Abstract
Heart disease is the leading cause of morbidity and mortality worldwide, and regenerative therapies that replace damaged myocardium could benefit millions of patients annually. The many cell types in the heart, including cardiomyocytes, endothelial cells, vascular smooth muscle cells, pericytes, and cardiac fibroblasts, communicate via intercellular signaling and modulate each other’s function. Although much progress has been made in generating cells of the cardiovascular lineage from human pluripotent stem cells, a major challenge now is creating the tissue architecture to integrate a microvascular circulation and afferent arterioles into such an engineered tissue. Recent advances in cardiac and vascular tissue engineering will move us closer to the goal of generating functionally mature tissue. Using the biology of the myocardium as the foundation for designing engineered tissue and addressing the challenges to implantation and integration, we can bridge the gap from bench to bedside for a clinically tractable engineered cardiac tissue.
Keywords: cardiovascular regenerative medicine, myocardial infarction, heart failure, coronary artery disease, cardiac tissue engineering, stem cells, microvessel engineering
1. INTRODUCTION
The heart is the first organ to form during embryogenesis, and yet this organ so essential for life has very little regenerative capacity in the adult (1). Rather, upon injury (such as a myocardial infarction), a wound-healing response in the heart creates an inflammatory bed where scar tissue is formed, replacing the contractile cardiomyocytes, healthy vasculature, and supportive stromal cells of the heart. With heart disease as the leading cause of morbidity and mortality worldwide (2), cardiac regeneration is an immense, multifaceted challenge in the biomedical sciences.
Multiple approaches are being pursued in clinical and preclinical studies to regenerate the myocardium, including cell delivery to the heart, cardiac tissue engineering, angiogenic therapies, and gene therapy. A fundamental goal of regeneration is the restoration of pumping function of the heart, which will require new cardiomyocytes to replace the one billion or so that are lost after myocardial infarction (3). However, the myocardium is a complex tissue with high metabolic demand, specialized vascular structure and function, great compliance, highly specialized electrical conduction, and an ability to quickly adapt to external demands (e.g., via beta-adrenergic stimulation). Therefore, ongoing research must appreciate this complexity and plan ahead for therapeutic regimens to be tailored to individual disease states.
Of the approaches used to date to regenerate the heart, cardiac tissue engineering has provided many advantages for developing new myocardium that contains the multiple cell types of the heart, and it is the primary focus of this review. In particular, native myocardium has capillaries adjacent to every cardiomyocyte, suggesting that success in cardiac tissue engineering will require the engineering of an organized vascular network within a bed of cardiomyocytes to create a truer myocardial tissue for heart repair. As we discuss, intercellular biochemical signaling between cell types is a fundamental aspect of myocardial biology that goes hand in hand with engineering the physical form of this multicellular tissue. Although the ultimate goal of cardiac tissue engineering may be to build a new organ that could be used for whole-heart transplants, the field is currently subdivided to address three general compartments of the heart: valves, vasculature, and cardiac patches. We refer the reader to a review by Sacks et al. on bioengineered heart valves (4) and examine here the engineering of a vascularized myocardial tissue.
2. HEART FUNCTION AND THE CARDIOVASCULAR UNIT
The healthy adult human heart weighs 200–350 g, is approximately the size of your fist, and contains 2–4 billion cardiomyocytes (5). The average cardiac output is 5 L/min at rest with a 60% ejection fraction, which increases with exercise to 15 L/min with up to an 85% ejection fraction (6). The architecture of the heart muscle enables efficient pumping of blood, exemplified by the fiber angle and orientation of cardiomyocytes within the extracellular matrix (ECM) that enable torsional squeezing to maximize ejection fraction (7). With this phenomenal pumping capacity, it is not surprising that a cardiomyocyte-centric approach to heart regeneration has been the predominant focus in the field, particularly because systolic dysfunction after myocardial infarction is common. However, our increasing appreciation of the cellular complexity of the heart is leading a change in our approach to tissue engineering to focus on creating a microvascular bed.
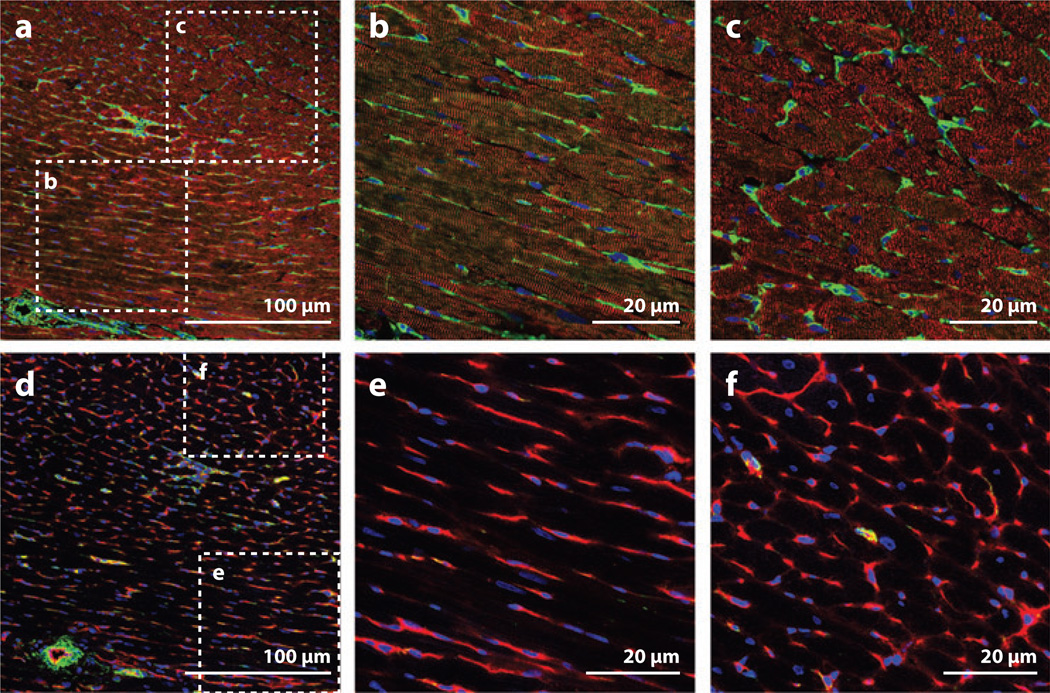
At the tissue level, the coronary circulation and cardiac fibroblasts follow the orientation of the cardiomyocytes, and the ratio and position of these components create a unique geometry that has been called a cardiovascular unit (CVU) (8, 9). The precise arrangement of these structures is shown in Figure 1, in which a changing fiber orientation through the thickness of the left ventricular wall displays cardiomyocytes, vasculature, and fibroblasts in longitudinal (Figure 1b,e) and cross-sectional (Figure 1c,f) views. Each cardiomyocyte is surrounded by 3–4 capillaries (10), which have a single layer of endothelial cells (ECs) stabilized by pericytes that share a common basement membrane (9, 11). Cardiac fibroblasts lie between cardiomyocytes, and larger coronary vessels provide blood flow to the CVU and are surrounded by vascular smooth muscle cells (VSMCs) and other perivascular cells. Using the CVU as a structural building block of myocardium, we should strive to engineer heart tissue with this complex architecture, as it is of great importance for heterotypic interactions and normal function.
Figure 1.
Architecture of myocardial cells in a healthy rat heart. (a–c) Alpha-actinin-positive cardiomyocytes (red) show striations in the longitudinal plane (b) and clearly make up the bulk of the tissue as viewed in cross section (c). Vimentin-positive cells (fibroblasts and ECs; green) are tucked between the cardiomyocytes. (d–f) In a serial section to panel a, ECs (RECA, red) show capillaries aligning with the main axis of the cardiomyocytes (e) and 3–4 capillaries surrounding each cardiomyocyte as viewed in cross section (f). Larger vessels have SMA-positive (green) SMCs surrounding the ECs (d). Abbreviations: ECs, endothelial cells; RECA, rat endothelial cell antigen; SMA, smooth muscle alpha-actin; SMCs, smooth muscle cells.
Recapitulating myocardium in engineered tissue will require sophisticated bioreactors for growth and survival of engineered myocardium in vitro, but the tissue’s in vivo survival upon transplantation is critically dependent upon efficient anastomosis with the host vasculature. Preparing for this eventual challenge will require both a well-developed microvasculature within the engineered tissue and surgical or other means of connecting graft to host vessels. Once integration of engineered myocardium addresses the vascular supply, it must be followed closely by electromechanical integration and reduction of scarring. We challenge colleagues in the field to investigate implantation techniques and focus our review on the development of integrated cardiac-microvascular tissues that may lead us into a new era of myocardial tissue engineering.
3. STEM CELLS AND CELL SOURCING
The cell populations in the adult heart originate from mesodermal precursors through spatially and temporally regulated events during embryonic heart formation. Cells destined to form the cardiovascular system are exposed to activin/nodal, bone morphogenetic protein (BMP), and Wnt signaling (reviewed in 12). Signaling pathways involved in defining the first and second heart fields, which give rise to cardiomyocytes, and the simultaneous morphological development of the four-chambered mammalian heart from a linear heart tube are reviewed elsewhere (13, 14). After the initial heart plan is established, mesodermal cells from a region called the proepicardium are recruited to form the epicardium, a single layer of cells that spreads over the heart (15). In an epithelial-to-mesenchymal transition, epicardial cells transform into migratory mesenchymal cells, invade the underlying myocardium, and differentiate into VSMCs and the fibroblasts of the adventitia and interstitium. Although the origin of coronary endothelium is debated, current knowledge suggests that endothelium has a distinct progenitor population from fibroblasts and smooth muscle cells (SMCs) in the proepicardium (16, 17). Remarkably, only after the main pattern of the coronaries is established do the ECs invade the aorta and create a functional coronary circulation (reviewed in 18). The distinct origins of the cell populations in the heart may influence organ function, which suggests that cell sourcing for tissue engineering and regenerative medicine should be carefully considered.
At present, only pluripotent stem cells such as human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) can generate definitive human cardiovascular cells in quantities sufficient for tissue engineering, so we focus on this cell source. Differentiation into cardiomyocytes has been greatly successful in recent years and has led to the identification of a cardiovascular progenitor population [of cells expressing both kinase insert domain receptor (KDR) and platelet-derived growth factor receptor-α (PDGFRα)] derived from hESCs/hiPSCs that can give rise to cardiomyocytes, SMCs, and ECs (19). Interestingly, ECs from hESCs/hiPSCs can be obtained from what appear to be several distinct progenitor populations. Derivation and characterization of myocardial stromal cells have received much less attention, although the importance of stromal/vascular cells has been gaining appreciation in the field of tissue engineering. Issues of autologous versus allogeneic cell sources, such as immune suppression, are discussed in Section 9: Challenges for Clinical Translation.
