Abstract
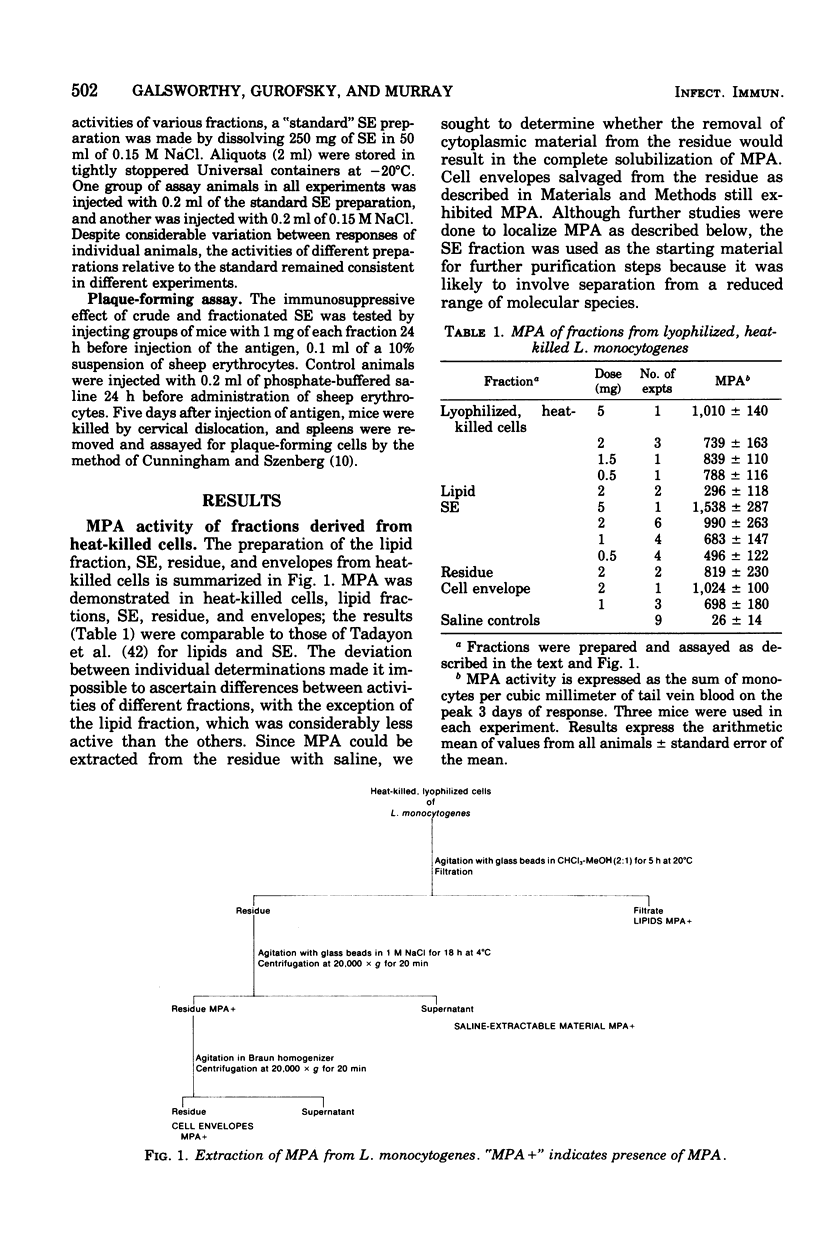
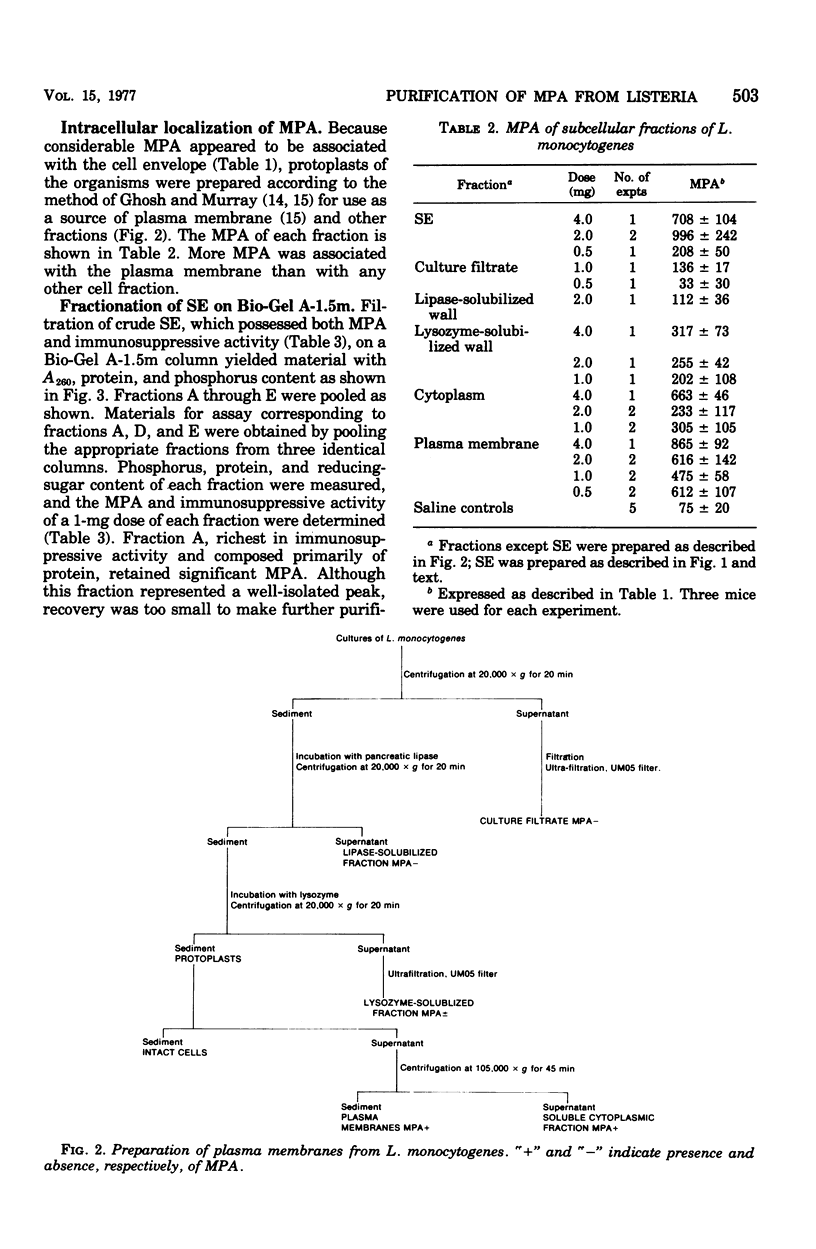
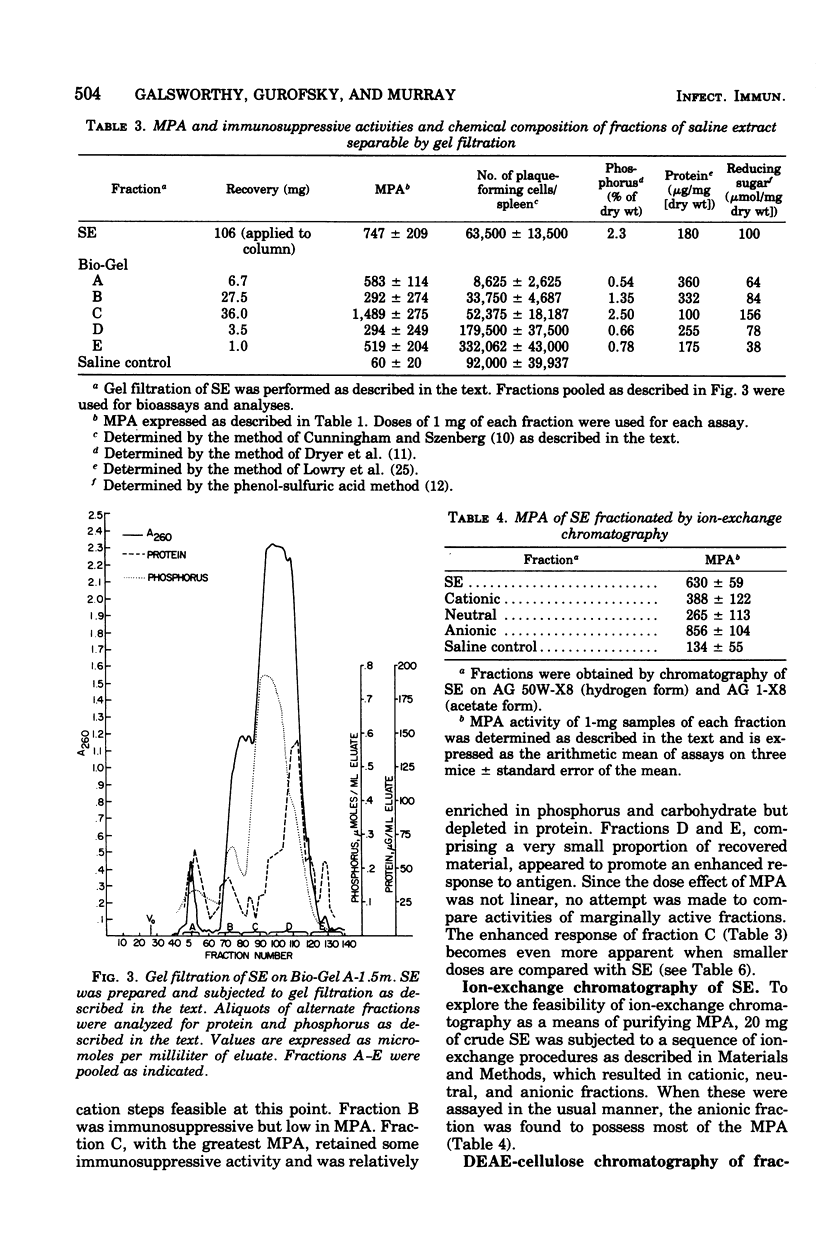
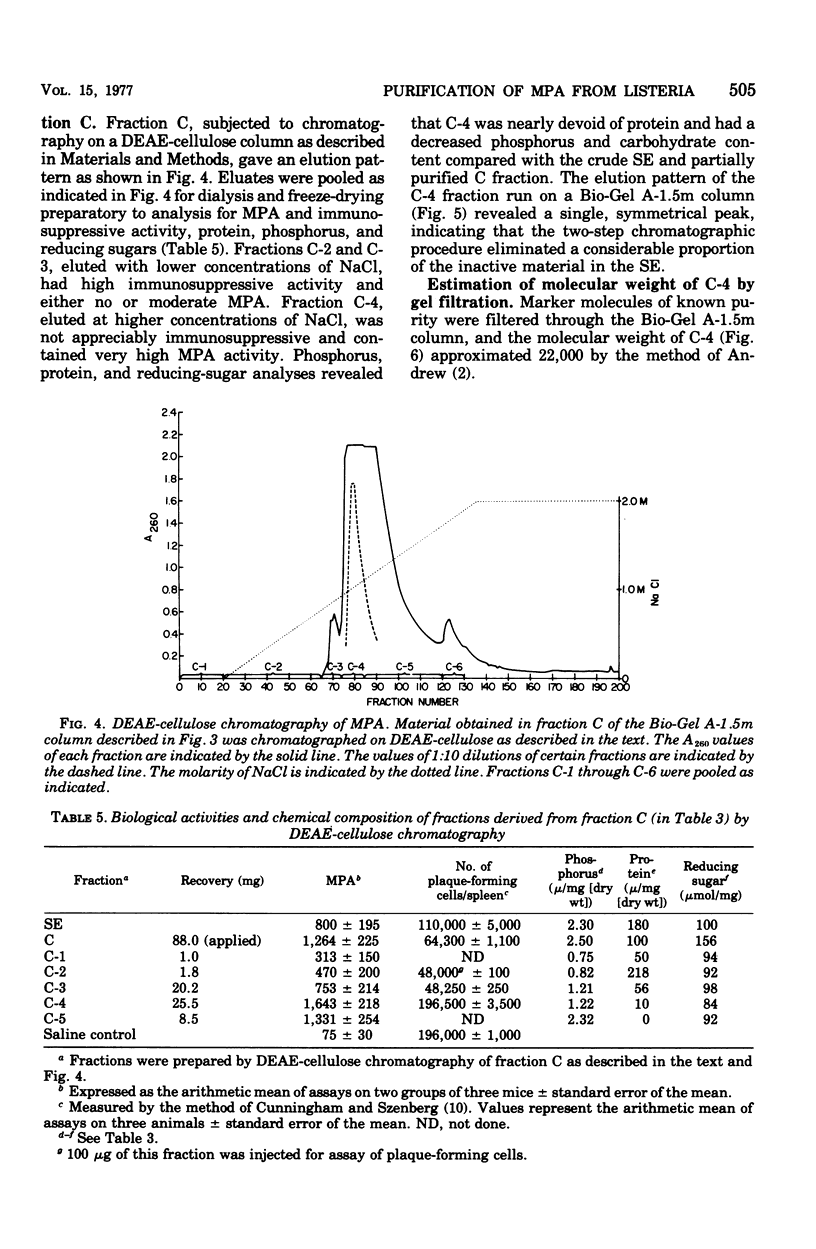
The monocytosis-producing activity (MPA) from Listeria monocytogenes is shown to be associated with the cell envelope. Both MPA and immunosuppressive activity were readily extracted with aqueous solvents and separated as two independent activities by gel filtration and ion-exchange chromatography. As little as 10 microng of the resulting MPA-containing fraction caused a ninefold elevation in the level of circulating monocytes. The molecular weight of the fraction was approximately 22,000, and it contained phosphorus and carbohydrate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Petit J. F., Lederer E. Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):851–854. doi: 10.1073/pnas.69.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bültmann B., Heymer B., Schleifer K. H., Seidl H. P., Haferkamp O. Migration inhibition of peritoneal macrophages by peptidoglycan. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):289–294. [PubMed] [Google Scholar]
- Campbell P. A., Martens B. L., Cooper H. R., McClatchy J. K. Requirement for bone marrow-derived cells in resistance to Listeria. J Immunol. 1974 Apr;112(4):1407–1414. [PubMed] [Google Scholar]
