Abstract
The arcuate nucleus (ARC) of the hypothalamus plays a key role in sensing metabolic feedback and regulating energy homeostasis. Recent studies revealed activation of microglia in mice with high-fat diet (HFD)-induced obesity (DIO), suggesting a potential pathophysiological role for inflammatory processes within the hypothalamus. To further investigate the metabolic causes and molecular underpinnings of such glial activation, we analyzed the microglial activity in wild-type (WT), monogenic obese ob/ob (leptin deficient), db/db (leptin-receptor mutation), and Type-4 melanocortin receptor knockout (MC4R KO) mice on either a HFD or on standardized chow (SC) diet. Following HFD exposure, we observed a significant increase in the total number of ARC microglia, immunoreactivity of ionized calcium binding adaptor molecule 1 (iba1-ir), cluster of differentiation 68 (CD68-ir), and ramification of microglial processes. The ob/ob mice had significantly less iba1-ir and ramifications. Leptin replacement rescued these phenomena. The db/db mice had similar iba1-ir comparable with WT mice but had significantly lower CD68-ir and more ramifications than WT mice. After 2 weeks of HFD, ob/ob mice showed an increase of iba1-ir, and db/db mice showed increase of CD68-ir. Obese MC4R KO mice fed a SC diet had comparable iba1-ir and CD68-ir with WT mice but had significantly more ramifications than WT mice. Intriguingly, treatment of DIO mice with glucagon-like peptide-1 receptor agonists reduced microglial activation independent of body weight. Our results show that diet type, adipokines, and gut signals, but not body weight, affect the presence and activity levels of hypothalamic microglia in obesity.
Keywords: leptin, obesity, high calorie diet
Introduction
Excessive energy intake is a major cause of obesity, which is recognized as one of the greatest threats to human health worldwide. Disproportionate hunger and food intake signals are generated as a result of an imbalance in the metabolic sensing and regulation networks of the central nervous system (CNS). Studies of the interaction between these networks and metabolism have suggested that malfunction of hypothalamic neurons is the leading candidate to explain the pathology of metabolic disease (Velloso and Schwartz, 2011; Yi et al., 2011). However, the specific causes of neuronal malfunction in these brain regions remain largely unknown, particularly regarding the influence from glia, which are important in maintaining a homeostatic microenvironment for neuronal survival.
Among the different types of glia, the resident macrophages—known as microglia—are responsible for cell debris clearance and release of cytokines to recruit other immune-responsive cells to the CNS (Neumann et al., 2009). In a recent study of metabolic syndrome induced by consumption of a high-fat diet (HFD), we showed that microglia in the hypothalamic arcuate nucleus (ARC) adopt a pro-inflammatory state. This hyperactivation is linked to an abnormal increase in the production of pro-inflammatory cytokines, which may be toxic to neurons, and results in loss of POMC neurons and decreased function of the neuronal network involved in maintaining energy balance (Smith et al., 2012; Thaler et al., 2012). Thus, in addition to neurons, microglia in the ARC could play an important role in maintaining energy homeostasis. The metabolic causes of hypothalamic microglial activation in obesity, however, remain to be elucidated.
In this study, we investigated microglial activity under diet induced or monogenic obese conditions, by using immunoreactivity of ionized calcium binding adaptor molecule 1 (iba1-ir), which is upregulated during microglial activation (Imai et al., 1996; Ito et al., 2001; Postler et al., 2000), and cluster of differentiation 68 (CD68-ir), which is associated with microglial phagocytosis (Betjes et al., 1991). We also analyzed the ramification of microglial processes under different obese conditions. We found that a high-calorie diet not only increases the number of iba1-ir and CD68-ir positive microglia but also increases non-iba1 microglia presence in the ARC of mice engineered to express green fluorescent protein (GFP) in microglia. Cell proliferation markers ki67 and BrdU do not differ in the ARC of either standardized chow (SC) diet or HFD fed mice, suggesting the increase of microglia under HFD is not due to local cell proliferation. Comparison of microglial activity between wild-type (WT), monogenic obese ob/ob (leptin deficient) (Halaas et al., 1995), db/db (leptin receptor mutation) (Chen et al., 1996), and Type-4 melanocortin receptor knockout (MC4R KO) mice (Gantz et al., 1993) revealed that microglial activity is not controlled by obesity per se but by HFD associated factors or leptin. Treating primary cultured hypothalamic microglia with serum from SC diet or HFD fed mice showed HFD—but not SC—serum stimulates microglial activity and production of cytokines. Furthermore, ob/ob and db/db mice hypothalami display different levels of microglial functional related gene expression. Treating diet-induced obese (DIO) mice with a glucagon-like peptide-1 receptor agonist was associated with a decrease of microglial iba1-ir and ramification. Together, these data suggest that metabolic hormones and diet, but not body weight, are major players of controlling the hypothalamic microglia activity under obese conditions.
