Abstract
Streptococcus pneumoniae infection is a common and serious health problem that is best prevented by the pneumococcal vaccine. The first vaccine approved by the U.S. Federal Drug Administration in 1977 contained 14 polysaccharide antigens. An improved vaccine introduced in 1983 included 23 polysaccharide antigens. Both vaccines were effective for immunocompetent adults; however, young children and immunocompromised adults remained susceptible. A pediatric vaccine was developed consisting of the capsular antigens of seven pneumococcal serotypes commonly found in children. The antigens in this preparation are covalently conjugated to diphtheria protein to make them more antigenic. The conjugate vaccine was expanded to include 13 serotypes by 2010. Although more immunogenic, the conjugate vaccine has fewer serotypes than the older 23-valent vaccine. The U.S. Centers for Disease Control and Prevention recommend that children at risk for pneumococcal pneumonia as defined by the presence of chronic disease should receive the 13-valent conjugated vaccine. Adults at risk for pneumococcal pneumonia, which includes those over 65 years of age and those who have a chronic disease, should receive the 23-polysaccharide vaccine. Immunosuppressed patients of any age should receive both vaccines. Adults should be revaccinated once at age 65 years or older with the 23-polysaccharide vaccine provided that at least 5 years have elapsed since the previous vaccination.
Keywords: pneumococcal vaccine, PCV13, PPV23, vaccination, adults
Pneumonia is the most common serious respiratory infection in the world, affecting 450 million people annually. It is the leading cause of death in the developing world and in children less than 5 years of age (1, 2). It frequently complicates many diseases and presents an enormous economic burden to society (3).
Streptococcus pneumoniae is the most common bacterial cause of pneumonia (4). It is commonly found on many mucosal surfaces. Colonization ordinarily causes no adverse health effect; however, the organism may invade almost any organ and cause serious disease. Invasive pneumococcal infections have a case–fatality rate of at least 10%, and this is much higher among the elderly and immunocompromised (5).
Although pneumococcal vaccination is the best available protection against invasive pneumococcal disease, many do not receive the vaccine. Although the U.S. Department of Health and Human Services set a target rate of 90% for pneumococcal vaccination of noninstitutionalized high-risk adults (age, 18–65 yr), only 32% of eligible adults received the vaccine in 2008 (6).
The most common reasons for failure of the adult immunization program include a lack of knowledge on the part of patients and physicians, concerns about side effects, needle phobia, and concerns regarding effectiveness. To overcome these barriers, the awareness of physicians of new recommendations is especially critical (7, 8).
The objectives of this review are to discuss the importance of pneumococcal infection, discuss currently available pneumococcal vaccines, and describe the Centers for Disease Control and Prevention (CDC) recommendation for individuals at high risk for invasive pneumococcal disease.
Streptococcus pneumoniae
S. pneumoniae, a bacterium discovered in the late nineteenth century, is an extracellular pathogen that resists phagocytosis in the absence of antibody. Experiments performed on the organism by the German scientist Fred Neufeld in the early twentieth century helped establish the concept of humoral immunity. Neufeld observed that serum from immunized laboratory animals caused microscopic capsular swelling and macroscopic agglutination of some pneumococcal organisms but not others. This capsular swelling effect, which became known as the Quellung reaction (from the German word for swelling), enabled the differentiation of specific capsular serotypes of Streptococcus pneumoniae.
In addition to being strongly antigenic, pneumococci are pathogenic in humans. Over the years, 91 different serotypes of S. pneumoniae have been discovered. About 23 serotypes are responsible for 80–90% of invasive infections (9). Certain serotypes are associated with disease in specific organs. For instance, serotypes 1 and 3 are more often isolated from patients with pneumonia, and serotypes 6, 10, and 23 are often isolated from patients with meningitis (10). Serotypes of invasive pneumococcal diseases also vary in different geographical locations, although serotype 14 is the most common isolate worldwide.
Pneumococcal disease can be classified as invasive or noninvasive. Invasive pneumococcal disease is associated with invasive strains and/or host defense factors and is defined by the involvement of a major organ or the presence of a bloodstream infection. An invasive infection is identified when S. pneumoniae is isolated from normally sterile biofluids such as blood, cerebrospinal fluid, pleural fluid, or peritoneal fluid. Less threatening noninvasive pneumococcal disease presents most commonly as bronchitis, otitis media, or sinusitis (11, 12).
S. pneumoniae has multiple virulence factors including a polysaccharide capsule and various surface proteins. Pneumococcal surface proteins vary between noninvasive and invasive strains. Approximately 96% of strains associated with invasive pneumococcal disease carry pneumococcal surface protein A (PspA) family 1 or family 2 (13).