- Campbell P. A., Rodriguez G. E., Schuffler C. Listeria cell wall fraction: adjuvant activity in vivo and in vitro. Cell Immunol. 1975 Jun;17(2):418–422. doi: 10.1016/s0008-8749(75)80045-1. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., Schuffler C., Rodriguez G. E. Listeria cell wall fraction: a B cell adjuvant. J Immunol. 1976 Mar;116(3):590–594. [PubMed] [Google Scholar]
- Ciorbaru R., Adam A., Petit J. F., Lederer E., Bona C., Chedid L. Isolation of mitogenic and adjuvant active fractions from various species of Nocardiae. Infect Immun. 1975 Feb;11(2):257–264. doi: 10.1128/iai.11.2.257-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Fulton A. M., Dustoor M. M., Kasinski J. E., Blazkovec A. A. Blastogenesis as an in vitro correlate of delayed hypersensitivity in guinea pigs infected with Listeria monocytogenes. Infect Immun. 1975 Sep;12(3):647–655. doi: 10.1128/iai.12.3.647-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD K. F., MURRAY E. G. The influence of a sustained monocytosis upon the antibody response in rabbits to various antigens. Can J Biochem Physiol. 1954 Jan;32(1):1–13. [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fractionation and characterization of the plasma and mesosome membrane of Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):426–440. doi: 10.1128/jb.97.1.426-440.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H. Requirement for a bone marrow-derived component in the expression of cell-mediated antibacterial immunity. Infect Immun. 