Abstract
Background
Vancomycin is drug of choice for treatment of Methicillin Resistant Staphylococcus aureus (MRSA) infections. S. aureus with reduced vancomycin susceptibility (SA-RVS) is on rise. Current guidelines of detection of SA-RVS are based on MIC (Minimum Inhibitory Concentration) by broth or agar dilution methods. Vancomycin MIC by E test (Epsilometer Test) is an alternative. A study was undertaken to know the prevalence of SA-RVS and compare vancomycin MIC by agar dilution and E test.
Methods
A prospective study was undertaken at tertiary care hospital; 232 clinical MRSA isolates were included. Vancomycin MIC was undertaken by agar dilution method and E test.
Results
All isolates were sensitive to Linezolid. Two MRSA isolates had vancomycin MIC ≥4 μg/ml; vancomycin MIC50 and MIC90 of MRSA isolates was 0.5 and 0.2 μg/ml respectively by agar dilution method. There was agreement over 93.5% isolates in vancomycin susceptibility by agar dilution and E test. E test had sensitivity and positive predictive value of 1.0 (CI – 0.34–1.0) and 0.5 (CI – 0.17–0.83) respectively compare to agar dilution method.
Conclusions
MRSA isolates continues to be susceptible to vancomycin and Linezolid. E test was found equally suitable in initial screening for vancomycin susceptibility. Due to geographic variation in prevalence, there is need of ongoing surveillance of SA-RVC.
Keywords: Staphylococcus aureus, MRSA, Vancomycin, Agar dilution method, E-test (Epsilometer Test)
Introduction
Methicillin Resistant Staphylococcus aureus (MRSA) is responsible for a sizable number of infections globally.1 A multi centric study from India reported a MRSA prevalence of 41% in 2008–2009 from 17 participating tertiary care hospitals from different parts of India.2 Vancomycin is treatment of choice for infections caused by MRSA. With increasing prevalence of MRSA infections, vancomycin use has increased many fold.3 There was emergence of vancomycin resistance enterococci in 1980s; leading to fear of wide spread vancomycin resistance in S. aureus. However, the first report of reduced susceptibility to vancomycin in clinical isolates of S. aureus was reported in 1996.4 Since then, there are many reports of reduced susceptibility to vancomycin from all over the world including India.3,5–8 There is a necessity of surveillance for S. aureus with reduced vancomycin susceptibility (SA-RVS); however, there are roadblocks since most microbiology laboratories perform disc diffusion test for antibiotic susceptibility which is not reliable for vancomycin testing.3 This is probably one of the reasons that many laboratories are not undertaking vancomycin susceptibility testing routinely. A survey by Centers for Diseases Control and Prevention (CDC) published in 2000, indicated that many laboratories participating in Emerging Infections Program were not using methods that can detect SA-RVS.9 CLSI (Clinical and Laboratory Standards Institutes) and EUCAST (European Committee on Antimicrobial Susceptibility Testing) guidelines for diagnosis of VSSA (Vancomycin Susceptible S. aureus), VISA (Vancomycin Intermediate S. aureus) and VRSA (Vancomycin Resistant S. aureus) are based on MIC (minimum inhibitory concentration) by micro-dilution or agar dilution methods.3,10,11 Susceptibility testing by micro-dilution or agar dilution methods is technically demanding. E test is also reported as one of the screening tests by CDC.12 The study was undertaken to know the prevalence of SA-RVS amongst a MRSA isolates at tertiary care hospital and compare agar dilution method and E test in diagnosis of SA-RVS.
Materials and methods
A prospective study was carried out at a tertiary care hospital of a teaching hospital from 01 Sep 2010 to 31 Mar 2013. Non-repeat clinical isolates of MRSA from various clinical specimens were included in the study. Isolates were identified as S. aureus based on morphology, colony characteristics and biochemical reactions as per the standard protocol.13 All isolates were tested for their susceptibility to various antibiotics as Primary, Primary selective, Supplementary and Urine by Kirby Bauer method using CLSI 2009 guidelines.10 Isolates of S. aureus were identified as MRSA by disc diffusion based on mecA mediated oxacillin resistance using cefoxitin disk as surrogate marker.10 S. aureus ATCC 25923 and ATCC 43300 strains were used as negative and positive controls respectively for standardization of procedure and quality control.