3.1. Cardiomyocytes
Neonatal rat ventricular cardiomyocytes have been extensively used for developing engineered cardiac tissue (20) since their debut in 1999 when they were seeded onto Gelfoam® and implanted onto an injured rat heart (21). The recent explosion in our knowledge and abilities to derive cardiomyocytes from hESCs and hiPSCs has fueled a transition to using human cardiomyocytes in engineered cardiac tissue, and these pluripotent stem cell–derived cardiomyocytes are the future of the field as we move into translational medicine. Derivation of cardiomyocytes from human pluripotent stem cells follows a well-defined developmental lineage through mesoderm induction. Two widely used cardiac differentiation protocols for hESCs add recombinant human activin A and BMP4 to induce cardiac mesoderm, reproducing the main elements of embryonic development (22, 23), and have been recently reviewed (24). It should be emphasized that all differentiation protocols occur in the context of the stem cells’ preexisting signaling environment. There seems to be a fair bit of variability in what constitutes pluripotency, so different pluripotent cell lines, and even the same lines grown by different laboratories, have different baseline signaling states. This means that there is no universal differentiation protocol but rather that a protocol needs to be taken as a guideline and the approach optimized in each user’s hands.
Using high-density monolayers of hESCs, Murry and colleagues generate cardiomyocyte populations with 30–70% cardiac purity that consist of ~20% nodal cells and ~80% working-type (ventricular and atrial) cells (22). This protocol induces endogenous expression of canonical Wnt ligands, and after Wnt induction of mesoderm, successful cardiac differentiation requires subsequent inhibition of this pathway (25). This biphasic Wnt profile, as occurs during development (26, 27), provides a means to evaluate efficiency of cardiac differentiation in different hESC/hiPSC lines and can be enhanced by addition of exogenous Wnt3a followed by inhibition of Wnt signaling with dickkopf homolog 1 (DKK1) or small molecules to increase cardiogenesis (25).
In a second approach to cardiac differentiation developed by Keller and colleagues (23), small cell aggregates (called embryoid bodies) are formed in suspension to mimic the three-dimensional (3D) environment of a developing embryo. Embryoid bodies are exposed to BMP4, activin A, basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and, after mesoderm induction, DKK1 under hypoxia (5% O2) for 10–12 days. A double-positive cardiovascular progenitor population expressing KDR and PDGFRα can be isolated at day 4 and has been shown to differentiate into >50% cardiac troponin T (cTnT)-positive cardiomyocytes in multiple hESC/hiPSC lines (19). Cardiovascular progenitors derived using this protocol also give rise to VSMCs (calponin-positive), fibroblasts [discoidin domain receptor 2 (DDR2)-positive], and low levels of ECs (CD31-positive).
A recent breakthrough in cardiac differentiation with hESCs/hiPSCs is the exclusive use of small molecules to induce mesoderm and the cardiac lineage by manipulating the biphasic Wnt pathway (28). A glycogen synthase kinase 3 (GSK3) inhibitor is used to activate Wnt/β-catenin signaling and mesoderm differentiation, followed by an inhibitor of Wnt production-4 to block Wnt secretion and promote high levels of cardiogenesis (>80% cTnT+ cardiomyocytes) (28). The use of small molecules to induce cardiomyocyte formation promises to be more consistent, more efficient, and more cost effective (versus differentiation with recombinant proteins). Thus, this protocol may be more amenable to the good manufacturing practices needed for clinical applications.
Once differentiation is complete, there are several strategies whereby cardiac purity can be enhanced. The earliest protocol involved density gradient centrifugation in Percoll (22), but in our hands, this is associated with injury to the cardiomyocytes and poor survival upon transplantation. Multiple investigators have used genetic selection strategies wherein a cardiac-specific promoter drives expression of an antibiotic resistance gene, enabling cardiomyocytes to survive while non-myocytes are killed (29). Cell-sorting strategies have been developed for cardiac-specific surface markers including signal regulatory protein alpha (SIRPA) (30) and VCAM1 (31). Other strategies take advantage of the relative abundance of mitochondria in cardiomyocytes, which can be exploited by sorting based on mitochondrial staining (32) or by placing cells in a lactate-rich medium that kills cells with too few mitochondria to survive (i.e., nonmyocytes) (33). However, 100% pure populations are likely not practical or necessary for clinical translation. In our hands, implantation of unsorted cardiomyocyte cultures with purities exceeding 50% has not led to teratomas (tumors containing tissues derived from all three germ layers) in preclinical animal studies (22), although occasional epithelial cysts have been observed (34). These outcomes are encouraging and suggest that development of reasonably high-purity hESC- and hiPSC-derived cardiomyocytes for future clinical use is within our scientific capacity.
In addition to the in vitro cell culturing techniques discussed above, new technologies are also evolving to address alternative (and complementary) avenues of therapeutic cardiac regeneration. For example, transdifferentiation in vivo using a gene therapy approach directly reprograms fibroblasts into cardiomyocytes, bypassing the pluripotent stage (35, 36). This breakthrough was inspired by hiPSC reprogramming technology and has generated much enthusiasm, although recapitulating this work has proven to be a challenge (37). At present, this method is inefficient (<1% of transduced cells exhibit contractile activity), and many of the cells exhibit a partially reprogrammed, cardiomyocyte-like phenotype (reviewed in 38). However, direct reprogramming of cardiomyocytes by gene therapy will provide diversity in therapeutic options and may be a valuable approach, particularly for patients not eligible for surgical heart repair with engineered tissue.
3.2. Endothelial Cells
There are a number of sources for human ECs for use in tissue engineering, including commercially available, host-derived, and hESC/hiPSC-derived ECs. Two common commercial lines include human umbilical vein ECs (HUVECs) and human microvascular ECs (HMVECs). These ECs are amenable to tube formation assays and self-assemble into vascular networks in vitro. However, these sources are not suitable for large-scale clinical translation because they are difficult to obtain in large numbers, necessitating alternative sources of ECs. It may be most advantageous to design tissues that encourage ingrowth of host ECs to form vessels, which reduces concerns about inflammation and rejection due to EC source and major histocompatibility complex mismatch. The greatest challenge with this approach will be to rapidly promote an efficiently perfused high-density vascular network in the engineered myocardium. Currently, there are much promise and excitement for angiogenic therapeutics and tissue engineering using hESC- and hiPSC-derived ECs. Recent work in developing directed differentiation protocols for ECs from hESCs and hiPSCs demonstrates that there are great interest and momentum in this area.
Like cardiac differentiation, EC differentiation from hESCs and hiPSCs has been developed in 3D embryoid bodies and two-dimensional (2D) monolayers using either medium with serum or chemically defined medium (without serum) with addition of cytokines and growth factors. Although the origin of coronary vascular ECs is still debated, most EC differentiation protocols induce mesoderm formation first. Many protocols currently use live-cell fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS) to isolate EC progenitor cells or maturing ECs. Isolation of hemangioblasts, precursors to both hematopoietic and vascular lineages, has been accomplished by a few groups (39, 40). White et al. (41) isolate KDR+ cells at day 6 of differentiation, and although this population is highly variable (7–73%) within and between hESC and hiPSC lines, >90% pure EC cultures can be obtained at day 21 by selective passaging of differentiating KDR+ precursors. Alternatively, mature ECs can be differentiated in embryoid bodies with VEGF and bFGF and sorted using CD31 and/or VE-cadherin (42, 43). Recently, ECs have been produced in defined growth medium supplemented with hydrocortisone, human epidermal growth factor, bFGF, and heparin (44). This monolayer differentiation protocol yielded 85% CD31+ VE-cadherin+ ECs by day 21 without sorting and has the advantages of being animal product–free and using defined media, which are both desirable attributes in performing EC differentiation for any clinical application. Recent studies suggest that ESC-derived ECs may be educated by their local environment to adopt organ-specific features in the liver and kidney (45), and if this is to be recapitulated in the heart, engineered tissue must provide the appropriate instructive myocardial microenvironment.
Functional assessment of hESC- and hiPSC-derived ECs has been demonstrated in vitro, in vivo, and in engineered tissues. Production of nitric oxide (NO), uptake of acetylated low-density lipoprotein (Ac-LDL), formation of EC networks in Matrigel™ assays, migration in transwell assays or across wounds/scratches, and sprouting into bulk collagen gels have all been used to validate EC function in vitro (41–44). Interestingly, White et al. (41) demonstrated that using their cardiovascular progenitor differentiation protocols, ECs derived from a panel of hESCs and hiPSCs were more uniform by microarray analysis than their pluripotent precursors were, and these ECs were more similar by hierarchical clustering to human atrial ECs than to either venous or lymphatic ECs. In vivo assessment of hESC- and hiPSC-derived ECs by subcutaneous gel plug assays (41, 42), hind-limb ischemia transplantation (44), or mouse dorsal windows (42) demonstrates that these human ECs form vessels perfused by red blood cells within 1 week, with some persisting up to 60 days even without stromal cell cotransplantation (42). Li et al. (42) transplanted ECs into mouse hearts after myocardial infarction and found a transient improvement in left ventricular fractional shortening by echocardiography at 2 weeks that did not persist at 8 weeks. Further analysis revealed that <1 % hESC-derived ECs survived beyond 8 weeks, suggesting that stable integration will be necessary for therapeutic benefits in ischemic disease. Similarly, engineered tissues have been formed in vitro using hESC-derived ECs and human stromal cells, and when implanted on the heart, human EC lumens had formed and were perfused by host red blood cells at short time points (43, 46). HESC-derived ECs have been used with hESC-derived cardiomyocytes and human fibroblasts in engineered cardiac tissue (47, 48), which demonstrates their utility in myocardial tissue engineering but has not yet seen widespread use. Certainly, issues of long-term vessel patency and perfusion need to be addressed in using hESC-derived ECs or any EC source for an effective, long-term therapy.
3.3. Stromal Cells
The supportive cells of a tissue or organ are the stromal cells, including fibroblasts and perivascular cells. Cells that support the function, viability, and stability of vascular ECs are called mural cells, which include pericytes and VSMCs. Pericytes support capillaries of the microvasculature and contribute to the formation of the basement membrane (reviewed in 11). Each cardiac pericyte is associated with two or three ECs with a star-like shape and long processes contacting ECs and other pericytes via gap junctions (9). Pericytes and VSMCs may share a common origin in the heart, deriving from an epithelial-to-mesenchymal transition of the epicardium (49), although other origins have been suggested. VSMCs are an essential component of larger vessels and reside within the vessel wall. Contractile VSMCs on arterial vessels encircle the ECs in concentric rings to modulate blood flow and pressure through changes in vessel diameter due to VSMC contraction and relaxation. Heterotypic interactions between ECs and these mural cells are an active area of research, as they are vitally important during sprouting angiogenesis for the establishment of stable vessels, as is desirable in therapeutic vascularization and tissue engineering.