1975 May;11(5):949–954. doi: 10.1128/iai.11.5.949-954.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiu I. J. Water-soluble and lipid-free fraction from BCG with adjuvant and antitumour activity. Nat New Biol. 1972 Aug 23;238(86):241–242. doi: 10.1038/newbio238241a0. [DOI] [PubMed] [Google Scholar]
- Holmström B. Studies on the cultivation of Listeria monocytogenes in batch and continuous cultures. Can J Microbiol. 1967 Nov;13(11):1551–1559. doi: 10.1139/m67-203. [DOI] [PubMed] [Google Scholar]
- Holton J. B., Schwab J. H. Adjuvant properties of bacterial cell wall mucopeptides. J Immunol. 1966 Jan;96(1):134–138. [PubMed] [Google Scholar]
- John C., Sprătkova I., Morávková M., Patocka F., Mára M. Effect of Listeria-factor Ei on spleen cells migration in rabbits hypersensitive to Listeria monocytogenes. J Hyg Epidemiol Microbiol Immunol. 1974;18(3):369–372. [PubMed] [Google Scholar]
- Kim J. J., St C Sinclair N. R., Singhal S. K., Carroll K. K. Immunosuppressive activity of an extract of Listeria monocytogenes. Int Arch Allergy Appl Immunol. 1976;50(6):641–650. doi: 10.1159/000231542. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mára M., Julăk J., Patocka F. Isolation and chemical properties of biologically active fractions of the surface of Listeria monocytogenes. J Hyg Epidemiol Microbiol Immunol. 1974;18(3):359–364. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B. Immunological control of macrophage proliferation in vivo. Infect Immun. 