Abstract
Background:
Tuberculosis is a leading cause of death in the world. A new alternative for the treatment of tuberculosis is urgently required, due to the emergence of multidrug resistant Mycobacterium tuberculosis.
Aim:
There is currently considerable interest in developing potential drugs from medicinal plants for treating tuberculosis. To evaluate anti-tubercular activity in the leaves of Kingiodendron pinnatum Rox. Hams., Humboldtia brunonis Wall., Indigofera cassioides Rottl.ex DC., Derris scandens Benth. and Ceasalpinia mimosoides Lamk.
Materials and Methods:
Non-polar and polar solvent extracts of leaves of these medicinal legumes were tested against M. tuberculosis H37RV and minimum inhibitory concentrations (MICs) were determined by the agar based proportion assay.
Results:
Phytochemical screening for secondary metabolites revealed the presence of saponins, steroids, anthro-quinones, terpinods, flavonoids and phlabotanins. Crude leaf extracts of these plants have shown MIC value of 50 μg/ml as against the standard drug Isoniazid value of 0.025 μg/ml.
Conclusion:
Results showed that crude extracts of legume leaves screened exhibited potential anti-tubercular activity against M. tuberculosis and further work is required to identify the active molecule of these legumes, to get a novel anti-tubercular drug. This is the maiden finding on anti-tubercular activity of these medicinal legumes.
Keywords: Bioactive compounds, medicinal legumes, secondary metabolites, tuberculosis
Introduction
One of the most serious infectious diseases that are currently being intensified by the existence of resistant strains is tuberculosis (TB), a disease caused by Mycobacterium tuberculosis.[1] Annually, 8 million people become ill with TB and two million people die from the diseases world-wide.[2] India accounts for nearly one-third of the global burden of TB and approximately 2 million people acquire TB every year.[3] In view of this a need for the development of new TB drugs is felt to control the spread of multidrug resistant TB (multi-drug resistance [MDR]-TB).[4] In spite of global research efforts, mechanisms underlying pathogenesis, virulence and persistence of M. tuberculosis infection remain poorly understood. The urgent need to find new drugs to reduce the global burden of TB is much discussed in the present biomedical research.[5] Natural products and their semi synthetic derivatives can play a vital role in curing TB.[6] There are several reasons that justify the need to search for new anti-tubercular drugs, such as shorten the treatment duration, to get efficient treatment for MDR-TB and to eradicate the latent infection.[7,8] Plants have supplied the foundation for traditional medicine for millennia dating back to information inscribed on clay tablets from Mesopotamia in 2600 BC. Today, traditional remedies continue to play an important role in modern medicine with approximately 80% of the world's inhabitants relying on them their primary health-care.[9] The union of traditional knowledge and modern science may provide an innovative and valuable bio prospecting tool for affordable, safe, novel and effective therapies[10] and to determine their potency against M. tuberculosis which will help to develop new drugs against the infection from these local plant extracts.
Traditionally, tribal people of the Western Ghats of Karnataka use these medicinal legumes for curing various diseases such as arthritis, diabetics, fever, cough, skin diseases etc., Among the selected medicinal plants, only Kingiodendron pinnatum Rox. Hams. found to be used in Ayurveda medicinal system as Samparni. In Ayurveda, the oleo gum-resin of K. pinnatum is used in catarrhal conditions of the genitourinary and respiratory tract infection.[11] In view of this, the present investigation was undertaken to know about the potential of K. pinnatum, Humboldtia brunonis Wall., Indigofera cassioides Rottl.ex DC., Derris scandens Benth. and Ceasalpinia mimosoides Lamk. for phytochemical and anti-tubercular properties.
Materials and Methods
Plant collection and authentication
Evergreen forests of the Western Ghats in Hassan district, Karnataka, India were explored to locate the population of K. pinnatum, (Accession no. 0004DK) H. brunonis, (Accession no. 0005DK), I. cassioides, (Accession no. 0006DK), D. scandens (Accession no. 0007DK) and C. mimosoides (Accession no. 0008DK). The leaves of these plants were carefully exercised from the plant, shade dried and placed in polythene bags. Herbaria of these plants are kept in Biodiversity Conservation Laboratory in the Department of Environmental Science, University of Mysore, India.