Materials and Methods
Animals
All studies were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Cincinnati. Wild type, Lepob/+ (for breeding of ob/ob mice), Lepob/ob (ob/ob), Leprdb/db (db/db), MC4R KO, and CX3CR1-enhanced green fluorescent protein (eGFP) mice (expresses eGFP in monocytes, dendritic cells, NK cells, and brain microglia under the control of the endogenous Cx3cr1 locus) were all obtained from the Jackson Laboratory with C57BL/6 background. Lepob/ob mice with microglial eGFP expression were generated by crossing Lepob/+ mice with CX3CR1-eGFP mice. All mice were group housed on a 12-h light, 12-h dark cycle (6 a.m.–6 p.m.) at 22°C, with free access to food and water.
Measuring Cell Proliferation Activity in the ARC
To investigate if iba1-ir, CD68-ir, or GFP positive microglia increased due to microglial proliferation inside the ARC, we placed intracerebral ventricular (ICV) infusion probes into the lateral intracerebral ventricle in SC diet and HFD fed mice and injected bromodeoxyuridine (BrdU) for 5 days (10 mg/mL, 5 μL/day). In a parallel group, we also injected BrdU intraperitoneally (i.p.) (10 mg/mL, 50 μL/10 g body weight/day, 5 days). Cell proliferation was also studied by measuring Ki67 expression in separate groups of SC diet and HFD fed mice 1 week after mechanical injury (as a positive control for cell proliferation induced by injury) induced by inserting a needle into mediobasal hypothalamus area next to the ARC.
Leptin Treatment of ob/ob Mice and Exendin-4 Treatment of DIO Mice
For leptin treatment, 10-week-old ob/ob mice and age-matched WT mice were divided into three subgroups (n = 5–7): vehicle treatment, leptin treatment, and vehicle-treated animals pair-fed to the leptin treatment group. In addition, 16 DIO mice with body weight matched to ob/ob mice were divided into two subgroups: vehicle treatment and leptin treatment. Each group of mice was matched for body weight, body fat mass, and food intake at baseline. Subcutaneous injections of leptin (1 mg/kg) or vehicle were administered daily for 5 days; food intake and body weight were monitored daily.
For exendin-4 treatment, 8-month-old DIO mice were divided into three groups (n = 5–7): vehicle treatment, exendin-4 treatment, and mice pair-fed to the exendin-4 treatment group. Exendin-4 (0.25 mg/kg) or vehicle subcutaneous injections were administered daily for 5 days; food intake and body weight were monitored daily.
Immunohistochemistry and Immunofluorescence of Mouse Brain Tissue
Mouse brain sections used for immunohistochemical and immunofluorescent staining were prepared by perfusion fixation as described before (Yi et al., 2012b). For iba1, CD68, Ki67, and BrdU immunohistochemistry, brain sections at the level between bregma −1.70 and −1.94 (Paxinos and Franklin, 2008) for staining in the ARC were divided into two groups by selecting alternating sections from 8 to 9 continuing sections; in addition, brain sections, including the damage areas caused by ICV probes (for BrdU) or needle insertion (for Ki67), were also selected. Immunohistochemistry and immunofluorescence staining are presented in Supporting Information data 1.
Leptin or Serum Treatment in Primary Cultured Hypothalamic Microglia
Primary cultured hypothalamic microglia were obtained from post-natal day 2 from dissected hypothalamus (Paxinos and Franklin, 2008) and cultured by a method adapted from Saura et al. (2003) (Supp. Info. data 2). Seventeen to 18 days afterward, cultured microglia were treated either with leptin (100 ng/mL) and vehicle for 120 min or with 1% serum collected from 6 months HFD or SC diet fed mice for 120 min (n = 3–4). Total RNA was isolated and real-time PCR was applied with TaqMan® probes (Applied Biosystems).
Low-Density Array Real-Time PCR
To broadly compare microglial function and the associated changes in the hypothalami of HFD induced obese mice or mice with leptin deficiency/leptin receptor mutation (body weight matched to DIO mice), we applied low-density array real-time PCR (Applied Biosystems, customized microfluidic card) in mice hypothalamic tissue (Supp. Info. data 3). Hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as house keeping gene. Data were analyzed by target genes relative expression/HPRT.