1973 Jul;8(1):68–73. doi: 10.1128/iai.8.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osebold J. W., Outteridge P. M., Pearson L. D., Dicapua R. A. Cellular Responses of Mice to Diffusion Chambers I. Reactions to Intraperitoneal Diffusion Chambers Containing Listeria monocytogenes and to Bacteria-Free Chambers. Infect Immun. 1970 Aug;2(2):127–131. doi: 10.1128/iai.2.2.127-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- ROWLEY D. Stimulation of natural immunity to Escherichia coli infection: observations on mice. Lancet. 1955 Jan 29;268(6857):232–234. doi: 10.1016/s0140-6736(55)90163-x. [DOI] [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Tadayon R. A., Carroll K. K., Murray R. G. Factors affecting the yield and biological activity of lipid extracts of Listeria monocytogenes. Can J Microbiol. 1969 May;15(5):421–428. doi: 10.1139/m69-074. [DOI] [PubMed] [Google Scholar]
- Tadayon R. A., Carroll K. K., Murray R. G. Purification and properties of biologically active factors in lipid extracts of Listeria monocytogenes. Can J Microbiol. 1970 Jun;16(6):535–544. doi: 10.1139/m70-090. [DOI] [PubMed] [Google Scholar]
- Taranta A., Cuppari G., Quagliata F. Dissociation of hemolytic and lymphocyte-transforming activities of streptolysin S preparations. J Exp Med. 1969 Apr 1;129(4):605–622. doi: 10.1084/jem.129.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]