Plant extraction and preparation of the extracts
The leaves were shade dried for 14 days at room temperature in a clean environment to avoid contamination. A total of 200 g leaves of each plant were powdered in a domestic grinder. Powdered leaves were extracted by percolation (cool maceration) under dark condition for 96 h at room temperature with petroleum ether, chloroform, ethyl acetate, methanol and distilled water (200 mL). All the extracts were filtered and the process was repeated 2 times for exhaustive extraction, to ensure that no metabolites were left in the residues. All the extracts for each set were combined and then concentrated under low pressure to dryness at 30°C using vacuum concentrator.[12] The dried solvent extracts obtained from each plant were air-dried then packed in 1 mL vials with proper labeling for future references. The extracts were kept refrigerated and away from the sunlight (wrapping with aluminum foil prior to further processing. Stock solutions of the extracts were prepared in Di-Methyl Sulfoxide (DMSO) at a concentration of 5 mg/ml and stored at -20°C until use. All the crude extracts of each plant species were tested for anti-tubercular activity.
Phytochemical analysis of extracts
The plant extracts were screened for phytochemicals using standard techniques for the detection of the saponins, tannins, terpenoids, alkaloids, steroids, flavonoids, anthraquinones and phlobatannins.[13,14]
Anti-tubercular activity against M. tuberculosis H37Rv American Type Culture Collection (ATCC 27294) by proportion assay
This assay measures the capability of the test compound to kill (or inhibit) the multiplication of pathogenic M. tuberculosis H37Rv (27294) was procured from the ATCC, USA. The extracts of selected five medicinal legumes were dissolved in DMSO separately to make stocks (5 mg/ml). Serial dilutions from stocks were also made in DMSO. Each test compound 0.1 ml or DMSO (negative control) or Isoniazid* (positive control) were added in to 1.9 ml Middle Brook (MB 7H10) agar medium (Experiment was conducted in Commercial (MB 7H10) medium purchased from Becton Dickinson Company (Difco™) supplemented with Oleic Albumin Dextrose Catalyse. The contents were mixed and allowed to solidify as slants. 3-week old culture of M. tuberculosis H37Rv (from L-J medium slant) was harvested and its suspension (0.1 mg/ml, equivalent to approx. 107 bacilli/ml) was made in normal saline containing 0.05% Tween-80. 10 μl of this suspension (~105 bacilli) was inoculated on to each tube and incubated at 37°C for 4 weeks. The lowest concentration of an extract up to which there was no visible growth of bacilli was its Minimum Inhibitory Concentration (MIC).[15]
Results
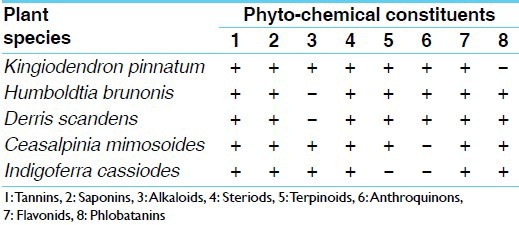
The crude extracts of medicinal legumes were evaluated for anti-tubercular activity containing Isoniazid as a positive control. Table 1 summarizes the anti-tubercular activity (MIC) of crude drugs along with Isoniazid (positive control) obtained from proportion assay. Results indicate that methanol extracts of leaves of all the five legumes, except I. cassioides completely inhibited the growth M. tuberculosis at the concentration of 50 μg/ml. The MIC for plants, which showed anti-tubercular activity was considered as 50 μg/ml [Figures 1 and 2]. Ethyl acetate extract of two legume leaves, K. pinnatum and D. scandens and chloroform extract of three legume leaves of H. brunonis, C. mimosoides and D. scandens showed anti-tubercular activity. However petroleum ether extract of I. cassioides, showed the activity, similarly, only methanol extract of H. brunonis leaves showed the activity. These observations suggest that the component responsible for the anti-tubercular activity of the two species were selectively got extracted in single solvent. However, same activity in all the three extracts (methanol, ethyl acetate and chloroform) of leaves from the two species, C. mimosoides and D. scandens indicate equal distribution of the active components in all the solvent extracts. The results of the phytochemical screening of medicinal legumes are shown in Table 2.
Table 1.
