Abstract
Background:
Guduchi Ghana is one of the unique Ayuvedic classical preparation which is prepared from aqueous of extract of Guduchi (Tinospora cordifolia Miers.) stem. It is one of the frequently used drugs to treat the Madhumeha, Pandu, Kamala, Amlapitta, Grahani, Kustha, Jirna Jwara and Viswamjwara, Trishna, Shool, Yakritavikara, etc. Looking to these indications, in market most of the Pharma industries prepared Guduchi Ghana by applying the various extraction process.
Aim:
To evaluate comparative anti-inflammatory activity of classically prepared and market sample of Guduchi Ghana.
Materials and Methods:
Both samples were evaluated for anti-inflammatory activity using carrageenan induced paw edema model in rats. Animals were divided in three groups, having six animals in each. Group A received test drug, Group B received market sample at a dose of 50 mg/kg orally, while Group C (control group) received tap water.
Results:
Reduction in edema was observed in Group A and B at 3 h interval by 33.06% and 11.71% respectively. Group A showed significant effects (P < 0.05) in comparison to control group.
Conclusion:
These experimental results have shown anti-inflammatory activity of Guduchi Ghana.
Keywords: Anti-inflammatory activity, aqueous extract, carrageenan, Ghana Kalpana, Guduchi, Tinospora cordifolia
Introduction
Tinospora cordifolia (Willd.) Miers (family Menispermaceae),[1] an important medicinal plant is also known as Guduchi. It is widely distributed in India, extending from the Himalayas down to the southern part of peninsular India.[2] It is categorized as “Rasayana”[3] and used for its anti-inflammatory,[4,5] immunomodulatory,[6] anti-allergic,[7] anti-diabetic,[8] properties etc. The whole plant is used medicinally; however, the stem is approved for use in medicine as listed by the Ayurvedic Pharmacopoeia of India. This is due to higher alkaloid content in the stems than in the leaves.[9]
Inflammation is defined as the local response of living mammalian tissues to injury due to any agent. It is a body defense reaction in order to eliminate or limit the spread of injurious agent. Depending upon the defense capacity of the host and duration of response, it is classified as acute and chronic. Among them the main features of acute inflammation are accumulation of fluid and plasma; Intravascular activation of platelets; and polymorphonuclear neutrophils as inflammatory cells.[10] Histamine, 5-hydroxytryptamine and bradykinin are the first detectable mediators in the early phase of carrageenan-induced inflammation, whereas prostaglandins are detectable in the late phase of inflammation.
Guduchi Ghana.[11] (concentrated form of decoction) is the secondary Kalpana (formulation) derived from the primary Kalpana, i.e. Kwatha (decoction). The other sample of Guduchi Ghana was purchased from the local market. Several research works have been carried out regarding the anti-inflammatory activity of the decoction,[12] alcohol extract,[13] water extract of the stem of Giloe (T. cordifolia that grow on Azadirachta indica)[5] and water extract.[14,15,16] The water extract of the plant is found to be more potent than the other extract.[17] Hence, it has been planned to study the comparative anti-inflammatory activity of classically prepared and market sample of Guduchi Ghana.
Materials and Methods
Collection of the plant material and preparation of the Ghana (extract)
For the preparation of Guduchi Ghana,[11] the stem was collected from the periphery of Jamnagar and authenticated by the Botanist of Phamacognosy Laboratory, I.P.G.T. and R.A., Jamnagar. The freshly collected stem was cut into small pieces; soaked in four times of water and made decoction of it. The decoction was reheated until it became semisolid and dried in the oven at 55°C.
The second sample of Guduchi Ghana was purchased from the market. This extract was prepared by centrifuge and spray drying method. First the decoction was made by using 16 times of water and reduced up to one-fourth in steam jacket vessel. Then, the liquid was centrifuged and the supernant liquid was discarded. Thick slurry was dried by spray drying method.
Animal and dose selection
Charles Foster strain albino rats of either sex weighing between 180 and 275 g were obtained from the animal house attached to Pharmacology Laboratory, IPGT and RA, Gujart Ayurved University, Jamnagar. They were housed at 22°C ± 2°C with constant humidity 50-60%, on a 12 h natural day and night cycles. They were fed with diet Amrut brand rat pellet feed (Pranav Agro Industries) and tap water ad libitum. This experiment was carried out after obtaining permission from “Institutional Animal Ethics Committee” (IAEC/2008/MD/03). The selected animals were grouped into three groups of 6 animals each. The test dose of the drugs for experimental study was calculated by extrapolating the human dose (500 mg/kg) to animals based on the body surface area ratio by referring to the standard table of Paget and Barnes.[18] The stock solution was prepared freshly by mixing adequate quantity of water with both samples and used for all the experimental purpose. Group A received Guduchi Ghana prepared from classical method, Group B received Guduchi Ghana market sample at the dose of 50 mg/kg orally and Group C received tap water as a control. The drugs were administered to overnight fasted animals in the dose of 1 ml/100 g body weight with the help of gastric catheter sleeved to syringe.