Statistical Analysis
All results are expressed as mean ± SEM. Statistical comparisons were performed using one-way or two-way ANOVA. A P value below 0.05 was considered statistically significant.
Results
Activity Levels as well as Total Numbers of Microglia Are Increased in the ARC of Mice Fed a HFD Diet
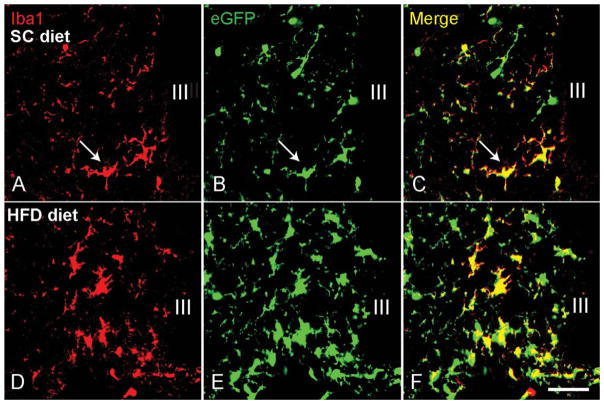
In a previous study, we showed that the number of iba1-immunoreactive microglia was increased in WT mice fed a HFD (Thaler et al., 2012). To determine whether this increase is due solely to the activation of resident microglial cells or if it is accompanied by an increase in the total microglia population, we quantified and compared the number of activated microglia to the total microglial population after 6 weeks of HFD or SC diet (started at age 6 weeks). In the ARC of mice fed a SC diet for 12 weeks, the average number of detectable iba1-positive microglia was 20.83 ± 1.03 per 0.06 mm2 field (Fig. 1A). The total number of microglia (revealed by eGFP expression under the control of the endogenous Cx3cr1 locus) was 27.33 ± 1.76 per 0.06 mm2 field (Fig. 1B). Thus, the total number of microglia was significantly higher than the detectable iba1-positive microglia (P = 0.013). When mice were exposed to 6 weeks of HFD, the total number of microglia increased to 50.50 ± 3.0/0.06 mm2 while the number of detectable iba1-positive cells was 31.92 ± 1.87 per 0.06 mm2 field (P < 0.001 vs. total eGFP and P < 0.001 vs. iba1-positive microglia in 10-week SC-fed mice, Fig. 1E,F). Increases occurring in the ARC were not observed in other areas of the hypothalamus. These data indicate that HFD increases the overall number of microglia number specifically in the ARC in addition to stimulating microglial activity.
FIGURE 1.
High-fat diet (HFD) increases the total number of microglia and the number of activated iba1-positive microglia in the arcuate nucleus of the hypothalamus. Wild-type mice fed a standard chow (SC) diet have a modest number of microglia (expressing eGFP (green) under the control of the endogenous Cx3cr1 locus) (B), including some iba1-positive cells (red, arrows in A and C). After 6 weeks of HFD diet, both the total number of microglia (E) and the number of iba1-positive microglia (D) are increased. Under these conditions, only a proportion of the total microglial population is still iba1-positive (F). III indicates third ventricle; scale bar = 25 μm.
Microglial Activation Is Mainly Induced by HFD Diet but Not by Increased Fat Mass
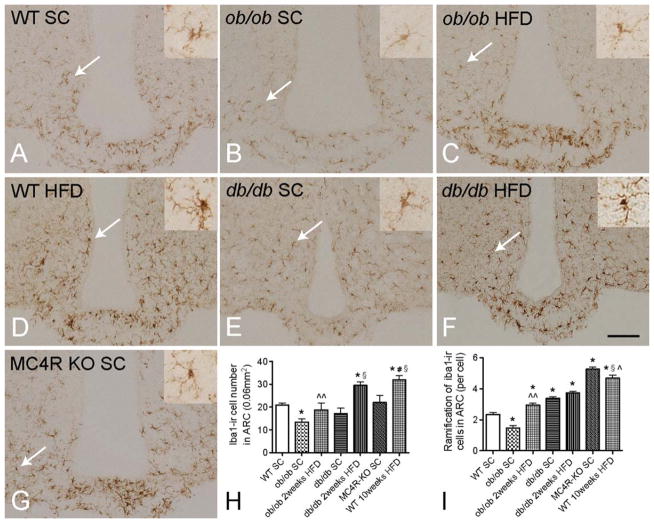
To determine whether microglial activation is caused by HFD diet or by obesity per se, we quantified and compared iba1-ir, iba1-ir ramified processes and CD68-ir between WT mice fed with SC diet or HFD and ob/ob, db/db, and MC4R KO mice fed with SC diet (otherwise matched), as well as ob/ob and db/db mice fed with HFD. The number of iba1-ir microglia was significantly lower in the ARC of SC-fed obese ob/ob mice than in age-matched SC-fed WT controls (Fig. 2A,B). Furthermore, the intensity of the iba1-ir in the soma of microglia from ob/ob mice was less than in WT controls. CD68-ir tended to be less in ob/ob mice than WT mice, but the levels did not reach significance (Fig. 3A,B). Also, there was less extensive ramification of iba1-positive microglial processes in ob/ob than in WT mice (Fig. 2I). Interestingly, the total microglial number did not differ between WT and ob/ob mice (Fig. 4), indicating that only microglia activity (demonstrated by iba1-ir) was influenced by leptin deficiency. Obese db/db mice fed