In vitro anti-tubercular activity of medicinal legumes against M. tuberculosis H37Rv
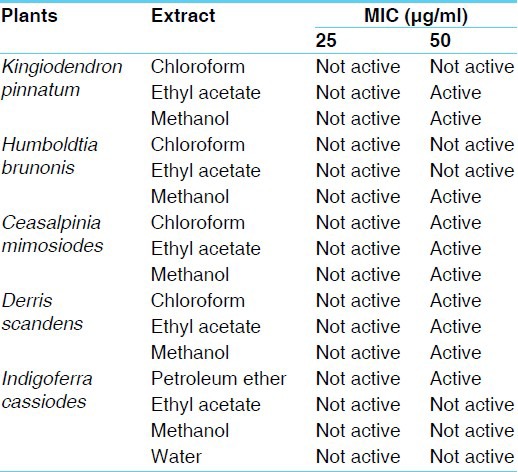
Figure 1.

Growth of Mycobacterium tuberculosis H37Rv on Lowenstein-Jensen midium
Figure 2.
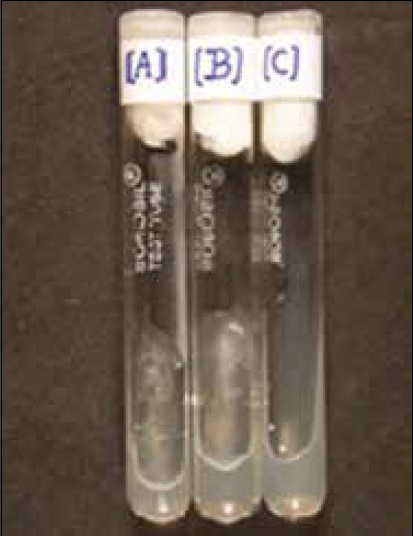
In vitro evaluation of anti-tubercular activity by Proportion assay. Tubes show-(A) Growth of Mycobacterium tuberculosis H37Rv (no drug control) (B) Partial growth (at Concentration < Minimum Inhibitory Concentration (MIC) of drug/compound) (C) Complete growth inhibition (at MIC of drug/compound)
Table 2.
Preliminary phytochemical screening of medicinal legumes
Discussion
TB has been a major health problem in developing countries including India. Due to increase in MDR and Extensive Drug Resistance strains of M. tuberculosis, there is an urgent need of finding newer anti-mycobacterial agents without side-effects to combat this problem. Plants are a valuable source of new anti-mycobacterial compounds. A number of studies have explored a wide range of natural products with strong activity against M. tuberculosis.[16] Ethnobotanical survey was carried out to study the plants used by traditional healers in Mexico to test the activity toward M. tuberculosis. Extracts of Citrus aurantifolia, Citrus sinensis and Olea europaea were found to be active against both drug-susceptible and drug-resistant strains of virulent M. tuberculosis with MICs ranging between 0.1 and 0.025 mg/ml.[17] There is a growing interest in identifying the compounds responsible for anti-mycobacterial activity of traditional medicine and developing them as potential TB drugs.[18] In the present scenario, drug resistance has become important medical crisis in many countries. In order to find a solution for the abatement of TB, the discovery of new anti-tubercular drugs is significantly important.[19] The plant extracts are considered as inactive if they could not prevent the growth of M. tuberculosis up to concentration of 200 μg/ml.[20] The MIC value of 128 μg/ml is defined as active against M. tuberculosis.[21] TB is one of the leading killers of humans world-wide. Its agent, M. tuberculosis causes more death than any other single infectious disease on earth. Search for new anti-TB drugs become obvious due to the shortage and expensive nature of TB drugs. Herbal remedies become the readily alternative in the search for new antimycobacterial compounds from Anogeissu sleiocarpus, Terminalia avicenniodes, Combretum spp. and Combretum. brassii have been reported as remedies for the management of TB.[22] Activity is considered to be significant if MIC values are below 100 μg/ml for crude extract and moderate when 100 < MIC < 625 μg/ml.[23,24] Therefore, the activity recorded from the selected medicinal legumes can be considered as significant. In the Solid Agar based Proportion assay, the Gold Standard’ in Anti-TB Drug Susceptibility testing, exact MIC values are not determined. In this assay, two-fold serial dilutions of the stock of the test sample (drug/test compound/extract) are tested against a defined mycobacterial inoculum and the concentration, which completely inhibits the mycobacterial growth as compared with the drug-free control is considered as the MIC of that sample.[19] In this study, none of the extract showed activity at 25 μg/ml concentration, whereas 10/16 showed activity at 50 μg/ml concentration. The front line anti-TB drugs have MICs in the range of 0.025-2.0 μg/ml and if we wish to compare activity of any new entity (compound/extract) its activity should also be in the same range. However, since extracts are crude preparations they may be considered active at higher concentrations also. In this perspective, activity determination up to 50 μg/ml is quite reasonable. Moreover, a clear discrimination of activity profile among different extracts could also be possible with the tested concentrations.