VSMCs have been derived from hESCs and hiPSCs through the mesodermal lineage but have received less attention than cardiomyocytes or ECs. Thus, the development of robust protocols to generate and isolate VSMCs is ongoing (reviewed in 50, 51). Notably, there is an inherent heterogeneity in VSMCs dependent upon their state (e.g., mature/quiescent versus differentiating/proliferative) that may complicate identification of VSMCs after differentiation from hESCs/hiPSCs. Mature, contractile VSMCs are identified by a panel of markers, including alpha-smooth muscle actin (αSMA), smooth muscle myosin heavy chain (SM-MHC), smoothelin, calponin, SM22α, and caldesmon. In a stage-specific differentiation scheme utilizing a mesenchymal stem cell (MSC)-like precursor state, Bajpai et al. (52) derived contractile VSMCs from hiPSCs and demonstrated contractile responses to vasoconstrictors in engineered vascular constructs. By contrast, El-Mounayri et al. (53) used the serum-free defined conditions for cardiac embryoid body differentiation through the KDR+ cardiovascular progenitor to derive VSMCs using VEGF and bFGF with passaging after 28 days. The resulting VSMC populations (20% in HES2 hESCs and 78% in H7 hESCs) showed gene expression profiles and elicited Ca2+ responses to vasocon-strictors most closely resembling those of human coronary SMCs. Finally, Cheung et al. (54) used hESCs to generate SMCs of high purity from different origins, including lateral plate mesoderm, paraxial mesoderm, and neuroectoderm. These cells, which reflect major sources of SMCs in the body, contracted in response to vasoconstrictors in vitro, invested endothelial tubes in vivo, and showed unique proliferative responses reflective of SMCs derived from their respective locations. Thus, there has been progress in generating mural cells for use in myocardial tissue engineering; however, the optimal protocols and throughput are not yet established. In the meantime, human bone marrow–derived MSCs have proven useful in myocardial tissue engineering owing to their plasticity, demonstrated support of EC network formation and stabilization, and expression of some pericyte markers (46, 55).
Although cardiomyocytes make up ~70% of the healthy adult heart by volume, the most common cells in the adult heart are cardiac fibroblasts. Despite their abundance, we understand surprisingly little about the function of cardiac fibroblasts in homeostasis and disease (reviewed in 56, 57). The complex role of cardiac fibroblasts, acting as a structural and signaling node between the ECM and cardiomyocytes, makes them a difficult cell population to study, particularly because isolation for in vitro study significantly alters their structure, physiology, and gene expression. Further, identifying fibroblasts in tissue is a challenge, as there is currently no fibroblast-specific marker that labels all fibroblasts. (As an aside, the lack of good markers to distinguish mesenchymal cell subtypes reflects more a lack of concerted effort than it does any intrinsic difficulty in developing them.) This dearth of specific markers complicates fate-mapping studies for lineage tracing and immunohistochemical identification. To circumvent this issue, multiple markers have been employed. Vimentin labels fibroblasts and ECs (and leukocytes in inflamed tissues); procollagen labels fibroblasts and SMCs; α-SMA labels activated fibroblasts and VSMCs; and fibroblast-specific protein 1 (FSP1; an inaccurate name, as other cell types express this protein), DDR2, and periostin each label a unique subset of fibroblasts (56, 58).
The primary function of cardiac fibroblasts is the maintenance of the ECM through production and remodeling of collagens and other macromolecules. The ECM is necessary for distributing mechanical stress in the heart, providing a scaffold for all cardiac populations, and electrically insulating the ventricles from the atria. Cardiac fibroblasts modulate production of ECM components in response to mechanical stimuli, acting as mechanosensors through receptors such as DDR2 and integrins. This creates a dynamic environment, as cardiac load changes with growth, exercise, and disease. As detailed above, cardiac fibroblasts arise at the time of ventricular compaction from proepicardial cells that undergo an epithelial-to-mesenchymal transition (58). Indeed, El-Mounayri et al. (53) observed DDR2-positive cells in their hESC-derived VSMC cultures from a KDR-positive progenitor population, but to our knowledge no group has further isolated or characterized hESC- or hiPSC-derived cardiac fibroblasts. Whereas the role of cardiac fibroblasts is essential in normal heart homeostasis, fibrosis is a result of excess ECM production by myofibroblasts, which are activated by inflammatory growth factors and cytokines, such as transforming growth factor-β (TGF-β). Targeting fibrosis may prevent progression of disease and be a complementary therapeutic for regeneration. For example, administration of withaferin-A, which binds and inhibits vimentin, reduced collagen mRNA half-life, collagen protein production, transcription of collagen genes, and interstitial fibrosis (59). Because engineered cardiac tissue will likely be delivered to a fibrotic environment, we must consider the role of fibroblasts when used as a cell source for tissue engineering (e.g., as a support cell for ECM maintenance) and take into account the host fibroblast and myofibroblast populations, which may act as barriers to or facilitators of integration of implanted engineered tissue.
4. INTERCELLULAR COMMUNICATION IN MYOCARDIUM
The use of multiple cell types in engineered cardiovascular tissue reflects our growing appreciation of the importance of tissue architecture and cell–cell communication in normal tissue homeostasis and function. Perturbations in cell–cell or cell–matrix signaling are associated with development and growth, but also disease and progression to heart failure. In order to build engineered tissues for heart regeneration, disease modeling, and drug screens, we must consider how choice of matrix, cell type(s), and cellular architecture affects the function of myocardial tissue. As suggested by the physical proximity of cardiomyocytes, ECs, mural cells, and cardiac fibroblasts, many signaling pathways between these cell types modulate their function in adult homeostasis.
Tissue perfusion, blood pressure, and vasomotor tone are modulated primarily by ECs that produce vasodilators and vasoconstrictors in response to physical stimuli such as shear stress. Examples of vasodilators that cause relaxation in VSMCs are NO and prostacyclin (also called prostaglandin I2). In cardiomyocytes, increased levels of NO also reduce force production, primarily by inducing an earlier onset of ventricular relaxation (60). During the cardiac cycle, enhanced ventricular relaxation due to NO promotes rapid early filling, increased diastolic compliance, and increased coronary vessel perfusion (61). Interestingly, prostacyclin causes positive inotropy in cardiomyocytes by prolonging twitch duration when NO synthesis is inhibited (62), which suggests that it counteracts the actions of NO to regulate cardiac contractility. Two potent vasoconstrictors, angiotensin II and endothelin (ET), are also positive inotropic agents for cardiomyocytes. Angiotensin II is both a circulating hormone of the renin-angiotensin-aldosterone system and produced locally by cardiomyocytes and fibroblasts in the heart to elicit local tissue-specific responses (reviewed in 63). Angiotensin II increases peak twitch tension and twitch velocity in cardiomyocytes and is synergistic with ET and regulated by ET; its effects are counterbalanced by prostacyclin (64–66). ET is also a positive inotropic agent, causing increased contraction in a dose-dependent manner (67) by increasing peak twitch tension, contraction velocity, time to peak tension, and relaxation kinetics (68). As a caution, ET is often used to induce hypertrophy in single cardiomyocytes, and enhanced ET signaling occurs in many cardiovascular pathologies (reviewed in 69), suggesting that interventions will reside within an environment where pathological signaling pathways are active. Although the details of these signaling interactions remain to be elucidated, cardiac contractility is clearly modulated with normal homeostatic changes in vascular tone.
The ECM is maintained primarily by cardiac fibroblasts and provides both a physical substrate for cells to assemble into tissue and a means by which intercellular signaling can be achieved (70). Cardiac fibroblasts communicate directly with cardiomyocytes via connexin 45-containing gap junctions (71), which pass electrical and chemical signals between the cells. The main components of the ECM, fibronectin and collagen types I and III, are produced by cardiac fibroblasts and promote cardiomyocyte attachment via β1 integrin binding to align cytoskeletal f-actin and initiate sarcomere assembly (72). Matrix metalloproteinases (MMPs) that degrade the ECM are produced by cardiac fibroblasts and cardiomyocytes in response to cytokines and mechanical stretch and are regulated by tissue inhibitors of metalloproteinases (TIMPS) (reviewed in 73). However, the balance of collagenase activity and production of new collagen is not well understood in the healthy adult heart. Degradation of the ECM releases bound growth factors that may participate in remodeling processes, such as fibroblast growth factor–driven angiogenesis (74), yet perturbations in the system are often required to learn about normal function. For example, neuregulin-1β (NRG-1β) is produced by microvascular ECs and acts upon cardiomyocytes to increase glucose uptake and protein synthesis (75). In a Phase III trial for the anticancer drug trastuzumab, an inhibitor of the receptor tyrosine-protein kinase erbB-2 (a NRG-1β receptor), up to 34% of patients had asymptomatic cardiac dysfunction, which suggests that NRG-1β signaling in the healthy adult heart is required for normal function (76). Although NRG-1β is well characterized during heart development, its distribution in the cells and ECM of the adult heart and its role in homeostatic regulation of normal heart function are largely unknown. Studies that develop our understanding of the complex and nuanced signaling environment in the heart must be pursued, as these will inform tissue engineering and a diversity of therapeutic approaches for cardiac regeneration.
5. THE REQUIREMENT TO PREVASCULARIZE ENGINEERED CARDIAC TISSUE
As the contractile cells of myocardium, cardiomyocytes are an essential component of engineered cardiac tissue. Historically, the field of cardiac tissue engineering began by utilizing neonatal rat cardiomyocytes that were harvested, purified (by removal of cardiac fibroblasts), cultured, and combined with various scaffolds to create tissues (recently reviewed in 20). The successful implantation of these tissues in vivo and the adoption of human PSC–derived cardiomyocytes into these systems have opened our eyes to the many possible uses of a biological engineered cardiac tissue—for example, in pediatric patients (77). However, cardiomyocytes alone in a scaffold is likely a system that is oversimplified (possibly to our own detriment). It has been long known that implanted scaffolds without any prior vasculature develop neovessels in the in vivo environment (78–81). Delivery of nutrients, particularly oxygen diffusion, has been a critical factor for graft survival upon implantation (82, 83). An avascular graft can accommodate ~280,000 cells/cm3 without experiencing appreciable hypoxia upon transplantation (83). However physiological cardiac tissue has 70-fold higher cardiomyocyte density (2 × 107 cells/cm3) (84, 85), suggesting an engineered graft with physiological cardiomyocyte density would require prevascularization for its survival. Further, infiltrating leukocytes from the host increases cellular load within the graft, thereby increasing the local metabolic demand. Therefore, limiting the inflammatory response could be helpful as far as diffusion requirements are concerned, but the positive role of inflammation in vascularization and infarct repair may demand an approach to balance the inflammatory response during cell grafting (86–89). Other complementary approaches that can impart transplanted cell resistance to hypoxic death should continue to be explored (90), including overexpression of prosurvival (82) or antiapoptotic proteins (91) and heat shock treatment prior to implantation (82). However, we surmise that only a perfused, hierarchical vascular bed will provide the necessary nutrients to maintain transplanted cardiomyocytes.
In order to provide a nutrient supply, the complexity of engineered myocardial tissue must increase. Thus, the focus of cell sourcing has shifted to using well-defined cocultures of cardiomyocytes in combination with other cell types in order to form viable myocardial tissues. These other cells may be vascular cells from a common cardiovascular progenitor cell, independently sourced cells (e.g., from human bone marrow), or host-derived vascular, stromal, and inflammatory cells. What is now required is the systems and approaches for assembling an efficient vascular tree within engineered myocardial tissue that recapitulates the coronary vascular size and structure.