a SC diet had similar numbers of iba1-ir microglia (Fig. 2E) but significantly less CD68-ir compared with WT controls (Fig. 3F). Moreover, there were significantly more iba1-ir ramified processes in db/db than in WT mice, indicating that leptin deficiency and leptin receptor mutations have diverse influences on microglial activity and phagocytic capacities. When ob/ob mice were exposed to 2 weeks of HFD, the number of iba1-ir ramified processes and CD68-ir in the ARC was greater than that of WT, SC-fed mice (Figs. 2C and 3C), suggesting that HFD diet alone can change microglial activation in ob/ob mice in the absence of leptin. In db/db mice fed with 2 weeks of HFD, the CD68-ir increased significantly compared with that of SC-fed mice (Fig. 3G); however, the values were still lower than in WT mice, in which no clear change in iba1-ir ramified processes was observed (Fig. 2F). In SC-fed, obese MC4R KO mice, iba1-ir microglial numbers in the ARC were similar to those of WT SC-fed controls (Fig. 2G) and significantly less than those of body-weight matched, 10-week HFD-fed WT DIO mice, however, the ramification of MC4R KO mice is similar to 10-week HFD-fed WT DIO mice; furthermore, CD68-ir was comparable with WT mice (Fig. 3D). These findings were particularly intriguing as the body weight of the MC4R KO mice matched the ob/ob, db/db and 10 weeks HFD DIO mice. Taken together, these data demonstrate that microglial activation is mainly a consequence of HFD but not obesity per se. Furthermore, either leptin deficiency or leptin receptor deficiency can cause different types of microglial underactivation.
FIGURE 2.
Iba1-ir microglia in the hypothalamic arcuate nucleus (ARC) of diet induced and monogenic obese mice. The numbers of iba1-positive microglia in the ARC of ob/ob mice (B) is reduced compared with wild-type (WT) mice (A) on standard chow (SC) diet as well as to WT mice on high-fat diet (HFD) (D, H), and the microglial processes are less extensively branched (high-magnification images for the cells pointed by white arrows are in the upper right panel) (I). The ARC of ob/ob mice fed a HFD contains an increased number of iba1-ir positive microglia, which also have more extensively branched processes (C). When body weight is matched to the ob/ob group (D), similar numbers of iba1-positive microglia are observed in db/db (E) and WT mice. Ramification of microglia in db/db mice is significantly higher than in SC diet fed WT mice and lower than in HFD fed WT mice. After feeding with 2 weeks HFD (F), the number of iba1-ir positive microglia is increased in the ARC, while ramification is not changed. In obese MC4R KO mice (G) with body weight matched to ob/ob and db/db mice and 10 weeks HFD-fed mice, the number of iba1-positive microglia are significantly lower than in WT HFD-fed controls, while ramification did not differ between MC4R KO and WT HFD mice. WT mice fed for 10 weeks with HFD have the largest number of iba1-positive microglia with the most extensive ramification (D). P < 0.05 for *: vs. WT SC group, ^^: vs. ob/ob SC group, §: vs. db/db SC group, #: vs. MC4 KO group, ^: vs. db/db HFD group; scale bar = 100 μm.
FIGURE 3.
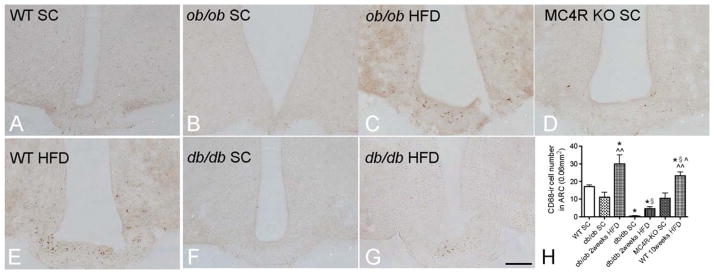
CD68-ir microglia in the hypothalamic arcuate nucleus of diet induced and monogenic obese mice. In comparison to iba1-ir, which stains both the soma and processes of microglia, CD68-ir is mainly visible inside the soma of the microglia. P < 0.05 for *: vs. WT SC group, ^^: vs. ob/ob SC group, §: vs. db/db SC group, ^: vs. db/db HFD group; scale bar = 100 μm.