MIC of vancomycin by agar dilution method: All MRSA strains were also tested for MIC of vancomycin by agar dilution method. Muller Hinton Agar (MHA) plates containing vancomycin concentrations of 0.25, 0.5, 1, 2, 4, 8, 16 and 32 μg/ml were prepared in house. MRSA isolates were inoculated in nutrient broth and incubated at 37 °C for 4 h. Adjusted 0.5 McFarland bacterial suspensions were inoculated onto these plates with the help of multipoint inoculators with 25 points. S. aureus ATCC 25923 and ATCC 700698 were included in all the test plates as control organisms. Plates were incubated at 35 °C for 24 h. Each spot was noted for the presence of growth or no growth. The least concentration of antibiotic that was able to inhibit visible growth of the organism was taken as MIC of the organism.
Vancomycin MIC by E-test (Epsilometer Test): Commercially available vancomycin E-test strips (AB BIODISK) were used. A 0.5 McFarland standard suspension of MRSA isolates were prepared as described above. Suspensions of isolates were lawn cultured on the MHA plates. Vancomycin E- test strip was applied over the plate with the help of applicator within 5 min of lawn culture. Plates were incubated at 37 °C for 24 h. A tear drop zone of inhibition was observed. The zone edge intersecting the graded strip at the minimum concentration of the antibiotic was interpreted as the MIC.
Definitions: VSSA, VISA, VRSA were defined as isolates having vancomycin MIC by agar dilution method as ≤2, 4–8 μg/ml and ≥16 μg/ml respectively.10 SA-RVS's were VISA or VRSA isolates. The MIC50 and MIC90 were defined as the vancomycin concentrations that inhibited growth of 50 and 90% of the isolates respectively.
Statistical analysis: Comparison of MIC of E test against agar dilution was undertaken for parameters as sensitivity, specificity, positive & negative predictive value (PPV & NPV), positive & negative likelihood ratios with confidence interval at 95%. Pearson product moment correlation coefficient was calculated to see the correlation between MIC's.
Results
A total of 232 non-repeat clinical isolates of MRSA were included in the study. Specimen wise distribution of these isolate is given in Table 1. It is apparent that the maximum MRSA isolates were from pus specimen. The location wise distribution of patients from whom MRSA were obtained is given in Table 2. A total of 26 (11.2%) and 48 (20.8%) of study subjects had more than 48 h stay in ICU or wards respectively indicative of hospital acquired infections. Antimicrobial susceptibility pattern of these isolates is presented in Table 3 and Fig. 1. Resistance to most of the commonly used antibiotics ranged from 60% to 90%. However, all isolates were sensitive to Linezolid.
Table 1.
Specimen wise distribution of samples.
| Nature of specimen | Number | Percentage (%) |
|---|---|---|
| Pus | 178 | 76.7 |
| Blood | 9 | 3.9 |
| Urine | 12 | 5.2 |
| Central line tip | 9 | 3.9 |
| Tracheal aspirate | 7 | 3.0 |
| Sputum | 6 | 2.6 |
| Joint aspirate | 4 | 1.7 |
| Other | 7 | 3.0 |
| TOTAL | 232 | 100 |
Table 2.
Location wise distribution of MRSA isolates.
| Location | No | % |
|---|---|---|
| ICU | 42 | 18.1 |
| Wards | 77 | 33.2 |
| OPD | 113 | 48.7 |
| Total | 232 | 100.0 |
Table 3.
Antimicrobial susceptibility pattern of MRSA isolates.
| Antibiotics | No of isolates tested | Sensitive | % | Resistant | % |
|---|---|---|---|---|---|
| Erythromycin | 220 | 51 | 23.2 | 169 | 76.8 |
| Clindamycin | 220 | 86 | 39.1 | 134 | 60.9 |
| Cefoxitin | 232 | 0 | 0.0 | 232 | 100.0 |
| Penicillin | 232 | 0 | 0.0 | 232 | 100.0 |
| Co-trimoxazole | 232 | 63 | 27.2 | 169 | 72.8 |
| Linezolid | 232 | 232 | 100.0 | 0 | 0.0 |
| Tetracyclin | 232 | 51 | 22.0 | 181 | 78.0 |
| Ciprofloxacin | 232 | 42 | 18.1 | 190 | 81.9 |
| Gentamicin | 232 | 81 | 34.9 | 151 | 65.1 |
| Norfloxacin | 12 | 3 | 25.0 | 9 | 75.0 |
| Nitrofurantoin | 12 | 7 | 58.3 | 5 | 41.7 |
Fig. 1.