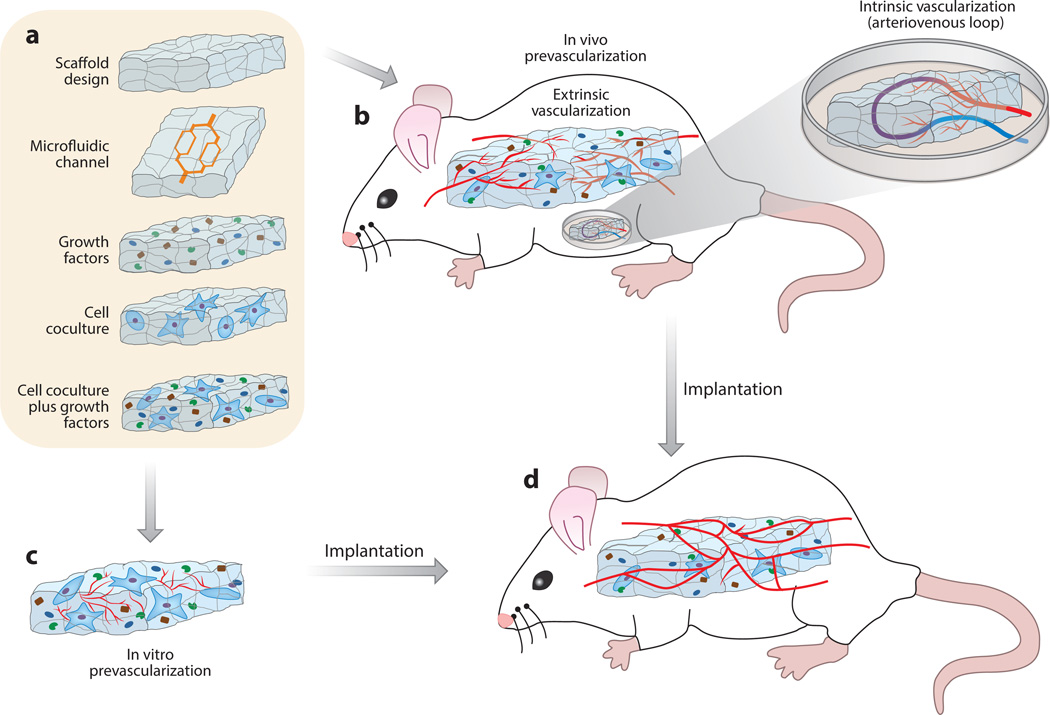
It is a desirable goal to emulate the sequential steps of angiogenesis—namely, vessel growth (EC sprouting), vessel maturation (stabilization with mural cells, flow-induced remodeling), and vessel growth suppression (quiescence)—to vascularize an engineered tissue. If left to its own devices, the heart will undergo vascular remodeling after cell engraftment that includes both arteriogenic remodeling outside of the graft and development of large and small smooth muscle–encoated vessels within the graft (92). Although much remains to be learned about the signaling pathways involved in arteriogenic remodeling and how to mimic the complex spatiotemporal interplay of cellular signaling cascades, significant progress has been made in recent years toward directing angiogenesis and providing larger arteries for vascularizing engineered tissue. In the following sections we discuss the main strategies used to vascularize an engineered cardiac graft, which are summarized in Figure 2.
Figure 2.
Schematic diagram depicting vascularization strategies for tissue-engineered myocardium. (a) Approaches to make a scaffold proangiogenic include tailoring scaffold porosity, microfluidic channel size and distribution, angiogenic growth factor release, cell coculture conditions, and combinations thereof. (b) In vivo prevascularization of scaffolds can be achieved extrinsically by implanting them into vascularized areas (such as in the subcutaneous space or onto the omentum or peritoneum) or intrinsically by implanting them using an artificially created arteriovenous loop in a protected chamber. (c) In vitro prevascularization is achieved by coculturing vascular cells within the (templated) scaffold in the presence of angiogenic factors. (d) Vascularized constructs are implanted onto infarcted myocardium and anastomose with the host microvasculature in order to restore lost tissue and cardiac function.
6. IN VIVO PREVASCULARIZATION
In vivo prevascularization can be carried out in two ways: extrinsic vascularization and intrinsic vascularization. Extrinsic vascularization involves growth of vessels from the periphery of the scaffold toward the center of the scaffold, whereas intrinsic vascularization involves engineering the tissue around a centrally located vascular axis and allowing a microvascular plexus to develop from central vessels within the engineered tissue (93).
6.1. Extrinsic Vascularization
Extrinsic vascularization is carried out by implanting the grafts into a well-vascularized area of the recipient’s body such as the subcutaneous space (94), peritoneum (95), or omentum (96, 97), then harvesting the prevascularized graft and implanting it at the desired site. At some sites, such as the omentum, a pedicle containing the afferent and efferent vessels can be swung into the new location with the vascular connections preserved. In other locations, this is not possible, and the afferent and efferent vessels must be severed and grown again after transplantation.
Laschke et al. (98) used poly(lactic-coglycolic acid) (PLGA) scaffolds (without cells) implanted into the flank of GFP-transgenic mice for 20 days and then harvested and reimplanted into dorsal skinfold chambers of wild-type isogenic mice to monitor microvasculature development with intravital fluorescence microscopy for 2 weeks. Their results demonstrated significantly increased functional capillary density (320 versus 160 cm/cm2), microvessel diameter (~35 versus 15 µm), and flow rates (235 versus 38 µm/s) for prevascularized versus nonvascularized (control) scaffolds on day 14 (98). Surprisingly, flow direction was reestablished in arterioles and venules in the prevascularized scaffolds, and capillary sprouting from preformed vessels developed functional interconnections, thus increasing capillary density. In another study, Amir et al. (99) utilized the peritoneal space for prevascularization of cylindrical cardiac patches (6-mm diameter × 1-mm thickness) composed of porous alginate seeded with rat neonatal cardiomyocytes. Patches kept in the peritoneum for 1 week developed large (up to ~50 µm) vessels coated with αSMA-positive cells in the periphery and smaller (~10 µm) vessels in the center. However, pronounced infiltration by myofibroblasts during prevascularization resulted in a collagen-dense graft after 1 month on the heart. Further, no grafted cardiomyocytes were identified in the patch at 1month, suggesting that the patch formulation in alginate was not appropriate for cardiomyocyte survival and engraftment, regardless of vascularization status. In a similar study, Dvir et al. (97) used a porous alginate scaffold but loaded it with prosurvival and angiogenic growth factors insulin-like growth factor 1 (IGF-1), VEGF, and stromal cell–derived factor 1 (SDF-1) in addition to the neonatal rat cardiomyocytes and then prevascularized this patch in the omentum of isogenic rats for 7 days. Prevascularized cardiac patches were transplanted onto an infarcted rat heart 7 days after permanent coronary ligation, resulting in improved cardiomyocyte engraftment, vessel density (~60 versus 25 vessels/mm2), and electrical connectivity with the host after 28 days, versus a nonvascularized control cardiac patch. As a result, left ventricular remodeling and dysfunction were attenuated, demonstrating that the appropriate combination of scaffold, cells, survival, and angiogenic factors and in vivo prevascularization were required. Further studies in this direction should focus on improving control over vessel size and distribution throughout the engineered patch and on minimizing inflammation and myofibroblast infiltration.
6.2. Intrinsic Vascularization
Intrinsic vascularization relies primarily on using an arteriovenous (AV) loop (or AV shunt) to provide blood flow through the engineered tissue, which is subsequently vascularized by angiogenesis. The potential of an AV shunt to support vascularization was first reported in 1979 by Erol & Spira (100), who observed that skin grafts placed around AV fistulae were infiltrated by neovessels emanating from the AV shunt. In this method, an artificial AV shunt is created surgically between artery and vein using a suitable vascular conduit (e.g., a vein or artery harvested from an accessible site), and this conduit is embedded within the scaffold and placed in a protected space (e.g., a polycarbonate chamber). An alternate surgical approach used extensively in clinical practice is the AV bundle method in which, instead of a shunt being created between the artery and vein, the existing artery and vein are both embedded within the scaffold (often bundled together) to provide arteriolar and venous blood flow through the growing tissue (101, 102). However, the AV loop method is superior to the AV bundle method in promoting neoangiogenesis and neotissue growth (103). Importantly, the AV shunt method has been successfully used clinically (104) to vascularize and promote the development of a living bone graft in the latissimus dorsi muscle, complete with a vessel pedicle for microsurgical connection to the vasculature upon transplantation to repair a >7-cm defect in a man’s mandible.
Angiogenesis from the AV shunt probably originates from surgery-induced ischemic injury and subsequent wound-healing response (93, 105). Lokmic et al. (105) studied the kinetics of AV loop–based angiogenesis in a rat model and noted that the AV loop generated its own fibrinous exudate by day 3 [even in the absence of any supplied ECM (106)], and this was subsequently invaded by αSMA-positive and VEGF receptor 2–positive cells that formed granulation tissue rich in new capillaries originating from the AV loop by day 7. Hypoxia peaked at day 7, and peak vascularization (maximum vessel density) occurred at day 10 (105). Tanaka et al. (107) first demonstrated that a type I collagen scaffold surrounding a femoral AV loop became vascularized and grew progressively with time. Early angiogenesis from the AV loop was found to be critically dependent upon the endogenously secreted fibrin matrix (108), whereas exogenously placed fibrin had slower vascularization kinetics versus PLGA (109). Further, treatment of scaffolds with growth factors such as bFGF, VEGF, and platelet derived growth factor (PDGF)-BB either alone or in combination increased vessel density and new tissue mass (110, 111).
The AV loop system has been used experimentally to engineer vascularized cardiac tissue. For example, Morritt et al. (112) seeded neonatal rat cardiomyocytes in Matrigel (6.5 × 106 cells in 150 µL per construct) into a protected femoral AV loop chamber, where this construct grew into a highly vascularized, spontaneously beating cardiac tissue that demonstrated length dependence of force with echocardiography. Of note, this study showed that a maximum thickness of 2 mm of vascularized cardiac grafts could be fabricated, suggesting the potential of AV loops in generating therapeutic-grade vascularized cardiac patches. Interestingly, in a study in which the alternate femoral AV bundle approach was used to fabricate a vascularized cardiac patch, Birla et al. (113) demonstrated similar contractile properties and noted vascularization. However, the different geometries, cell input numbers, scaffold materials, and limited quantification of vessel density make comparison of these two approaches difficult.
In summary, an AV loop vascularization strategy promotes angiogenic sprouting and the creation of microvascular beds in thick tissues that can be modulated by scaffold choice and growth factors, with the benefit of providing a vascular pedicle for microsurgical attachment to the circulation. Evaluation of intrinsic versus extrinsic vascularization has shown similar neovascular bed volume and kinetics of angiogenesis (114), and combining these two strategies may enhance vascularization of thick tissues (115). Studies must be done to determine how an engineered vascular graft may perform in an AV loop system to provide vascularization to engineered tissue.
7. IN VITRO PREVASCULARIZATION
In vitro prevascularization creates a vascular plexus within the engineered tissue to establish a “plug and play” network for enhancing perfusion after implantation. Although some vascular aficionados object to labeling these in vitro structures as vessels, we find the nomenclature sufficiently useful to justify this small imprecision.