FIGURE 4.
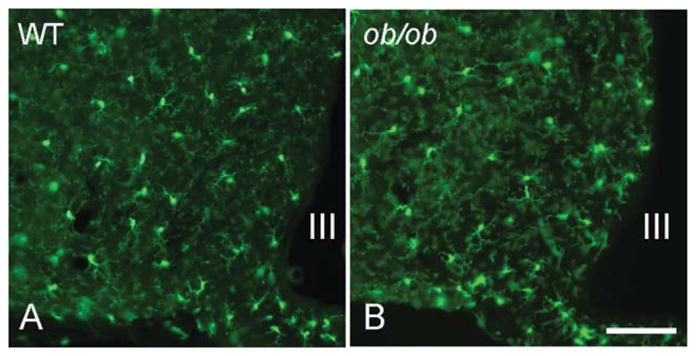
Total microglial population in the hypothalamic arcuate nucleus (visualized by eGFP expression under the control of the endogenous Cx3cr1 locus) does not differ between WT mice (A) and ob/ob (B) mice. III indicates third ventricle; scale bar = 100 μm.
Total Cell Proliferation Activity is Low in the ARC
After 5 days of ICV or i.p. administration, the BrdU positive nuclei are expressed abundantly around the area damaged by ICV probes; however, there are very few BrdU labeled nuclei in the ARC of SC diet and HFD fed mice. Similarly, Ki67 immunohistochemistry staining shows clear, positive immunoreactivity in the nuclei of cells in the brain region injured by mechanical injury, but not in the ARC of SC diet or HFD fed mice (Supp. Info. Fig. 1). This indicates that there are very few cell proliferation events taking place in the ARC under either diet. The increased iba1 and CD68 immunoreactive microglia may be due to higher expression of these proteins, migration of microglia from other brain regions, or infiltration of peripheral macrophages into the ARC.
Leptin and GLP-1 Receptor Agonist Normalize Microglial Activity in the ARC
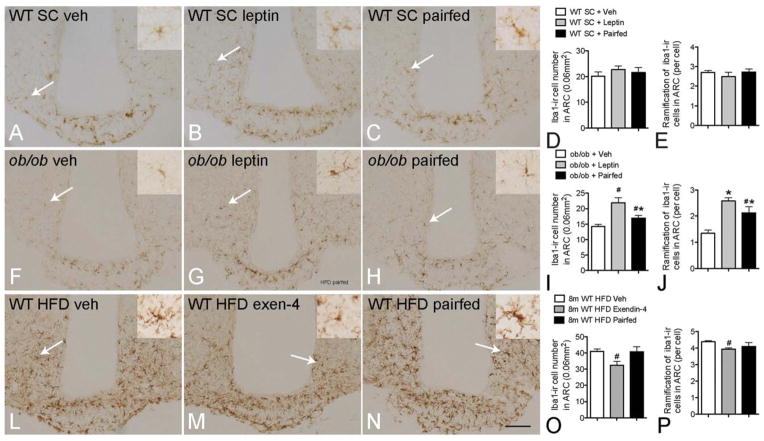
Given the under-activation of microglia observed in ob/ob mice, we examined whether leptin replacement can rescue microglial activation. After 5 days of leptin treatment, in age-matched WT (Fig. 5A–E) and body-weight matched DIO groups, there was no effect on either number or morphology of the microglia. The ob/ob mice treated by leptin had significantly reduced food intake and body weight (data not shown), as shown previously (Halaas et al., 1995). The number of iba1-ir microglial and iba1-ir ramified processes in the ARC was significantly increased compared with that of vehicle controls (Fig. 5F–J). The number of iba1-positive microglia and iba1-ir ramified processes in the pair-fed group (restricted food intake matched to leptin-treated group) was slightly increased but less than in the leptin treated group. These data indicate that leptin is important for maintaining normal microglial activity.
FIGURE 5.