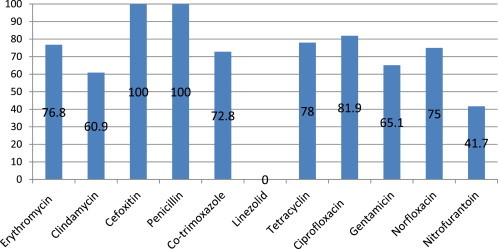
Drug resistance pattern of MRSA isolates.
Vancomycin MIC by agar dilution method: A total of 230 (99.1%) isolates were having vancomycin MIC ≤2 μg/ml i.e. VSSA; amongst them 80.9% (186/230) strains were having vancomycin MIC ≤1 μg/ml. Only 2 (0.9%) isolates were having MIC ≥2 μg/ml i.e. VISA. MIC50 and MIC90 of the study isolates were 0.5 and 2 μg/ml respectively.
MIC by E test: All isolates were also subjected to E test. A total of, 215 (92.7%) isolates were having MIC ≤2 μg/ml. 13 (5.6%) and 4 (1.7%) isolates had MIC in the of 2.5–3.5 and 4 μg/ml respectively. MIC50 and MIC90 of these isolates by E test were 0.75 and 2 μg/ml respectively.
Comparison of vancomycin MIC by agar dilution and E test: There was an agreement over 217 (93.5%) isolates in diagnosis of VSSA or VISA by agar dilution and E test i.e. 215 and 2 respectively. There was non-agreement over 15 isolates. All these isolates had MIC ≤2 by agar dilution; amongst them 13 and 2 isolates had MIC 2.5–3.5 and 4 by E test respectively. The data is presented in Table 4. Pearson product moment correlation coefficient r = 0.862 was found statistically highly significant at df = 230 (<0.05). Thus correlation between MIC by agar dilution and E test was concluded; same is presented in Fig. 2. As compared to MIC by agar dilution method sensitivity, specificity, PPV, NPV, Positive and Negative likely hood ratios were calculated and they were 1.0 (CI – 0.34–1.0), 0.99 (CI – 0.969–0.998), 0.5 (CI – 0.17–0.83), 1.0 (CI – 0.98–1), 115.0 (CI – 28.935–457.052) and 0.17 (CI – 0.01–2.12) respectively.
Table 4.
Comparison of vancomycin MIC by agar dilution and E test.
| MIC by agar dilution |
|||||||
|---|---|---|---|---|---|---|---|
| 4 μg/ml VISA |
≤2 μg/ml VSSA |
Total |
|||||
| No | % | No | % | No | % | ||
| MIC by E test | ≥4 μg/mla | 2 | 0.9 | 2 | 0.9 | 4 | 1.7 |
| <4 μg/mla | 0 | 0.0 | 228 | 98.3 | 228 | 98.3 | |
| Total | 2 | 0.9 | 230 | 99.1 | 232 | 100.0 | |
0.5 was added to all values in calculations of some of the statistical parameters.
Fig. 2.
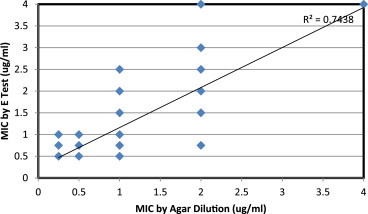
Vancomycin MIC by E test against agar dilution method.