7.1. Cell Coculture
In 1980, Folkman & Haudenschild (116) first demonstrated that ECs are capable of making capillary-like networks on plastic in vitro and, further, that collagen promotes this capillary formation (117). Notably, structures developed in 2D systems are typically solid cords, and fibroblasts and other nonvascular cells can form cord networks in 2D as well (118). Conversely, only ECs will form lumen-containing capillary-like structures in 3D, making 3D systems much more reliable assays. These capillaries, made of ECs only, regress and undergo apoptosis after a few days (119). ECs alone seeded on biodegradable polymeric scaffolds also give rise to microvessels upon implantation, but these also undergo apoptosis, suggesting that ECs alone are not sufficient to form a stable microvasculature (120). Coculture of ECs with SMCs was initially shown to inhibit EC growth, suggesting that mural cells may stabilize capillaries by inducing a quiescent EC phenotype (121). Indeed, HUVECs and mesenchymal precursor cells (mouse 10T1/2 cells) in 3D fibronectin/collagen gels formed durable vessels lasting for 1 year in vivo, highlighting the role of mural cells in the stability of vasculature (122). Overexpression of antiapoptotic protein Bcl-2 in HUVECs also produced stable vessels in vivo by facilitating their survival long enough for the cells to recruit host-derived mural cells (123).
To the best of our knowledge, the first attempt to engineer a vascularized tissue was carried out in 1998 by Black et al. (124), who cultured keratinocytes with fibroblasts and HUVECs in order to develop a vascularized engineered skin equivalent. Since then, coculturing angiogenic cells (ECs alone or in combination with pericytes, mural cells, SMCs, fibroblasts, or MSCs) within 3D scaffolds has been utilized as a viable strategy to prevascularize engineered tissues. In a study in which ECs were cocultured with cardiomyocytes in a 3D scaffold in the absence of flow, ECs promoted cardiomyocyte survival, alignment, and coordinated contractions, highlighting an instructive role of vasculature in tissue organization and function (125). The Okano group (126) showed that coculture of ECs with cardiomyocytes increased capillary network formation in scaffold-free cell sheets formed on pNIPAAM-coated dishes in a manner dependent on EC dose. These prevascularized cell sheets almost doubled capillary density (up to 120 capillaries/mm2) in the infarcted wall of a rat heart after 4 weeks and improved fractional shortening (~25% versus 10% in a cardiac-only control) (126). Levenberg et al. (127) engineered vascularized skeletal muscle tissue by coculturing myoblasts, ECs, and mouse embryonic fibroblasts (MEFs) and demonstrated that inclusion of MEFs as a stromal support cell increased EC lumen number and area in vitro. The benefits of using a stromal cell were verified by Caspi et al. (48) in engineered cardiac tissue with hESC-derived cardiomyocytes in a PLLA/PLGA scaffold. In scaffold-free cardiac patches with hESC-derived cardiomyocytes, Stevens et al. (47) showed increased EC networks and increased anastomosis with host (rat) vasculature upon implantation when HUVECs and MEFs were included in the engineered tissue, which resulted in a 10-fold increase in cardiomyocyte graft area in vivo. Further, the stromal cell source in these scaffold-free tri-cell patches influenced the vascular potential of ECs in vitro and in vivo (46). Together, these studies clearly demonstrate that co-culture of vascular and stromal cells with cardiomyocytes provides an avenue for creating in vitro EC networks capable of increasing vascularization of the graft upon implantation to promote cardiomyocyte graft size. Although this approach is limited by the lack of a hierarchical organization of EC networks, utilizing coculture with improved scaffold designs and growth factor delivery may improve control over in vitro vascularization.
7.2. Decellularized Tissues as a Source of Microvasculature
Instead of de novo generation of microvasculature in vitro, tissues with intact vessels can be decellularized and used for reconstructing vasculature. For example, Chang et al. (128) harvested whole microcirculatory beds (containing arterioles, capillaries, and venules), seeded them with adult stem cell populations, and reimplanted these into rats, demonstrating the feasibility of harvesting autologous capillaries for tissue engineering applications. Decellularization of a whole rat heart provided an intact cardiac matrix that could be reseeded with rat cardiomyocytes via intramural injection and with ECs via perfusion through the coronary vessels (129). This engineered heart displayed contractile activity after 8 days in a perfusion bioreactor, generating pressure within the left ventricle equivalent to about 2% of that in an adult rat heart. The modest force production doubtlessly relates to the modest amounts of myocardium generated with a coronary perfusion cardiomyocyte reseeding approach, but it nonetheless demonstrates the feasibility of this approach for whole-heart tissue engineering (129). Notably, the scalability that is required for application of this strategy in the significantly larger human heart remains to be demonstrated.
7.3. Microfabrication of Microvasculature
The majority of approaches aimed at engineering a microcirculation that are discussed above give rise to unorganized microvessels, instead of a physiological, organized, hierarchical vascular network. In this regard, microfabrication of organized networks within scaffolds is a very attractive approach to generate a physiologically organized microvascular network. Early studies attempted to fabricate branched microchannels on silicon and borosilicate glass (130, 131) surfaces using photolithography. These channels were seeded with ECs and preconditioned with fluid flow conditions mimicking the microcirculation (130). In other studies, SMCs were cultured on nylon fibers (127-µm diameter) (132) or cellulose microfibers (200-µm diameter) (133), which were subsequently removed by mechanical force or enzymatic degradation with cellulase, respectively, to give rise to open microvessels.
Golden & Tien (134) embedded micromolded gelatin meshes in ECM (collagen/fibrin/matrigel) hydrogels before polymerization. Gelatin meshes were melted (by raising the temperature above ~28°C), leaving behind an interconnected branched tubular network (~6-µm diameter) within the hydrogels, which could be seeded with ECs and supported transport of macromolecules under low pressure gradients. Choi et al. (135) determined the diffusivity of molecules within the calcium alginate microfluidic hydrogels by systematically studying diffusion profiles under pulsed flow at varying flow rates. Dimensions and distribution of microfluidic channels and porosity of the hydrogels could be manipulated to achieve adequate convective mass transfer through the channels as well as effective diffusion of the molecules in the bulk of the scaffold. Zheng et al. (136) used photolithographic patterning to create microfluidic channels in high-density collagen gels. After seeding with ECs, these engineered microvessels displayed barrier function and antithrombogenic properties in response to whole blood flow. Furthermore, these vascular networks displayed angiogenic sprouting and perivascular cell recruitment from the bulk collagen to the vessels and produced an appropriate thrombogenic response under inflammatory conditions akin to normal inflammatory responses of the vasculature (136). These studies suggest that a microfluidic-based approach can give rise to microvascular networks that are responsive to physiological stimuli, which may facilitate development of well-integrated, vascularized tissue-engineered constructs. Microfabrication is one of the most promising approaches for maintaining precise control over the organization, architecture, and hierarchy of branching vascular networks, and as such it is likely to play a significant role in advancing the field of cardiac tissue engineering as well.
8. STRATEGIES PROMOTING NEOANGIOGENESIS
Improving the rate of vascularization of implanted engineered tissue to promote cell survival and tissue integration has been pursued with a number of strategies, including scaffold design, delivery of angiogenic growth factors, and controlled release of angiogenic factors from the scaffold itself. In principle, these approaches utilize sophisticated strategies to enhance the host’s native angiogenic response in vivo and develop a new vascular bed throughout the implant.
8.1. Scaffold Design
Pore size, distribution, and interconnectivity are critical factors that determine mass transfer of oxygen and nutrients to support effective vascular ingrowth within scaffolds (137). Scaffold porosity may also facilitate seeding of cardiomyocytes and other cells for engineering cardiac tissue. Standard methods of porous scaffold fabrication [e.g., phase separation, gas foaming, freeze drying, and particulate leaching (137)] have been used widely in tissue engineering applications. However, these methods give rise to randomly organized pores, resulting in tortuous vessel ingrowth within the scaffold.
To provide better control of structure and generate compartmentalized scaffolds with defined architecture, Madden et al. (138) microtemplated composite scaffolds of poly (2-hydroxyethyl methacrylate-comethacrylic acid) containing parallel channels to allow for directional cardiomyocyte growth and spherical channels for vascular ingrowth (138). The 40-µm pore size resulted in the highest vessel density with reduced fibrosis (versus larger 80-µm pores) upon implantation (138). Porous elastomeric scaffolds with a parallel array of channels (370-µm diameter) enhanced the oxygen supply to the cardiomyocyte/fibroblast constructs when perfluorocarbon was used as an oxygen carrier (139). In an in vivo study, larger pore size (>250 µm) gave rise to higher microvessel density (140). These studies suggest that the field is still determining how pore and channel size should be optimized for microvessel development to recapitulate the CVU architecture in an engineered myocardial tissue.
Rapid prototyping uses computer-aided design (CAD) to print scaffolds in a layer-by-layer format to mimic the 3D architecture of native tissues (141). Recent advancement in 3D printing technologies has allowed fabrication of cell-encapsulated scaffolds with defined geometries and porous architecture (142). In particular, Cui & Boland (143) demonstrated the feasibility of printing microvessels using HMVECs embedded in fibrin as bio-ink. Though promising, this approach has not realized its full potential in engineering vascularized tissues, as biomaterials and cells are not easily processed. Development of more sophisticated strategies is required to print multicellular vascularized tissues with physiological architecture and organization (142). As this technology advances, it will become an even more exciting and promising direction to pursue.
8.2. Angiogenic Growth Factor Delivery
Angiogenic growth factors such as VEGF, bFGF, TGF-β, PDGF, and angiopoeitins (reviewed in 144, 145) are proteins with short half-lives (minutes to hours for soluble form) that bind to their cognate receptors and initiate downstream angiogenic signaling. Growth factors are required at very low concentrations (10−9–10−11 M) (146) over long periods of time (hours to days) for effective angiogenesis (147). Bolus injections intended to induce therapeutic angiogenesis at ischemic sites have performed poorly in the clinic, with problems including the induction of angiogenesis at undesired sites and tumor formation (148, 149). By contrast, targeted delivery has shown promising results (150), but both the concentration and the gradient of one or more growth factors within a scaffold are important factors determining the effectiveness of neoangiogenesis (147, 151, 152). Two strategies have been employed to date to achieve site-directed release of growth factor(s) in vivo: (a) implantation of genetically modified cells overexpressing angiogenic growth factor(s) and (b) incorporation of growth factors within the scaffold for controlled released through a number of physicochemical cell-mediated mechanisms.
8.2.1. Growth factor overexpression from genetically modified cells
Bone marrow cells (153), myoblasts (154), and rat cardiomyocytes (155) have been modified ex vivo to constitutively express VEGF, which upon implantation into infarcted hearts significantly improved vascularization. Delivery of multiple factors may be more effective, as they may have synergistic effects or can be released sequentially to mimic in vivo angiogenesis (156). For example, bone marrow–derived mesenchymal cells cotransfected with both VEGF and bFGF gave rise to higher capillary densities upon implantation into infarcted hearts versus cells expressing a single factor (157). However, therapeutic cells that have been engineered to transiently express growth factors may be a better strategy to avoid potential long-term side effects of permanently modified cells, such as unresolved angiogenesis or tumor formation, which have hindered application of this approach in the clinic.