Effect of anti-obesity pharmacotherapy on hypothalamic microglial activation in obese mice. Compared with vehicle control (A), no effects on iba1-ir and ramification were observed in WT mice treated with 5 days of leptin or pair-fed (high-magnification images for the cells pointed by white arrows are in the upper right panel) (A–E); 5 days of leptin treatment significantly increased the iba1-ir microglial number and ramification in the arcuate nucleus (ARC) of ob/ob mice in comparison with the vehicle group. The pair-fed (to leptin) group also had significantly increased microglial activation but with less-extensive ramification (F–J). Exendin-4 treatment in mice with diet-induced obesity significantly reduced the number of iba1-positive microglia and ramification in the ARC compared with vehicle control and pair-fed control groups (L–P). P < 0.05 for #: vs. vehicle in (I), (J), (O) and (P); *: vs. leptin group; scale bar = 100 μm.
As the rescued microglial activity in leptin-treated ob/ob mice suggests that abnormal microglia activity can be normalized, we tested whether hyperactivation of microglia also can be normalized with the glucagon-like peptide-1 agonist exendin-4 (Flint et al., 1998; Gutniak et al., 1992; Thorens et al., 1993) in DIO mice fed HFD for 8 months. Five days of exendin-4 treatment in DIO mice significantly reduced food intake (data not shown) and body weight (Supp. Info. Fig. 2). As expected, the pair-fed group also showed significantly reduced body weight. However, compared with the vehicle group, the number of iba1-positive microglia and their ramifications in the ARC was only significantly reduced in the exendin-4 treated group, not in the pair-fed control group (Fig. 5L–P). These data indicate that normalization of microglial activation occurs in parallel with improvement of the metabolic syndrome following exendin-4 treatment but not reduction of HFD intake.
Leptin or High-Fat Diet Fed Mice Derived-Serum Stimulates Cytokines Production in Primary Cultured Hypothalamic Microglia
In primary cultured hypothalamic microglia, after 120 min of leptin treatment, the gene expression levels of two cytokines that have been shown to increase in the hypothalamus upon HFD (Thaler et al., 2012), TNFα, and IL-1β, both increased significantly. The expression level of iba1 did not show a significant difference (Fig. 6A). This suggests that leptin is one of the factors that can stimulate microglial activation and cytokine production. Next, we treated primary cultured hypothalamic microglia with serum collected from SC diet or HFD fed mice, to check whether and to what extent HFD derived circulating factors influence microglial activity. With 1% concentration, HFD-serum significantly increased iba1, TNFα, and IL-1β gene expression (Fig. 6B), suggesting HFD derived circulating factors can stimulate hypothalamic microglial activity and cytokine production.
FIGURE 6.
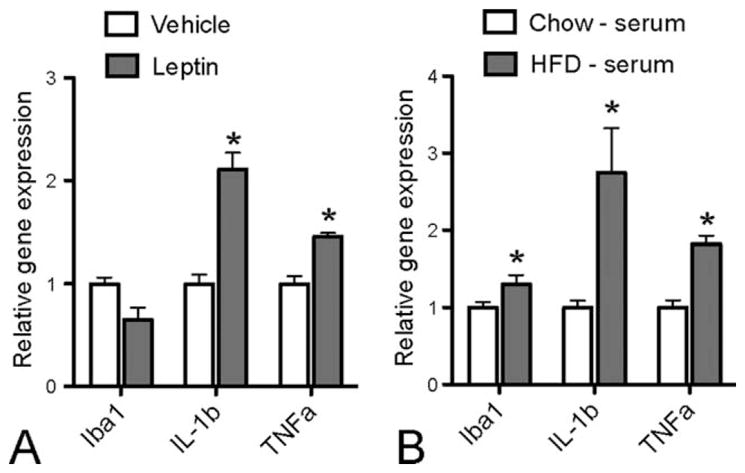
In cultured primary hypothalamic microglia, leptin treatment stimulates IL-1β and TNFα, but not iba1 gene expression (A), whereas HFD-fed mice serum treatment stimulates iba1, IL-1β, and TNFα genes expression compared with SC diet fed mice serum (B). P < 0.05 for *: vs. vehicle group or Chow - serum group.
Lack of Leptin Signaling Affects Microglial Function in Hypothalamus
In homogenized, whole hypothalamic tissue, ob/ob mice have significantly less Emr1 (F4/80) (Kwakkenbos et al., 2002) (Fig. 7), nucleotide binding and oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) (Heneka et al., 2013), integrin-alpha X (Itgax) (Akiyama and McGeer, 1990), ras-related protein Rab-4A (Rab4a), and IL-1β gene expression. The db/db mice have significantly lower activation and transcription factor-3 (ATF-3), Itgax, Rab4a, IL-1β, and TNFα gene expression, while cyclin-dependent kinase inhibitor 1A, involved in microglial proliferation (Yamamoto et al., 2012), is higher in both ob/ob and db/db mice. In addition, HFD fed mice have significantly higher IL-1β gene expression than SC diet fed mice. This indicates that a lack of leptin signaling can affect different aspects of microglial function that may be involved in an abnormality of the hypothalamic network in controlling metabolic homeostasis.