The phytochemical screening carried out on these medicinal plants showed that it contained major classes of natural products. The activity shown by the crude extracts may be a synergistic action of complex phytochemicals. Investigation of wild medicinal plants is an efficient way of searching for new candidate chemotherapeutic drugs. Plant extracts are attractive and effective sources of new drugs.[25] The use of herbs for the treatment of TB is increasing due to increased incidence of resistance to the available antibiotics. Natural products play a significant role in drug discovery and development of highly active anti-mycobacterial metabolites.[26,27] Our investigation indicate the presence of saponins, steroids, tannins, terpenes, anthraquinone, flavones, phlobatannins and these phytochemicals are responsible for the anti-tubercular activity. Similar observations have been made in plants employed for traditional medicines, which were known to contain the said mentioned bioactive components.[28] Literature survey indicated that these compounds have bioactivity. Anti-mycobacterial herbal medicine where some metabolites such as terpenes, steroids and alkaloids are found to be abundant in the plant extracts and possess potential structural skeletons that could provide useful scaffolds or templates for the development of new anti-mycobacterial drugs has been revived.[29] The bioactivity demonstrated by the extracts is attributable to the presence of secondary metabolites. There are reports about the potent activity of tannins isolated from Combretum molle against M. tuberculosis.[30] Alkaloids isolated from medicinal plant Alstonia scholaris showed the anti-mycobacterial activity.[31] The antibacterial, antifungal and anti-TB activity exhibited by the extracts of the plant Cissampelos owariensis (P. Beauv.) A study proved it is a potential antibiotic agent and confirms the folklore uses of the plant in medicine.[32] This study is the first report of anti-mycobacterial activity on theses selected medicinal plants collected from the Western Ghats of Karnataka against M. tuberculosis by using agar based proportion assay. The preliminary results presented in this study showed the potential of extracts from these medicinal plants to be used against M. tuberculosis. However, further in-vitro studies are needed to understand the mechanism of action of crude drugs.
Conclusion
Results of the present research work showed that leaf extracts of these medicinal legumes possess anti-tubercular activity and offers the scope for the development of potential anti-tubercular crude drugs. Heat labile phytochemicals present in these legumes may be responsible for the anti-tubercular activity. In view of these findings, it can be concluded that medicinal plants play astonishing role in curing mycobacterial disease; hence, there is a need for multiplication and conservation of these medicinal legumes.
Acknowledgment
The authors are thankful to Institute of Excellence, University of Mysore, for the financial support of this project references no UOM.DV2/30/PDF/PA.IOE.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Donald PR, van Helden PD. The global burden of tuberculosis - Combating drug resistance in difficult times. N Engl J Med. 2009;360:2393–5. doi: 10.1056/NEJMp0903806. [DOI] [PubMed] [Google Scholar]
- 2.Angela GK. Research advances: Onions battle osteoporosis; New weapon in war on TB; smokers beware: Study shows increased cadmium levels in the brain may cause severe neurological disorders. J Chem Educ. 2005;82:1114–5. [Google Scholar]
- 3.Young F, Critchley J, Unwin N. Diabetes and tuberculosis: A dangerous liaison and no white tiger. Indian J Med Res. 2009;130:1–4. [PubMed] [Google Scholar]
- 4.Tripathi RP, Tewari N, Dwivedi N, Tiwari VK. Fighting tuberculosis: An old disease with new challenges. Med Res Rev. 2005;25:93–131. doi: 10.1002/med.20017. [DOI] [PubMed] [Google Scholar]
- 5.Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, Winkler H, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–7. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 6.Cechinel FV, Yunes RA. Evaluation of antifungal activity of Piper solmsianum C. DC. var. solmsianum (Piperaceae) Quim Nova. 1998;21:99–105. doi: 10.1248/bpb.28.1527. [DOI] [PubMed] [Google Scholar]