8.2.2. Scaffold-based angiogenic growth factor release
Angiogenic growth factors have been incorporated into various scaffolds, and choice of scaffold and method of growth factor incorporation enable localized delivery and differential release kinetics (158–162). Growth factors can be physically entrapped during scaffold fabrication or immobilized on scaffolds by physical adsorption (e.g., electrostatic interaction between the growth factor and scaffold), chemical bonding (e.g., covalent attachment of a functional group on the growth factor with the scaffold), or secondary association with the help of an intermediate molecule (e.g., heparin or proteolytically cleavable peptides). In all cases, growth factor release in the local microenvironment depends upon scaffold degradation and/or the interactions of enzymes to release the protein, which then becomes bioactive. Various biomaterials and strategies that have been used for controlled release applications in tissue engineering are discussed elsewhere (158–160).
VEGF adsorbed to biopolymeric scaffolds such as PLGA or alginate hydrogels promotes neo-vascularization by a sustained release that mimics angiogenic signaling (163, 164). Similarly, different formulations of bFGF-releasing microspheres have enhanced blood vessel formation in vivo (165) and shown promising results in promoting therapeutic angiogenesis in the clinical setting (150). However, spatiotemporally controlled release to deliver multiple growth factors more closely replicates the physiological angiogenic cascade. To this end, Mooney and colleagues (156) developed composite scaffolds of PLGA microspheres containing PDGF-BB embedded in a PLGA porous sponge containing VEGF. Rapid VEGF release recruited ECs and was followed by PDGF-BB recruitment of mural cells to the new endothelial tubes, resulting in enhanced neovascularization upon implantation in vivo (156). Another approach is to create concentration gradients of angiogenic growth factors to spatially control angiogenesis. In one study, micro-spheres were generated with a core of low VEGF concentration surrounded by a layer of high VEGF concentration that produced a VEGF gradient perpendicular to the ischemic hind limb of mice, resulting in neoangiogenesis in the desired direction away from the femoral artery ligation site (166). Another group used controlled photopolymerization of poly(ethylene glycol) (PEG) hydrogels to generate spatial gradients of bFGF that induced SMC alignment and directional migration (167). Similarly, gradients formed within scaffold materials themselves instruct angiogenesis, as demonstrated by hydrogels with RGDS peptide gradients to promote attachment of ECs (168) or by a hyaluronic acid gradient within collagen scaffolds to provide spatial cues for angiogenic sprouting (169).
Covalent attachment of proteins and peptides (including cell-adhesive peptides and protease cleavage sites) to scaffolds has been employed to facilitate spatially controlled signaling and cell attachment and to engineer cell-controlled release of proteins from the scaffolds. Covalent conjugation of TGF-β1 in fibrin hydrogels utilizing plasmin cleavage sites induced sustained signaling to fibrin-embedded cells, yielding increased contractile function of vascular constructs prepared from MSCs (162). Immobilized TGF-β1 promoted uniform cell distribution, increased vascular contractility, and elastin synthesis, thereby providing an elegant way to engineer elastin into the vascular wall (170). Lutolf et al. (171) chemically cross-linked bioinert PEG hydrogels with cell-adhesive peptides (containing RGDS peptides to facilitate cell attachment) and MMP-cleavable peptides for selective cleavage by tissue MMPs. Subcutaneous implantation of these PEG hydrogels with covalently linked VEGF created vascularized tissue as the matrix was remodeled (172). Similarly, matrices of PEG diacrylate (PEGDA) containing an MMP-cleavable peptide, cell-adhesive peptide, and VEGF have also been shown to improve blood circulation in an ischemic hind-limb mouse model (173).
Several growth factors (e.g., FGFs, VEGF, and PDGFs) have a basic heparin-binding domain that electrostatically binds with sulfate and carboxylic acid residues present on heparan sulfate, making heparin an attractive linker protein to the scaffold. Heparin binding also prevents inactivation (174) and proteolytic degradation (175) of growth factors, thereby enhancing growth factor half-life. Heparan sulfate acts as a storage system (176) and releases growth factors as per cellular demand. Interestingly, heparan sulfate binding to VEGF was shown to be critical for generating spatial VEGF gradients that determine the extent of angiogenic sprouting and capillary branching (177). Chemical immobilization of heparin on collagen scaffolds prolonged bFGF release and increased endothelialization (178). Similarly, covalent incorporation of heparin within glycosaminoglycans (hyaluronan or chondroitin sulfate) prolonged bFGF release and enhanced neovascularization significantly upon subcutaneous implantation (179). Heparin-immobilized PEGDA scaffolds sustained release of bFGF and VEGF up to 42 days in vitro and demonstrated sustained vascularization in vivo (180). Finally, incorporation of bFGF and VEGF in a heparin-containing collagen scaffold enhanced mature vessel formation when the scaffold was implanted subcutaneously (181).
9. CHALLENGES FOR CLINICAL TRANSLATION
Despite the progress reviewed in this article, significant challenges remain to the clinical use of tissue engineering for heart repair, and we discuss the most important challenges here. Regarding cell sourcing, we believe that hESC-derived cardiomyocytes will be the first human cardiomyocytes to make it to clinical trials for therapeutic development, and this will be as injected cells rather than as engineered tissue. Immune suppression probably will be required, as is true with heart transplant patients. Of relevance to this review, transplantation of prevascularized constructs may be particularly challenging because ECs are highly immunogenic owing to their ability to present antigens in the context of class II human leukocyte antigen (HLA) molecules (182). The use of HLA matching between patient and cells stored in cell banks (as is being pursued in Japan) should allow for much lower doses of immunosuppression compared with those for whole-heart transplantation. Alternatively, one of the holy grails in stem cell biology is the generation of the universal donor cell, akin to type O-negative blood. This might be achieved by knocking out HLA loci (183) or by engineering the cells to secrete locally an immunosuppressive molecule. In lieu of this, it may be possible to generate donor-specific tolerance for the allogeneic myocardial tissue by establishing hematopoietic microchimerism from stem cell–derived blood or thymus progenitors (184, 185). However, approaches encouraging host vascular ingrowth into new myocardium may be best suited to vascularization for long-term therapy without adverse rejection events.
Of course hiPSCs raise the potential of using autologous cells for generating perfectly matched tissues for individual patients. We, like most investigators in the field, are captivated by these cells and expect them to have a large impact on science and medicine. At present, however, it is hard to imagine how hiPSCs can be used for autologous therapy in any large-scale manner. Generating hiPSCs currently is a painstaking process that takes weeks to months, and one needs to ensure that the resulting product is fully reprogrammed and is genetically normal before starting expansion and differentiation. The quality control required for each line is considerable and, if current good manufacturing practices are used, could cost millions of dollars per patient. This clearly is not affordable for a disease as common as heart failure. For this reason, we favor an off-the-shelf product that can be produced and quality controlled in large batches. An off-the-shelf product has the advantage of being used to treat acute illnesses (e.g., myocardial infarction), whereas autologous hiPSC-derived products could be used primarily to treat chronic illness owing to the inevitable lag time in production.
Finally, it is instructive to consider the patient population for whom the regenerative therapy is intended. (Readers interested in a more in-depth treatment of this topic are referred to 186.) Most animal studies have focused on models of acute myocardial infarction, yet most patients with acute infarction do reasonably well in the near term with conventional treatment. This may make them difficult to study in clinical trials unless a subset that has a particularly poor prognosis can be identified prospectively. Patients who develop chronic systolic heart failure due to progressive loss of cardiomyocytes have the most pressing need for a therapeutic that replaces human cardiomyocytes. However, in our experience, it is much harder to restore function in the failing heart than it is to prevent heart failure in the first place (34). Another class of patient is those with preserved myocardial viability but poor perfusion owing to macro- or microvascular disease. In these patients, a therapy that improves angiogenesis or facilitates arterial enlargement (arteriogenesis) would be most useful. Finally, we are increasingly aware of patients who have diastolic heart failure, more strictly defined as heart failure with preserved systolic function. This is a rapidly growing population that includes elderly and diabetic patients, and to our knowledge there has been little thought given to how regenerative strategies might repair the hearts of such patients.
10. CONCLUDING REMARKS
In the heart, the close apposition of cardiomyocytes with capillaries demands a regenerative medicine approach that includes vascularized cardiac tissue in its design. The necessity of an efficient microvascular network cannot be overstated. Simply put, capillaries are relatively easy to grow nowadays, but most of us are growing rudimentary plexuses from postcapillary venules that conduct blood very slowly. The challenge for the next decade will be engineering approaches to achieve hierarchical arterial and arteriolar inputs. In addition, integration into the host of a myocardial tissue will require anastomosis via microsurgical techniques or angiogenic approaches for the vasculature, as well as electromechanical integration of the contractile cardiomyocytes. Integration of graft into the host should also be approached as a challenge in the reverse: using the graft to influence or modulate the host environment. This may be critically important in a diseased heart, in which remodeling after an ischemic event needs to be stopped and/or reversed to achieve functional recovery.
Technology development continues to expand the horizon of tissue engineering, and our knowledge of human myocardial function and stem cell biology deepens. It is with these assets that we must remain diligent and curious in our quest to engineer myocardial tissue for cardiac regeneration.
ACKNOWLEDGMENTS
We gratefully acknowledge grant support from NIH K99 HL115123 (K.L.K.C.); NIH R01 HL086582 (S.T.A.); the New York Stem Cell Science Fund (NYSTEM #C024315 to S.T.A.); and NIH R01 HL084642, P01 HL094374, P01 GM81619, and U01 HL100405 (C.E.M.).
Footnotes
DISCLOSURE STATEMENT
C.E.M. is a cofounder and equity holder in BEAT BioTherapeutics.
Contributor Information
Kareen L.K. Coulombe, Email: kareen_coulombe@brown.edu.
Charles E. Murry, Email: murry@uw.edu.