FIGURE 7.
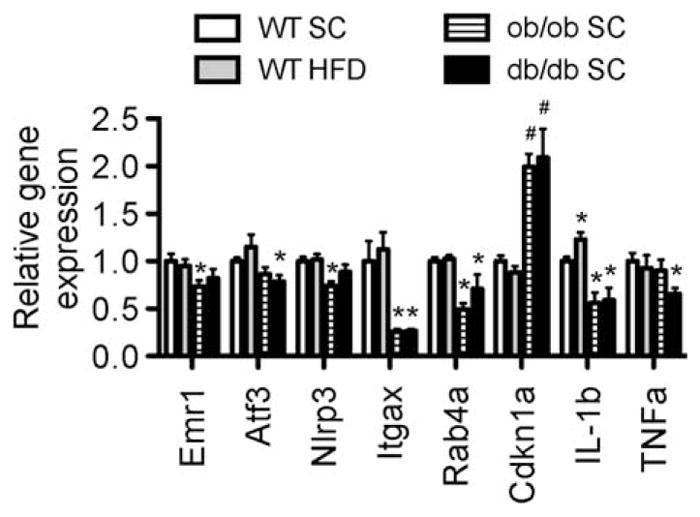
Microglial functional related gene expression profiles in the hypothalamus of diet-induce obese and monogenic obese mice. *P < 0.05 vs. WT SC, #P < 0.05 vs. both WT SC and WT HFD groups.
Discussion
We have shown that in addition to enhanced microglial activation, there is an increase in the total number of microglia in the ARC of mice following induction of obesity through exposure to a HFD. This effect on hypothalamic microglia is induced by the HFD itself rather than obesity or adiposity per se. We have also shown that leptin is important for maintaining normal levels of microglial activity and is involved in cytokine production. Lack of leptin signaling in ob/ob and db/db mice causes deficiencies in various aspects of microglial function in the hypothalamus. Furthermore, we have demonstrated that both under- and over-activation of microglia in ob/ob and DIO mice can be normalized by administration of leptin and a GLP-1 receptor agonist. Taken together, these data provide important insights into the control of hypothalamic microglial activation in obesity and highlight a potential new target for the treatment of obesity and related diseases.
We observed that the total number of microglia in the ARC is significantly increased in HFD-fed DIO mice, likely due to migration of microglia from other brain regions, or infiltration of macrophages from the periphery (Nelson et al., 2002) as no clear cell proliferation profiles can be detected in the ARC of HFD fed mice. The possibility of infiltration is supported by our recent observation that during HFD feeding there is extensive deposition of IgG inside the ARC microglia (Yi et al., 2012a), indicating increased permeability of the blood–brain barrier (BBB). This functional change in the BBB is consistent with the observation that hypervascularization and angiopathy, along with endothelial damage, are induced in the hypothalamus by exposure to HFD (Yi et al., 2012a,b). It is possible that a malfunction of the BBB during HFD feeding leads to increased recruitment of macrophages from the general circulation to assist the resident microglia with maintaining an adequate immune response. Whether or not these pathological changes in the vascular system are solely responsible for the observed effects on hypothalamic microglia, it seems clear that calorie-rich dietary conditions have deleterious effects on the microenvironments that are required to support neuronal survival. Interestingly, in ob/ob and/or db/db mice, although most of the microglial activity markers in the total hypothalamic homogenized tissue are lower than in the WT mice, one of the cell proliferation genes, cdkn1a, has a higher expression level, suggesting there are factors stimulating cdkn1a but further cell proliferation processes are blocked by other factors associated with lack of leptin signaling. The exact mechanism under this phenomenon requires further study.