Anti-inflammatory activity in rats
The standard method for screening anti-inflammatory effect was followed.[19] Rats of either sex weighing between 180 and 275 g were used. Rats were provided with food and tap water up to the start of the experiment. The test drug was administrated at a dose of 50 mg/kg body weights to three groups for 7 days. On 7th day, initially left hind paw volume up to the tibiotarsal articulation was recorded by using plethysmograph. The plethysmograph employed consisted of a 10 ml glass vessel (25 × 65 mm) fixed to a 2 ml glass syringe through pressure tubing. A total volume of 4 ml of mercury was filled in the syringe and the mercury level was adjusted to zero mark on the micropipette. The space between the zero mark and the fixed mark on the glass vessel was filled with water and few drops of teepol. The initial level of fluid was adjusted and set at zero. The paw was immersed in water exactly up to the tibiotarsal articulation. The increased level of water in the glass vessel was adjusted to the prefixed mark by releasing the pressure of the connected syringe. The level where water and mercury interface in the micropipette was recorded as paw volume. On 5th day 1 h after drug administration, edema was produced by injecting 0.1 ml of freshly prepared 1% carrageenan in sterile saline solution to the sub-plantar aponeurosis of the left hind limb. The rats were administered tap water in the dose of 2 ml/100 g body weight to ensure uniform hydration and hence to minimize variations in edema formation. Paw volume was recorded 3 h after carrageenan injection. Results were expressed as an increase in paw volume in comparison to the initial paw volume and also in comparison with the control group.
Statistical analysis
All the values were expressed as mean ± standard error of mean. The data was analyzed by unpaired t-test. A level of P < 0.05 was considered to be statistically significant. Level of significance was noted and interpreted accordingly.
Results and Discussion
Anti-inflammatory activity
The effect of test drugs on carrageenan induced paw edema is depicted in Table 1. In Group A statistically significant decrease (P < 0.05) was observed in comparison to control group; while moderate decrease was observed in Group B which was statistically non-significant. The difference in suppression observed in Group A was found to be significant in comparison to Group B.
Table 1.
Effect of test drug on carrageenan induced hind paw edema in albino rats
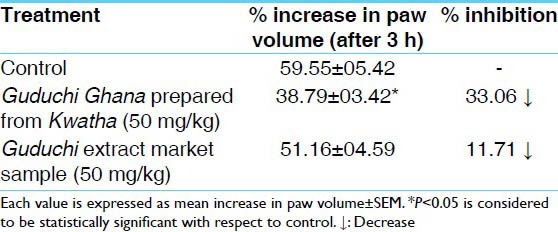
Considering the preliminary nature of the study and also fundamentally inflammatory process is the same in the majority of the tissue, the test drugs were evaluated against Carrageenan induced paw edema to determine whether they have anti-inflammatory activity, especially against acute inflammation or not. Carrageenan induced paw edema is considered to represent the first phase of the inflammatory reaction, which is characterized by fluid and cell exudation. The development of edema in the paw of the rat after injection of carrageenan is a biphasic event. The initial phase of the edema has been attributed to the release of histamine and serotonin, the edema maintained during the plateau phase to kinin like substances and the second accelerating phase of swelling to the release of prostaglandin like substances.[20,21,22] Among the two test formulations, Guduchi Ghana prepared by classical method produced significant suppression of carrageenan-induced edema indicating that it inhibits fluid exudation and thus acute inflammation. Market sample failed to suppress the carrageenan induced paw edema to significant extent in comparison to control group, while Guduchi Ghana prepared by classically produced a considerable suppression in edema formation. Inhibition of edema observed in inflammatory models in the present study, may be attributed to the ability of Guduchi Ghana to modify the role of various chemical mediators of inflammation like histamine and 5 HT during the initial phase of inflammation dry up through attenuation of their formation or through activity at the receptor levels.