LITERATURE CITED
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu. Rev. Biomed. Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 5.Adler CP, Friedburg H. Myocardial DNA content, ploidy level and cell number in geriatric hearts: post-mortem examinations of human myocardium in old age. J. Mol. Cell. Cardiol. 1986;18:39–53. doi: 10.1016/s0022-2828(86)80981-6. [DOI] [PubMed] [Google Scholar]
- 6.Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: Cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69:203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 7.Young AA, Cowan BR. Evaluation of left ventricular torsion by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012;14:49. doi: 10.1186/1532-429X-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ausoni S, Sartore S. The cardiovascular unit as a dynamic player in disease and regeneration. Trends Mol. Med. 2009;15:543–552. doi: 10.1016/j.molmed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Nees S, Weiss DR, Juchem G. Focus on cardiac pericytes. Pflügers Arch. 2013;465:779–787. doi: 10.1007/s00424-013-1240-1. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko N, Matsuda R, Toda M, Shimamoto K. Three-dimensional reconstruction of the human capillary network and the intramyocardial micronecrosis. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H754–H761. doi: 10.1152/ajpheart.00486.2010. [DOI] [PubMed] [Google Scholar]
- 11.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 2012;18:68–80. doi: 10.1017/S1431927611012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Pomares JM, de la Pompa JL. Signaling during epicardium and coronary vessel development. Circ. Res. 2011;109:1429–1442. doi: 10.1161/CIRCRESAHA.111.245589. [DOI] [PubMed] [Google Scholar]
- 16.Tian X, Hu T, Zhang H, He L, Huang X, et al. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013;23:1075–1090. doi: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cossette S, Misra R. The identification of different endothelial cell populations within the mouse proepicardium. Dev. Dyn. 2011;240:2344–2353. doi: 10.1002/dvdy.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomanek RJ. Formation of the coronary vasculature: a brief review. Cardiovasc. Res. 1996;31:E46–E51. [PubMed] [Google Scholar]
- 19.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Ye L, ZimmermannWH, Garry DJ, Zhang J. Patching the heart: cardiac repair from within and outside. Circ. Res. 2013;113:922–932. doi: 10.1161/CIRCRESAHA.113.300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:II63–II69. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 22.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 24.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/β-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, et al. Biphasic role forWnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, et al. Developmental stage-specific biphasic roles ofWnt/β-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim C, Majdi M, Xia P, Wei KA, Talantova M, et al. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 2010;19:783–795. doi: 10.1089/scd.2009.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, et al. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE. 2011;6:e23657. doi: 10.1371/journal.pone.0023657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat. Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 33.Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell. Cardiol. 2010;49:941–949. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song K, Nam YJ, Luo X, Qi X, Tan W, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ. Res. 2012;111:50–55. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Addis RC, Epstein JA. Induced regeneration—the progress and promise of direct reprogramming for heart repair. Nat. Med. 2013;19:829–836. doi: 10.1038/nm.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Li L, Shojaei F, Levac K, Cerdan C, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the heman-gioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White MP, Rufaihah AJ, Liu L, Ghebremariam YT, Ivey KN, et al. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2013;31:92–103. doi: 10.1002/stem.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Wilson KD, Smith B, Kraft DL, Jia F, et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE. 2009;4:e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler. Thromb. Vasc. Biol. 2010;30:80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kane NM, Meloni M, Spencer HL, Craig MA, Strehl R, et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2010;30:1389–1397. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 45.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, et al. Molecular signatures of tissuespecific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev. Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreutziger KL, Muskheli V, Johnson P, Braun K, Wight TN, Murry CE. Developing vasculature and stroma in engineered human myocardium. Tissue Eng. Part A. 2011;17:1219–1228. doi: 10.1089/ten.tea.2010.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc. Natl. Acad. Sci. USA. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ. Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 49.Vrancken Peeters MPFM, Gittenberger-de Groot AC, Mentink MMT, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat. Embryol. 1999;199:367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- 50.Descamps B, Emanueli C. Vascular differentiation from embryonic stem cells: novel technologies and therapeutic promises. Vasc. Pharmacol. 2012;56:267–279. doi: 10.1016/j.vph.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Cheung C, Sinha S. Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation. J. Mol. Cell. Cardiol. 2011;51:651–664. doi: 10.1016/j.yjmcc.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Bajpai VK, Mistriotis P, Loh YH, Daley GQ, Andreadis ST. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc. Res. 2012;96:391–400. doi: 10.1093/cvr/cvs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Mounayri O, Mihic A, Shikatani EA, Gagliardi M, Steinbach SK, et al. Serum-free differentiation of functional human coronary-like vascular smooth muscle cells from embryonic stem cells. Cardiovasc. Res. 2013;98:125–135. doi: 10.1093/cvr/cvs357. [DOI] [PubMed] [Google Scholar]
- 54.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin–dependent disease susceptibility. Nat. Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul JD, Coulombe KL, Toth PT, Zhang Y, Marsboom G, et al. SLIT3–ROBO4 activation promotes vascular network formation in human engineered tissue and angiogenesis in vivo. J. Mol. Cell. Cardiol. 2013;64:124–131. doi: 10.1016/j.yjmcc.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ. Res. 2010;107:1304–1312. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 58.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ. Res. 2009;105:934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Challa AA, Vukmirovic M, Blackmon J, Stefanovic B. Withaferin-A reduces type I collagen expression in vitro and inhibits development of myocardial fibrosis in vivo. PLoS ONE. 2012;7:e42989. doi: 10.1371/journal.pone.0042989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cotton JM, Kearney MT, MacCarthy PA, Grocott-Mason RM, McClean DR, et al. Effects of nitric oxide synthase inhibition on basal function and the force-frequency relationship in the normal and failing human heart in vivo. Circulation. 2001;104:2318–2323. doi: 10.1161/hc4401.098515. [DOI] [PubMed] [Google Scholar]
- 61.Paulus WJ, Vantrimpont PJ, Shah AM. Paracrine coronary endothelial control of left ventricular function in humans. Circulation. 1995;92:2119–2126. doi: 10.1161/01.cir.92.8.2119. [DOI] [PubMed] [Google Scholar]
- 62.Mohan P, Brutsaert DL, Sys SU. Myocardial performance is modulated by interaction of cardiac endothelium derived nitric oxide and prostaglandins. Cardiovasc. Res. 1995;29:637–640. [PubMed] [Google Scholar]
- 63.Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ. Res. 1999;85:643–650. doi: 10.1161/01.res.85.7.643. [DOI] [PubMed] [Google Scholar]
- 64.Meulemans AL, Andries LJ, Brutsaert DL. Does endocardial endothelium mediate positive inotropic response to angiotensin I and angiotensin II? Circ. Res. 1990;66:1591–1601. doi: 10.1161/01.res.66.6.1591. [DOI] [PubMed] [Google Scholar]
- 65.Chua BH, Chua CC, Diglio CA, Siu BB. Regulation of endothelin-1 mRNA by angiotensin II in rat heart endothelial cells. Biochim. Biophys. Acta. 1993;1178:201–206. doi: 10.1016/0167-4889(93)90010-m. [DOI] [PubMed] [Google Scholar]
- 66.Kusaka Y, Kelly RA, Williams GH, Kifor I. Coronary microvascular endothelial cells cosecrete angiotensin II and endothelin-1 via a regulated pathway. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1087–H1096. doi: 10.1152/ajpheart.2000.279.3.H1087. [DOI] [PubMed] [Google Scholar]
- 67.McClellan G, Weisberg A, Rose D, Winegrad S. Endothelial cell storage and release of endothelin as a cardioregulatory mechanism. Circ. Res. 1994;75:85–96. doi: 10.1161/01.res.75.1.85. [DOI] [PubMed] [Google Scholar]
- 68.Li K, Stewart DJ, Rouleau JL. Myocardial contractile actions of endothelin-1 in rat and rabbit papillary muscles: role of endocardial endothelium. Circ. Res. 1991;69:301–312. doi: 10.1161/01.res.69.2.301. [DOI] [PubMed] [Google Scholar]
- 69.Drawnel FM, Archer CR, Roderick HL. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br. J. Pharmacol. 2013;168:296–317. doi: 10.1111/j.1476-5381.2012.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ. Res. 2009;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, et al. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun. Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hilenski LL, Terracio L, Borg TK. Myofibrillar and cytoskeletal assembly in neonatal rat cardiac myocytes cultured on laminin and collagen. Cell Tissue Res. 1991;264:577–587. doi: 10.1007/BF00319047. [DOI] [PubMed] [Google Scholar]
- 73.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc. Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 74.Vlodavsky I, Fuks Z, Ishai-Michaeli R, Bashkin P, Levi E, et al. Extracellular matrix-resident basic fibroblast growth factor: implication for the control of angiogenesis. J. Cell. Biochem. 1991;45:167–176. doi: 10.1002/jcb.240450208. [DOI] [PubMed] [Google Scholar]
- 75.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1α and β isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp. Cell Res. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Cote GM, Sawyer DB, Chabner BA. ERBB2inhibition and heart failure. N. Engl. J. Med. 2012;367:2150–2153. doi: 10.1056/NEJMcibr1203156. [DOI] [PubMed] [Google Scholar]
- 77.Kreutziger KL, Murry CE. Engineered human cardiac tissue. Pediatr. Cardiol. 2011;32:334–341. doi: 10.1007/s00246-011-9888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta M, et al. Site-directed neovessel formation in vivo. Science. 1988;241:1349–1352. doi: 10.1126/science.2457952. [DOI] [PubMed] [Google Scholar]
- 79.Wake MC, Patrick CW, Jr, Mikos AG. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994;3:339–343. doi: 10.1177/096368979400300411. [DOI] [PubMed] [Google Scholar]
- 80.Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, Langer R. Prevascularization of porous biodegradable polymers. Biotechnol. Bioeng. 1993;42:716–723. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 81.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J. Biomed. Mater. Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 82.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 83.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J. Bone Joint Surg. Am. 2004;86:1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 84.Giraud MN, Armbruster C, Carrel T, Tevaearai HT. Current state of the art in myocardial tissue engineering. Tissue Eng. 2007;13:1825–1836. doi: 10.1089/ten.2006.0110. [DOI] [PubMed] [Google Scholar]
- 85.Pessanha MG, Mandarim-de-Lacerda CA. Influence of the chronic nitric oxide synthesis inhibition on cardiomyocytes number. Virchows Arch. 2000;437:667–674. doi: 10.1007/s004280000276. [DOI] [PubMed] [Google Scholar]
- 86.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trial J, Rossen RD, Rubio J, Knowlton AA. Inflammation and ischemia: Macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp. Biol. Med. (Maywood) 2004;229:538–545. doi: 10.1177/153537020422900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Troidl C, Möllmann H, Nef H, Masseli F, Voss S, et al. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J. Cell. Mol. Med. 2009;13:3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freytes DO, Santambrogio L, Vunjak-Novakovic G. Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs. 2012;195:171–182. doi: 10.1159/000331392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura Y, Yasuda T, Weisel RD, Li RK. Enhanced cell transplantation: Preventing apoptosis increases cell survival and ventricular function. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H939–H947. doi: 10.1152/ajpheart.00155.2006. [DOI] [PubMed] [Google Scholar]
- 92.Weyers JJ, Schwartz SM, Minami E, Carlson DD, Dupras SK, et al. Effects of cell grafting on coronary remodeling after myocardial infarction. J. Am. Heart Assoc. 2013;2:e000202. doi: 10.1161/JAHA.113.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cassell OC, Hofer SO, Morrison WA, Knight KR. Vascularisation of tissue-engineered grafts: the regulation of angiogenesis in reconstructive surgery and in disease states. Br. J. Plast. Surg. 2002;55:603–610. doi: 10.1054/bjps.2002.3950. [DOI] [PubMed] [Google Scholar]