Discussion
Drug resistance in MRSA: MRSA continues to be an important cause of community and hospital acquired infections. A multicentre study from India reported overall prevalence of 41% of MRSA from tertiary care centers.2 Various other studies have also reported similar high prevalence of MRSA in hospital and community acquired infections.14–16 MRSA are routinely isolated from soft tissue and skin infections however invasive infections due to MRSA are not uncommon. Multi drug resistance is common in MRSA isolates. Resistance to commonly used antibiotics such as erythromycin, clindamycin, gentamicin, co-trimoxazole, ciprofloxacin, and tetracycline is reported from all over India.2 Similar were findings seen in this study. Linezolid, a recently introduced antibiotic is one of the alternatives to vancomycin in treatment of skin and soft tissue infections, pneumonia, and urinary tract infections with or without bacteremia due to MRSA.17 All study isolates were sensitive to Linezolid. Very high susceptibility of MRSA isolates to Linezolid was reported from different parts of India.2 Linezolid can be one alternative in treatment of MRSA; however, studies have shown more adverse effect in patients on Linezolid with long-term therapy.18
Vancomycin MIC by agar dilution method and E test: In vitro sensitivity results suggest that vancomycin continues to be an effective drug in the treatment of MRSA infections in our population. MIC50 & MIC90 were in VISS range; monitoring these values over time will help in delineate institutional vancomycin MIC creep (upward trend), if any in future.19 It is well known fact that MIC creep is associated with higher vancomycin MIC with poorer treatment outcomes.19 We have simultaneously processed isolates for MIC E test and agar dilution. Mean MIC by E test was marginally higher at 1.06 (±0.73) against 0.89 (±0.68); however, it was statistically not significant. There was good sensitivity, specificity and MIC correlation between MIC by E test against agar dilution method; however, PPV of E test was 0.5. This could due to very low prevalence of VISA isolates in the study subjects. Various other studies have reported higher MIC by E test compared to MIC by agar dilution method.20,21 One of the reasons for this could be due to the fact that in E test dilutions are in arithmetic progression as against MIC by micro-dilution/agar dilution method where dilutions are in geometric progression. Some of workers postulated that the higher vancomycin MIC results provided by the E test appear to be more reliable in predicting vancomycin treatment responses.22 CDC has adopted E test vancomycin MICs of ≥4 μg/ml as one of the method in screening of VISA.12 We found, E test is more convenient method compared to agar dilution method and can be undertaken for routine clinical use.
Surveillance of S. aureus with reduced vancomycin susceptibility: Surveillance data of >300,000 S. aureus isolates from the United States and Europe showed that vancomycin MICs of 4 μg/ml are very unusual and represent <0.3% of all vancomycin MIC values for that species.23 Howden et al have showed that isolates of S. aureus with vancomycin MICs of 4–8 μg per ml are rare, while S. aureus isolates with vancomycin MICs of 2 μg per ml are relatively common.3 There are few surveillance studies of SA-RVS from India; some of them have reported 100% vancomycin sensitivity amongst MRSA isolates24,25; however, there are reports of VRSA and VISA from India.5–8 Recently published studies from south India have reported sizable VRSA and VISA amongst hospitalized patients.8 Two studies reported VRSA and VISA isolates from normal flora.7,26 15.9% of VISA prevalence was reported by Gowrishankar et al from southern India; they studied 63 MRSA isolates from in patients of group A Streptococcal pharyngitis,7 the highest reported prevalence by any available Indian study. There is lot of variation in prevalence of SA-RVS from different geographic parts of country or even in patient groups. This highlights necessity of active surveillance program in India.
To conclude, the result of our study indicated high antibiotic resistance to commonly used antibiotic by MRSA isolates. MRSA continues to be sensitive to linezolid and vancomycin. E test was found equally sensitive as compared to agar dilution method in screening MRSA for vancomycin susceptibility. With emergence and varying prevalence of S. aureus with reduced vancomycin susceptibility from different geographical regions of India, there is a necessity of an active surveillance program.
Conflicts of interest
All authors have none to declare.
Acknowledgement
This paper is based on Armed Forces Medical Research Committee Project No 4075/2010 granted by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organisation, Government of India.
References
- 1.Enright M.C., Robinson D.A., Randle G., Feil E.J., Grundmann H., Spratt B.G. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci USA. 2002;99(11):7687. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Indian Network for Surveillance of Antimicrobial Resistance (INSAR) Group, India Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res. 2013;137:363–369. [PMC free article] [PubMed] [Google Scholar]