Conclusion
It was observed that classically prepared Guduchi Ghana produced significant anti-inflammatory activity. Though market sample of Guduchi Ghana exhibited similar activity but the magnitude of the effect was much less. Classical method has been found much better in comparison to the market sample.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.X. Sp-W. New Delhi: Publication and Information Directorate, CSIR; 1989. The Wealth of India: Raw Materials; p. 251. [Google Scholar]
- 2.Geetha KA, Josphin M, Maiti S. Gender instability in Tinospora cordifolia: An immunomodulator. Current Science. 2007;92:591. [Google Scholar]
- 3.Bhava Mishra. Varanasi: Chaukhambha Bharti Academy; 2008. Bhava Prakasha Nighantu, Part-1, Guduchyadi Varga, Commentary by Chunekar KC; p. 228. [Google Scholar]
- 4.Rai M, Gupta SS. The deposition of the secondary saltsover the five pellets in rats was inhibited by the aqueous extract of T. cordifolia. J Res Indian Med. 1966;10:113–6. [Google Scholar]
- 5.Pendse VK, Dadhich AP, Mathur PN, Bal MS, Madam BR. Anti-inflammatory, immunosuppressive and some related pharmacological actions of the water extract of Neem Giloe (Tinospora cordifolia): A preliminary report. Indian J Pharm. 1977;9:221–4. [Google Scholar]
- 6.Thatte UM, Dahanukar SA. Immunotherapeutic modification of experimental infections by Indian medicinal plants. Phytother Res. 1989;3:43–9. [Google Scholar]
- 7.Nayampalli SS, Desai NK, Ainapure SS. Anti-allergic properties of Tinospora cardifolia in animal models. Indian J Pharm. 1986;18:250. [Google Scholar]
- 8.Wadood N, Wadood A, Shah SA. Effect of Tinospora cordifolia on blood glucose and total lipid levels of normal and alloxandiabetic rabbits. Planta Med. 1992;58:131–6. doi: 10.1055/s-2006-961414. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous. 1st ed. Part I. Vol. 1. NewDelhi: Department of AYUSH, Ministry of Health and FW; 2001. The Ayurvedic Pharmacopoeia of India; pp. 53–4. [Google Scholar]
- 10.Mohan H. 5th ed. Vol. 6. New Delhi: Jaypee Brothers Medical Publisher (P) Ltd; 2010. Textbook of Pathology; p. 133. [Google Scholar]
- 11.Vaidya Yadavji Trikamji Acharya, Siddha Yoga Samgraha, Jwaradhikara 1. Varanasi: Shri Baidhnath Ayurveda Bhavan; 2006. p. 4. [Google Scholar]
- 12.Sharma AK, Singh RH. Screening of anti inflammatory activity of certain indigenous drugs on carrageenin induced hind paw edema in rats. Bull Medico Ethnobot res. 1980;1:93. [Google Scholar]
- 13.Wesley JJ, Christina AJ, Chidambaranathan N. Effect of alcoholic extract of Tinospora Cordifolia on acute and subacute inflammation. Pharmacologyonline. 2008;3:683–7. [Google Scholar]
- 14.Jana U, Chattopadhyay RN, Shw BP. Preliminary studies on anti-inflammatory activity of Zingiber officinale Rosc., Vitex negundo Linn and Tinosporacordifolia (Willid) Miers in albino rats. Indian J Pharm. 1999;31:232–3. [Google Scholar]
- 15.Gulati OD, Pandey DC. Anti-inflammatory activity of Tinospora cordifolia. Rheum. 1982;17:76–83. [Google Scholar]
- 16.Gulati OD. Clinical trial of Tinospora cordifolia in rheumatoid arthritis. Rheum. 1980;15:143–8. [Google Scholar]
- 17.Thatte U, Chabria S, Karandikar SM, Dahanukar S. Immunotherapeutics modification of E. coli abdominal sepsis and mortality in mice by Indian medicinal plants. Indian drugs. 1987;25:95. [Google Scholar]
- 18.Paget GE, Barnes JM. Evaluation of Drug Activities and Pharmacometrics. In: Laurence DR, Bacharach AL, editors. Vol. 1. Academic Press: London NY; 1964. pp. 135–66. Toxicity tests. [Google Scholar]
- 19.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 20.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 21.Di Rosa M, Willoughby DA. Screens for anti-inflammatory drugs. J Pharm Pharmacol. 1971;23:297–8. doi: 10.1111/j.2042-7158.1971.tb08661.x. [DOI] [PubMed] [Google Scholar]
- 22.Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]