In contrast to the effect of HFD on the accumulation and activation of microglia in the ARC of WT mice, the absence of leptin signaling in obese ob/ob mice obstructs normal microglial activity and production of cytokines such as IL-1β. Interestingly, when compared with WT mice, the expression of the microglial-derived cytokine TNFα is lower in db/db but not in ob/ob mice. Furthermore, db/db mice do not show lower iba1-ir in the ARC, revealing another phenotypic distinction, in addition to persistent hyperglycemia, between ob/ob and db/db mice. Whether hyperglycemia in the db/db mice is involved in maintaining some aspects of microglial activity or normal iba1-ir is unknown. Regardless, the different microglial phenotypes between db/db and ob/ob mice raise the possibility that the abnormal activity of microglia is one of the causes of the ob/ob and db/db phenotypes. Mixed cultures of macrophages from ob/ob and db/db mice have been shown to have deficient phagocytic activity due to lack of leptin signaling (Lafrance et al., 2010; Loffreda et al., 1998). This deficient phagocytic activity probably also applies to microglia in the CNS. The ob/ob and db/db mice have lower IL-1β and/or TNFα in the hypothalamus, which could be the direct cause of the inability of these mice to maintain or recruit other microglia or macrophages into the hypothalamus for endocytic clearance of debris and maintenance of a healthy microenvironment (Napoli and Neumann, 2009). Leptin is able to stimulate TNFα and IL-1β production in primary cultured hypothalamic microglia, providing evidence that leptin is one of the essential triggers of cytokine production in microglia. Whether reactivating cytokine production in ob/ob and db/db mice can rescue the obese and/or diabetic phenotypes remains unanswered.
The GLP-1 receptor agonist exendin-4 is a well-characterized anti-obesity and anti-diabetic agent (Barrera et al., 2011). It acts through the GLP-1 receptor, which is expressed by microglia (Iwai et al., 2006) to deactivate microglial hyperactivity induced by consumption of a HFD. In other animal models of neurodegenerative diseases, GLP-1 treatment can protect neurons from degeneration via the reduction of pro-inflammatory molecules and cytokines arising from suppression of microglial activity (Kim et al., 2009; McClean et al., 2011). Thus, in the present study, by suppressing microglial hyperactivity, exendin-4 could also protect ARC neurons from the effects of inflammatory cytokines.
Overall, our results reveal that hypothalamic microglial activation is induced by HFD independent of body weight. This activation can be suppressed by GLP-1 receptor agonism while improving other metabolic abnormalities. Thus, microglial activation in the hypothalamus is an important cellular readout of the metabolic syndrome. As a major player in controlling immunity and homeostasis in the CNS, microglia may represent a relevant target for the prevention and treatment of metabolic disorders. Thus, combinatorial pharmacotherapy aimed at not only modifying neuronal function but also normalizing microglia and other components of the neuronal “supporting unit” may represent a promising therapeutic approach.
Supplementary Material
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: An integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betjes MG, Haks MC, Tuk CW, Beelen RH. Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology. 1991;183:79–87. doi: 10.1016/S0171-2985(11)80187-7. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55:352–360. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol. 2009;202:431–439. doi: 10.1677/JOE-09-0132. [DOI] [PubMed] [Google Scholar]
- Kwakkenbos MJ, Chang GW, Lin HH, Pouwels W, de Jong EC, van Lier RA, Gordon S, Hamann J. The human EGF-TM7 family member EMR2 is a heterodimeric receptor expressed on myeloid cells. J Leukocyte Biol. 2002;71:854–862. [PubMed] [Google Scholar]
- Lafrance V, Inoue W, Kan B, Luheshi GN. Leptin modulates cell morphology and cytokine release in microglia. Brain Behav Immun. 2010;24:358–365. doi: 10.1016/j.bbi.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. New York: Elsevier; 2008. [Google Scholar]
- Postler E, Rimner A, Beschorner R, Schluesener HJ, Meyermann R. Allograft- inflammatory-factor-1 is upregulated in microglial cells in human cerebral infarctions. J Neuroimmunol. 2000;104:85–91. doi: 10.1016/s0165-5728(99)00222-2. [DOI] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int J Obes (Lond) 2011;35:1455–1465. doi: 10.1038/ijo.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kohsaka S, Nakajima K. Role of cell cycle-associated proteins in microglial proliferation in the axotomized rat facial nucleus. Glia. 2012;60:570–581. doi: 10.1002/glia.22291. [DOI] [PubMed] [Google Scholar]
- Yi CX, Gericke M, Krüger M, Alkemade A, Kabra DG, Hanske S, Filosa J, Pfluger P, Bingham N, Woods SC, Herman J, Kalsbeek A, Baumann M, Lang R, Stern JE, Bechmann I, Tschöp MH. High calorie diet triggers hypothalamic angiopathy. Mol Metab. 2012a;1:95–100. doi: 10.1016/j.molmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Tschop MH, Woods SC, Hofmann SM. High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Dis Model Mech. 2012b;5:686–690. doi: 10.1242/dmm.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Zeltser L, Tschop MH. Metabolic Syndrome ePoster—Brain and Neuron. Nat Med [Online] 2011;(Supplementary 17)(7) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.