- 7.WHO. USA: World Health Organisation and the Stop TB Partnership; 2006. Global Plan to Stop TB. [Google Scholar]
- 8.Dye C, Espinal MA, Watt CJ, Mbiaga C, Williams BG. Worldwide incidence of multidrug-resistant tuberculosis. J Infect Dis. 2002;185:1197–202. doi: 10.1086/339818. [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 10.Patwardhan B. Ethnopharmacology and drug discovery. J Ethnopharmacol. 2005;100:50–2. doi: 10.1016/j.jep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Khare CP. Verlag-Heidelberg, Berlin: Springer Science, LLC Media; 2007. Indian Medicinal Plants: An Illustrated Dictionary; pp. 3–4. [Google Scholar]
- 12.Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem Cent J. 2007;1:13. doi: 10.1186/1752-153X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harborne JB. London, UK: Chapman and Hall; 1984. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; pp. 49–50. [Google Scholar]
- 14.Gibbons S. Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem Rev. 2005;4:63–78. [Google Scholar]
- 15.Camacho-Corona Mdel R, Ramírez-Cabrera MA, Santiago OG, Garza-González E, Palacios Ide P, Luna-Herrera J. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother Res. 2008;22:82–5. doi: 10.1002/ptr.2269. [DOI] [PubMed] [Google Scholar]
- 16.Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol. 2007;110:200–34. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Earl EA, Altaf M, Murikoli RV, Swift S, O’Toole R. Native New Zealand plants with inhibitory activity towards Mycobacterium tuberculosis. BMC Complement Altern Med. 2010;10:25. doi: 10.1186/1472-6882-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trease GE, Evans WC. 11th ed. London: Brailliar Tiride Land Mac Millian Publishers; 1989. The Pharmacognosy; pp. 710–84. [Google Scholar]
- 19.McClachy JK. Susceptibilty testing in mycobacteria. Lab Med. 1978;9:47–52. [Google Scholar]
- 20.osun F, Kizilay CA, Sener B, Vural M, Palittapongarnpim P. Antimycobacterial screening of some Turkish plants. J Ethnopharmacol. 2004;95:273–5. doi: 10.1016/j.jep.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Gu JQ, Wang Y, Franzblau SG, Montenegro G, Yang D, Timmermann BN. Antitubercular constituents of Valeriana laxiflora. Planta Med. 2004;70:509–14. doi: 10.1055/s-2004-827149. [DOI] [PubMed] [Google Scholar]
- 22.Mann A, Amupitan JO, Oyewale AO, Okoun JI, Ibrahim K. An ethnobotanical survey of indigeneous flora for treating tuberculosis and other respiratory diseases in Niger State. Nigeria J Phytomed Therap. 2007;12:1–12. [Google Scholar]
- 23.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010;76:1479–91. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 24.Kuete V, Efferth T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front Pharmacol. 2010;1:123. doi: 10.3389/fphar.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celio TH, Fernando R, Clarice QF, Miriam S, Wagner V, Sergio RA, et al. Triterpenes and antitubercular activity of Byrsonima crassa. QuíNova. 2008;7:1719–21. [Google Scholar]
- 26.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 27.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990-2000. Bull World Health Organ. 1994;72:213–20. [PMC free article] [PubMed] [Google Scholar]
- 28.Otshudi AL, Foriers A, Vercruysse A, Van Zeebroeck A, Lauwers S. In vitro antimicrobial activity of six medicinal plants traditionally used for the treatment of dysentery and diarrhoea in Democratic Republic of Congo (DRC) Phytomedicine. 2000;7:167–72. doi: 10.1016/s0944-7113(00)80090-2. [DOI] [PubMed] [Google Scholar]
- 29.Copp BR. Antimycobacterial natural products. Nat Prod Rep. 2003;20:535–57. doi: 10.1039/b212154a. [DOI] [PubMed] [Google Scholar]
- 30.Asres K, Bucar F, Edelsbrunner S, Kartnig T, Höger G, Thiel W. Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phytother Res. 2001;15:323–6. doi: 10.1002/ptr.724. [DOI] [PubMed] [Google Scholar]
- 31.Macabeo AP, Krohn K, Gehle D, Read RW, Brophy JJ, Cordell GA, et al. Indole alkaloids from the leaves of Philippine Alstonia scholaris. Phytochemistry. 2005;66:1158–62. doi: 10.1016/j.phytochem.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Akande R, Okwute SK, Iliya I, Efiom O. Chemical constituents and anti-tuberculosis activity of the root extracts of Cissampelos owariensis (P. Beauv.) Menispermaceae. Afr J Pure Appl Chem. 2013;7:21–30. [Google Scholar]