In addition to pneumococcal virulence factors, the host microbiome may play a role in determining S. pneumoniae carriage and invasiveness. Pneumococcal infection starts with adherence of bacteria to the nasopharyngeal epithelium. Licciardi and colleagues proposed that commensal bacteria interact with underlying dendritic cells and trigger a cascade of immunologic pathways in T and B cells to protect against S. pneumoniae carriage (14). Interestingly, the nasopharyngeal colonization of noninvasive S. pneumoniae induces a local helper T-cell type 17 adaptive response that prevents subsequent colonization by invasive strains (15).
The invasion mechanism of S. pneumoniae infection is not well understood (16). It is proposed that the bacterium enters epithelial and endothelial cells using pneumococcal adherence and virulence factor A (PavA) and pneumococcal surface protein C (PspC) (17, 18). It has also been shown that some epithelial receptors such as laminin are crucial to initiate bacterial attachment to endothelial cells (19). Clathrin, a protein that facilities endocytosis in mammalian cells (20), helps S. pneumoniae to reach into cells via clathrin-mediated endocytosis (16). These proteins and receptors determine whether colonization in nasopharynx escalates to an invasive disease.
Pneumococcal Vaccines
The identification of highly antigenic pneumococcal capsular antigens fostered intense research on vaccine development in the early twentieth century. Exploiting the immunogenicity of the organisms’ polysaccharide capsule garnered modest early successes (21). Vaccination was instrumental in reducing an outbreak of pneumonia at a Massachusetts state hospital in 1938 (22) and in preventing pneumococcal pneumonia among soldiers in the Second World War (23). The latter study confirmed the importance of the protective effect of the various polysaccharide serotypes—the vaccine did not protect against pneumonia caused by serotypes not included in the vaccine.
With the advent of effective antibiotics, research on pneumococcal vaccines decreased. The development of resistance to penicillin and better vaccine technology caused interest to again resurge. The first vaccine, which included antibodies to 14 capsular antigenic serotypes, pneumococcal polysaccharide vaccine 14 (PPV14), was approved for use in the United States in 1977. An improved pneumococcal polysaccharide vaccine was expanded to include 23 serotypes (PPV23) in 1983. The vaccine was effective in adults, but did not consistently generate immunity in children less than 2 years of age.
In 2000, a pneumococcal conjugate vaccine (PCV7) was developed for children. Using seven of the most common S. pneumoniae cell membrane polysaccharide antigens that caused invasive pneumococcal disease in children, the vaccine was developed by covalently conjugating these antigens with diphtheria proteins to increase immunogenicity (24). The conjugate vaccine was expanded to include 13 serotypes (PCV13) and was licensed for use in the United States in 2010. This vaccine exists in various forms and became available for use at various times in other countries.
The conjugate vaccines have substantially reduced the rate of asymptomatic carriage and invasive pneumococcal disease. Use of conjugate vaccines has also promoted “herd immunity” that extends to unvaccinated persons (25).
Duration of Protection
Protective type-specific antibody levels of PPV23 appear within 2 to 3 weeks of vaccination and decline to the prevaccination level about 4 to 7 years later (26, 27). The clinical relevance of this decline has not been established. Immunocompromised patients such as those who are infected with HIV, recipients of a renal transplant, and those with systemic lupus erythematosus have a lower antibody response after vaccination with polysaccharide pneumococcal vaccine, and antibody levels return to baseline within a shorter time interval of approximately 3 years (28–31).
Adverse Effects
Pneumococcal vaccination is generally safe and well tolerated (32). Some individuals may experience a local adverse reaction at the injection site including mild pain, redness, and itching, particularly after revaccination. Severe local reactions occur in 2.5–15% of subjects (32). Systemic adverse reaction including mild fever, body aches, and chills occurs in 22–35% of subjects. Those side effects are manageable with reassurance and short-term analgesics (32–34).
Current Status of the Vaccines
Although effective in relatively healthy individuals, many studies have shown that PPV23 is ineffective in reducing invasive pneumococcal disease in elderly (35) and immunocompromised patients (36), including transplant patients (37) and HIV-infected patients with low CD4+ cell counts (38).
Although designed for children, the serotypes found in PCV13 were responsible for half of invasive pneumococcal diseases in immunocompromised adults in 2010 (39). In the same year, less than one-fourth of invasive pneumococcal diseases were caused by serotypes found in PPV23 (40). Accordingly, in December 2011, the U.S. Food and Drug Administration licensed PCV13 for prevention of pneumonia and invasive pneumococcal disease in adults who are 50 years of age and older (40). Although PCV13 covers many serotypes that are prevalent in immunocompromised individuals, it does not cover several serotypes occurring in noncompromised patients (41), which raises a question about which vaccine physicians caring for adult patients should use.
According to latest CDC recommendations, patients with chronic liver disease, cirrhosis, alcoholism, and diabetes are at high risk for pneumococcal disease and require vaccination with PPV23. There is no current CDC recommendation on dual vaccination with PPV23 and PCV13. Further investigation is needed to better understand the most effective vaccine strategy in immunocompromised patients.
