):87–102. doi: 10.2165/00128071-200910020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Yardi S.S., Khopkar U.S., Phadke V.A., Idgunji S.S. Tap water iontophoresis for palmoplantar hyperhidrosis. Indian J Dermatol. 1997;42(3):164–167. [PubMed] [Google Scholar]
- 5.Togel B., Greve B., Raulin C. Current therapeutic strategies for hyperhidrosis: a review. Eur J Dermatol. 2002;12(3):219–223. [PubMed] [Google Scholar]
- 6.Pena M.A., Alam M., Yoo S.S. Complications with the use of botulinum toxin type a for cosmetic applications and hyperhidrosis. Semin Cutan Med Surg. 2007;26:29–33. doi: 10.1016/j.sder.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Strutton D.R., Kowalski J.W., Glaser D.A., Stang P.E. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241–248. doi: 10.1016/j.jaad.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 8.Solish N., Benohanian A., Kowalski J.W. Prospective open-label study of botulinum toxin type A in patients with axillary hyperhidrosis: effects on functional impairment and quality of life. Dermatol Surg. 2005;31(4):405–413. [PubMed] [Google Scholar]
- 9.Lowe N.J., Glaser D.A., Eadie N., Daggett S., Kowalski J.W., Lai P.Y. Botulinum toxin type A in the treatment of primary axillary hyperhidrosis: a 52-week multicenter double-blind, randomized, placebo-controlled study of efficacy and safety. J Am Acad Dermatol. 2007;56(4):604–611. doi: 10.1016/j.jaad.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski J.W., Eadie N., Dagget S., Lai P.Y. Validity and reliability of the hyperhidrosis disease severity scale (HDSS) J Am Acad Dermatol. 2004;50(3):P51. [Google Scholar]
- 11.Vadoud-Seyedi J., Heenen M., Simonart T. Treatment of idiopathic palmar hyperhidrosis with botulinum toxin. Dermatology. 2001;203:318–321. doi: 10.1159/000051780. [DOI] [PubMed] [Google Scholar]
- 12.Solomon B.A., Hayman R. Botulinum toxin type A therapy for palmar and digital hyperhidrosis. J Am Acad Dermatol. 2000;42:1026–1029. [PubMed] [Google Scholar]
- 13.Schieman C., Gelfand G.J., Grondin S.C. Hyperhidrosis: clinical presentation, evaluation and management. Expert Rev Dermatol. 2010;5(1):31–44. [Google Scholar]