- 3.Howden B.P., Davies J.K., Johnson P.D.R., Stinear T.P., Grayson M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin- intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–138. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiramatsu K., Aritaka N., Hanaki H. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari H.K., Sen M.R. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis. 2006;6:156–162. doi: 10.1186/1471-2334-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha B., Singh A.K., Ghosh A., Bal M. Identification and characterization of a vancomycin-resistant Staphylococcus aureus isolated from Kolkata (South Asia) J Med Microbiol. 2008;57:72–79. doi: 10.1099/jmm.0.47144-0. [DOI] [PubMed] [Google Scholar]
- 7.Gowrishankar S., Thenmozhi R., Balaji K., Pandian S.K. Emergence of methicillin-resistant, vancomycin-intermediate Staphylococcus aureus among patients associated with group A Streptococcal pharyngitis infection in southern India. Infect Genet Evol. 2013;14:383–389. doi: 10.1016/j.meegid.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Thati V., Shivannavar C.T., Gaddad S.M. Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad. Indian J Med Res. 2011;134:704–708. doi: 10.4103/0971-5916.91001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Laboratory capacity to detect antimicrobial resistance, 1998. Morb Mortal Wkly Rep. 2000;48:1167–1171. [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute . 7th ed. CLSI Document M7-A8. CLSI; Wayne, PA: 2009. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard. [Google Scholar]
- 11.EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. The European Committee on Antimicrobial Susceptibility Testing; Sweden: 2012. www.eucast.org [cited 2013 May 15]. Available at: [Google Scholar]
- 12.Hageman J.C., Patel J.B., Carey R.C., Tenover F.C., McDonald L.C. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2006. Investigation and Control of Vancomycin-intermediate and Resistant Staphylococcus aureus; a Guide for Health Departments and Infection Control Personnel.http://www.cdc.gov/ncidod/dhqp/pdf/ar/visa_vrsa_guide.pdf [cited 2013 May 15]. Available from: [Google Scholar]
- 13.Baird D. Staphylococcus: cluster-forming gram-positive cocci. In: Collee J.G., Fraser A.G., Marmion B.P., Simmons A., editors. Mackie & McCartney Practical Medical Microbiology. 14th ed. Elsevier; New Delhi: 2007. pp. 253–261. [Google Scholar]
- 14.Kini A.R., Shetty V., Kumar A.M., Shetty S.M., Shetty A. Community-associated, methicillin-susceptible, and methicillin-resistant Staphylococcus aureus bone and joint infections in children: experience from India. J Pediatr Orthop. 2013;22:158–166. doi: 10.1097/BPB.0b013e32835c530a. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Uria G., Reddy R. Prevalence and antibiotic susceptibility of community-associated methicillin-resistant Staphylococcus aureus in a rural area of India: is MRSA replacing methicillin-susceptible Staphylococcus aureus in the Community? ISRN Dermatol. 2012;2012:248951–248955. doi: 10.5402/2012/248951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsering D.C., Pal R., Kar S. Methicillin-resistant Staphylococcus aureus: prevalence and current susceptibility pattern in Sikkim. J Glob Infect Dis. 2011;3:9–13. doi: 10.4103/0974-777X.77289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falagas M.E., Manta K.G., Ntziora F. Linezolid for the treatment of patients with endocarditis: a systematic review of the published evidence. J Antimicrob Chemother. 2006;58:273–280. doi: 10.1093/jac/dkl219. [DOI] [PubMed] [Google Scholar]
- 18.Beekmann S.E., Gilbert D.N., Polgreen P.M. Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections Network survey. Diagn Microbiol Infect Dis. 2008;62:407–410. doi: 10.1016/j.diagmicrobio.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Joana S., Pedro P., Elsa G., Filomena M. Is vancomycin MIC creep a worldwide phenomenon? Assessment of S. aureus vancomycin MIC in a tertiary university hospital. BMC Res Notes. 2013;6:65. doi: 10.1186/1756-0500-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruzel M.C., Lewis C.T., Welsh C.T. Determination of vancomycin and daptomycin MICs by different testing methods for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2011;49(6):2272–2273. doi: 10.1128/JCM.02215-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tandel K., Praharaj A.K., Kumar S. Differences in vancomycin MIC among MRSA isolates by agar dilution and E test method. Ind J Med Microbiol. 2012;30:453–455. doi: 10.4103/0255-0857.103768. [DOI] [PubMed] [Google Scholar]
- 22.Hsu D.I., Hidayat L.K., Quist R. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of methicillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2008;32:378–385. doi: 10.1016/j.ijantimicag.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Tenover F.C., Moellering R.C., Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari S., Sahu M., Rautaraya B., Karuna T., Mishra S.R., Bhattacharya S. Prevalence of methicillin-resistant Staphylococcus aureus and its antibiotic susceptibility pattern in a tertiary care hospital. J Indian Med Assoc. 2011;109:800–801. [PubMed] [Google Scholar]
- 25.Pai V., Rao V.I., Rao S.P. Prevalence and Antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus [MRSA] isolates at a tertiary care hospital in Mangalore, South India. J Lab Physicians. 2010;2:82–84. doi: 10.4103/0974-2727.72155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goud R., Gupta S., Neogi U. Community prevalence of methicillin and vancomycin resistant Staphylococcus aureus in and around Bangalore, southern India. Rev Soc Bras Med Trop. 2011;44:309–312. doi: 10.1590/s0037-86822011005000035. [DOI] [PubMed] [Google Scholar]