- 94.Arkudas A, Tjiawi J, Saumweber A, Beier JP, Polykandriotis E, et al. Evaluation of blood vessel ingrowth in fibrin gel subject to type and concentration of growth factors. J. Cell. Mol. Med. 2009;13:2864–2874. doi: 10.1111/j.1582-4934.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu ZC, Chang TM. Artificial cell microencapsulated stem cells in regenerative medicine, tissue engineering and cell therapy. Adv. Exp. Med. Biol. 2010;670:68–79. doi: 10.1007/978-1-4419-5786-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saxena AK, Marler J, Benvenuto M, Willital GH, Vacanti JP. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: preliminary studies. Tissue Eng. 1999;5:525–532. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 97.Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl. Acad. Sci. USA. 2009;106:14990–14995. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laschke MW, Rucker M, Jensen G, Carvalho C, Mulhaupt R, et al. Improvement of vascularization of PLGA scaffolds by inosculation of in situ-preformed functional blood vessels with the host microvasculature. Ann. Surg. 2008;248:939–948. doi: 10.1097/SLA.0b013e31818fa52f. [DOI] [PubMed] [Google Scholar]
- 99.Amir G, Miller L, Shachar M, Feinberg MS, Holbova R, et al. Evaluation of a peritoneal-generated cardiac patch in a rat model of heterotopic heart transplantation. Cell Transplant. 2009;18:275–282. doi: 10.3727/096368909788534898. [DOI] [PubMed] [Google Scholar]
- 100.Erol OO, Spira M. New capillary bed formation with a surgically constructed arteriovenous fistula. Surg. Forum. 1979;30:530–531. [PubMed] [Google Scholar]
- 101.Khouri RK, Upton J, Shaw WW. Prefabrication of composite free flaps through staged microvascular transfer: an experimental and clinical study. Plast. Reconstr. Surg. 1991;87:108–115. doi: 10.1097/00006534-199101000-00017. [DOI] [PubMed] [Google Scholar]
- 102.Morrison WA, Penington AJ, Kumta SK, Callan P. Clinical applications and technical limitations of prefabricated flaps. Plast. Reconstr. Surg. 1997;99:378–385. doi: 10.1097/00006534-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka Y, Sung KC, Tsutsumi A, Ohba S, Ueda K, Morrison WA. Tissue engineering skin flaps: Which vascular carrier, arteriovenous shunt loop or arteriovenous bundle, has more potential for angiogenesis and tissue generation? Plast. Reconstr. Surg. 2003;112:1636–1644. doi: 10.1097/01.PRS.0000086140.49022.AB. [DOI] [PubMed] [Google Scholar]
- 104.Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 105.Lokmic Z, Stillaert F, Morrison WA, Thompson EW, Mitchell GM. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB. J. 2007;21:511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- 106.Mian R, Morrison WA, Hurley JV, Penington AJ, Romeo R, et al. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000;6:595–603. doi: 10.1089/10763270050199541. [DOI] [PubMed] [Google Scholar]
- 107.Tanaka Y, Tsutsumi A, Crowe DM, Tajima S, Morrison WA. Generation of an autologous tissue (matrix) flap by combining an arteriovenous shunt loop with artificial skin in rats: preliminary report. Br. J. Plast. Surg. 2000;53:51–57. doi: 10.1054/bjps.1999.3186. [DOI] [PubMed] [Google Scholar]
- 108.Lokmic Z, Thomas JL, Morrison WA, Thompson EW, Mitchell GM. An endogenously deposited fibrin scaffold determines construct size in the surgically created arteriovenous loop chamber model of tissue engineering. J. Vasc. Surg. 2008;48:974–985. doi: 10.1016/j.jvs.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 109.Cassell OC, Morrison WA, Messina A, Penington AJ, Thompson EW, et al. The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann. N. Y. Acad. Sci. 2001;944:429–442. doi: 10.1111/j.1749-6632.2001.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 110.Rophael JA, Craft RO, Palmer JA, Hussey AJ, Thomas GP, et al. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am. J. Pathol. 2007;171:2048–2057. doi: 10.2353/ajpath.2007.070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arkudas A, Tjiawi J, Bleiziffer O, Grabinger L, Polykandriotis E, et al. Fibrin gel-immobilized VEGF and bFGF efficiently stimulate angiogenesis in the AV loop model. Mol. Med. 2007;13:480–487. doi: 10.2119/2007-00057.Arkudas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 113.Birla RK, Borschel GH, Dennis RG, Brown DL. Myocardial engineering in vivo: formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Eng. 2005;11:803–813. doi: 10.1089/ten.2005.11.803. [DOI] [PubMed] [Google Scholar]
- 114.Polykandriotis E, Horch RE, Arkudas A, Labanaris A, Brune K, et al. Intrinsic versus extrinsic vascularization in tissue engineering. Adv. Exp. Med. Biol. 2006;585:311–326. doi: 10.1007/978-0-387-34133-0_21. [DOI] [PubMed] [Google Scholar]
- 115.Arkudas A, Pryymachuk G, Beier JP, Weigel L, Korner C, et al. Combination of extrinsic and intrinsic pathways significantly accelerates axial vascularization of bioartificial tissues. Plast. Reconstr. Surg. 2012;129:55e–65e. doi: 10.1097/PRS.0b013e3182361f97. [DOI] [PubMed] [Google Scholar]
- 116.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 117.Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J. Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vernon RB, Angello JC, Iruela-Arispe ML, Lane TF, Sage EH. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab. Investig. 1992;66:536–547. [PubMed] [Google Scholar]
- 119.Ilan N, Mahooti S, Madri JA. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J. Cell Sci. 1998;111:3621–3631. doi: 10.1242/jcs.111.24.3621. [DOI] [PubMed] [Google Scholar]
- 120.Nor JE, Peters MC, Christensen JB, Sutorik MM, Linn S, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab. Investig. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 121.Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 123.Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. USA. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Black AF, Berthod F, L’Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB. J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 125.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–S152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 127.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 128.Chang EI, Bonillas RG, El-ftesi S, Ceradini DJ, Vial IN, et al. Tissue engineering using autologous microcirculatory beds as vascularized bioscaffolds. FASEB. J. 2009;23:906–915. doi: 10.1096/fj.08-114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 130.Frame MD, Sarelius IH. A system for culture of endothelial cells in 20–50-µm branching tubes. Microcirculation. 1995;2:377–385. doi: 10.3109/10739689509148282. [DOI] [PubMed] [Google Scholar]
- 131.Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, et al. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000;6:105–117. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 132.Neumann T, Nicholson BS, Sanders JE. Tissue engineering of perfused microvessels. Microvasc. Res. 2003;66:59–67. doi: 10.1016/s0026-2862(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 133.Ko IK, Iwata H. An approach to constructing three-dimensional tissue. Ann. N. Y. Acad. Sci. 2001;944:443–455. doi: 10.1111/j.1749-6632.2001.tb03854.x. [DOI] [PubMed] [Google Scholar]
- 134.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 135.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 136.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 138.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, et al. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 140.Druecke D, Langer S, Lamme E, Pieper J, Ugarkovic M, et al. Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: long-term investigations using intravital fluorescent microscopy. J. Biomed. Mater. Res. A. 2004;68:10–18. doi: 10.1002/jbm.a.20016. [DOI] [PubMed] [Google Scholar]
- 141.Hollister SJ. Porous scaffold design for tissue engineering. Nat. Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 142.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 143.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 144.Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J. Cardiovasc. Med. (Hagerstown) 2008;9:1190–1221. doi: 10.2459/JCM.0b013e3283117d37. [DOI] [PubMed] [Google Scholar]
- 145.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 146.Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 147.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Investig. 2004;113:516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Epstein SE, Fuchs S, Zhou YF, Baffour R, Kornowski R. Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovasc. Res. 2001;49:532–542. doi: 10.1016/s0008-6363(00)00217-0. [DOI] [PubMed] [Google Scholar]
- 149.Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc. Res. 2001;49:522–531. doi: 10.1016/s0008-6363(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 150.Ruel M, Laham RJ, Parker JA, Post MJ, Ware JA, et al. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J. Thorac. Cardiovasc. Surg. 2002;124:28–34. doi: 10.1067/mtc.2002.121974. [DOI] [PubMed] [Google Scholar]
- 151.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Helm CL, Fleury ME, Zisch AH, Boschetti F, Swartz MA. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl. Acad. Sci. USA. 2005;102:15779–15784. doi: 10.1073/pnas.0503681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang J, Zhou W, Zheng W, Ma Y, Lin L, et al. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology. 2007;107:17–29. doi: 10.1159/000093609. [DOI] [PubMed] [Google Scholar]
- 154.Ye L, Haider H, Tan R, Su L, Law PK, et al. Angiomyogenesis using liposome based vascular endothelial growth factor-165 transfection with skeletal myoblast for cardiac repair. Biomaterials. 2008;29:2125–2137. doi: 10.1016/j.biomaterials.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 155.Yau TM, Fung K, Weisel RD, Fujii T, Mickle DA, Li RK. Enhanced myocardial angiogenesis by gene transfer with transplanted cells. Circulation. 2001;104:I218–I222. doi: 10.1161/hc37t1.094896. [DOI] [PubMed] [Google Scholar]
- 156.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 157.Yau TM, Kim C, Li G, Zhang Y, Fazel S, et al. Enhanced angiogenesis with multimodal cell-based gene therapy. Ann. Thorac. Surg. 2007;83:1110–1119. doi: 10.1016/j.athoracsur.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 158.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 160.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 161.Geer DJ, Swartz DD, Andreadis ST. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am. J. Pathol. 2005;167:1575–1586. doi: 10.1016/S0002-9440(10)61242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liang MS, Andreadis ST. Engineering fibrin-binding TGF-β1 for sustained signaling and contractile function of MSC based vascular constructs. Biomaterials. 2011;32:8684–8693. doi: 10.1016/j.biomaterials.2011.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 164.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 165.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. A. 2003;65:489–97. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 166.Chen RR, Silva EA, Yuen WW, Brock AA, Fischbach C, et al. Integrated approach to designing growth factor delivery systems. FASEB. J. 2007;21:3896–3903. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 167.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 168.Burdick JA, Khademhosseini A, Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir. 2004;20:5153–5156. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- 169.Borselli C, Oliviero O, Battista S, Ambrosio L, Netti PA. Induction of directional sprouting angiogenesis by matrix gradients. J. Biomed. Mater. Res. A. 2007;80:297–305. doi: 10.1002/jbm.a.30896. [DOI] [PubMed] [Google Scholar]
- 170.Liang MS, Koobatian M, Lei P, Swartz DD, Andreadis ST. Differential and synergistic effects of mechanical stimulation and growth factor presentation on vascular wall function. Biomaterials. 2013;34:7281–7291. doi: 10.1016/j.biomaterials.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cellinvasion characteristics. Proc. Natl. Acad. Sci. USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell in growth matrices for vascularized tissue growth. FASEB. J. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 173.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc. Natl. Acad. Sci. USA. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J. Cell. Physiol. 1986;128:475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 175.Sommer A, Rifkin DB. Interaction of heparin with human basic fibroblast growth factor: protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J. Cell. Physiol. 1989;138:215–220. doi: 10.1002/jcp.1041380129. [DOI] [PubMed] [Google Scholar]
- 176.Bashkin P, Doctrow S, Klagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989;28:1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- 177.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, et al. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J. Control. Release. 2000;64:103–114. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 179.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 180.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 181.Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, Van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen–heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–1131. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 182.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1348. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Riolobos L, Hirata RK, Turtle CJ, Wang PR, Gornalusse GG, et al. HLA engineering of human pluripotent stem cells. Mol. Ther. 2013;21:1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Parent AV, Russ HA, Khan IS, Laflam TN, Metzger TC, et al. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell. 2013;13:219–229. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun X, Xu J, Lu H, Liu W, Miao Z, et al. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell. 2013;13:230–236. doi: 10.1016/j.stem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 186.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu. Rev. Pathol. 2007;2:307–339. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]