Although the cost of PCV13 (Prevnar 13) is $135 and that of PPV23 (Pneumovax 23) is $64 in the United States (1), a cost analysis of PVC13 immunization showed that it is more cost effective than PPV23 in immunocompromised persons (42). Also, using both vaccines was determined to be cost effective in certain circumstances.
Recommendations
23-Valent Nonconjugated Vaccine (PPV23)
The Advisory Committee on Immunization Practices (ACIP) and the CDC recommend PPV23 immunization for all individuals who are at increased risk of invasive pneumococcal disease. This includes persons older than 65 years of age and those with diabetes and other significant chronic diseases (40) (Table 1). Persons should receive a second dose of PPV23 if they received their first dose before the age of 65 years and at least 5 years has elapsed since their first immunization with PPV23.
Table 1.
Recommendations for administration of 23-valent pneumococcal vaccine and 13-valent pneumococcal conjugate vaccine in adults
| Immunologic Status | Condition | PPV23 | PCV13 |
|---|---|---|---|
| Good immunologic responder | Chronic pulmonary diseases, including sarcoidosis | * | |
| Chronic heart disease including congestive heart failure and cardiomyopathies | * | ||
| Chronic liver disease | * | ||
| Tobacco smoking | * | ||
| Alcoholism | * | ||
| Diabetes mellitus | * | ||
| Nephrotic syndrome | * | * | |
| Chronic renal failure | * | * | |
| Poor immunologic responder | CSF leaks | * | * |
| Cochlear implants | * | * | |
| Sickle cell disease and other hemoglobinopathies | * | * | |
| Congenital or acquired asplenia | * | * | |
| Primary immunodeficiencies: Including all types of B- and T-cell lymphocyte deficiencies, complement (C1, C2, C3, C4), and phagocyte disorders, except chronic granulomatous disease | * | * | |
| Iatrogenic immunodeficiencies: Any condition for which patient takes chronic systemic corticosteroids (such as prednisone, 15 mg/d, for more than 6 mo) or immunosuppressant agents such as methotrexate, or radiation therapy | * | * | |
| HIV infection | * | * | |
| Hematologic malignancies | * | * | |
| Generalized malignancy | * | * | |
| Solid organ transplant | * | * |
Definition of abbreviations: PCV13 = 13-valent pneumococcal conjugate vaccine; PPV23 = 23-valent pneumococcal polysaccharide vaccine.
Note: Adapted from Reference 40.
Pneumococcal Conjugate Vaccine (PCV13)
The ACIP recommends PCV13 for children with chronic medical conditions regardless of whether they may have previously received PCV7 or PPV23. The committee also recommends PCV13 vaccination for immunocompromised adults (39) (Tables 1 and 2), including individuals with cerebrospinal fluid leak, cochlear implant, sickle cell disease or other hemoglobinopathies, congenital immunodeficiency, HIV, immunosuppressive therapy including long-term corticosteroids and radiation therapy, advanced chronic renal disease, solid organ transplantation, and active cancer.
Table 2.
Recommendations for administration of 23-valent pneumococcal vaccine and 13-valent pneumococcal conjugate vaccine in children
| Age | PPV23 | PCV13 |
|---|---|---|
| >2 yr | 4 doses, one dose at 2, 4, 6, 12–15 mo | |
| 2–4 yr | 1 dose if not vaccinated previously | |
| 2–5 yr | 1 or 2 doses if not vaccinated previously and has chronic medical condition including sickle cell disease, a damaged spleen or no spleen, cochlear implant, cerebrospinal fluid leaks, HIV or other diseases that affect the immune system (such as diabetes, cancer, and liver disease), chronic heart or lung disease; children who take medications that affect the immune system (such as chemotherapy or corticosteroids) | |
| 2 yr and above | One dose if patient has chronic medical condition including chronic heart disease, chronic lung disease, sickle cell disease, diabetes, alcoholism, cirrhosis, leakage of cerebrospinal fluid or cochlear implant, immunocompromised condition, and taking immunosuppressant drugs |
Definition of abbreviations: PCV13 = 13-valent pneumococcal conjugate vaccine; PPV23 = 23-valent pneumococcal polysaccharide vaccine.
Note: Adapted from Reference 39.
Both PPV23 and PCV13
The ACIP recommends that both PPV23 and PCV13 be given to persons with sickle cell disease and other hemoglobinopathies, primary immunodeficiency disorders, and persons receiving immunosuppressive therapy. S. pneumoniae is removed by the spleen and is associated with overwhelming disease in persons without a functioning spleen, such as in sickle cell disease. Cochlear implants and cerebral spinal fluid leakage are associated with central nervous system disease. Patients receiving immunosuppressive therapy have a greater than 10-fold risk of developing invasive pneumococcal disease (43).
The rationale for using both PPV23 and PCV13 stems in part from a report that PCV13 covered 84% of the serotypes and PPV23 covered 92% of serotypes of invasive pneumococcal disease (44).
Pneumococcal Revaccination
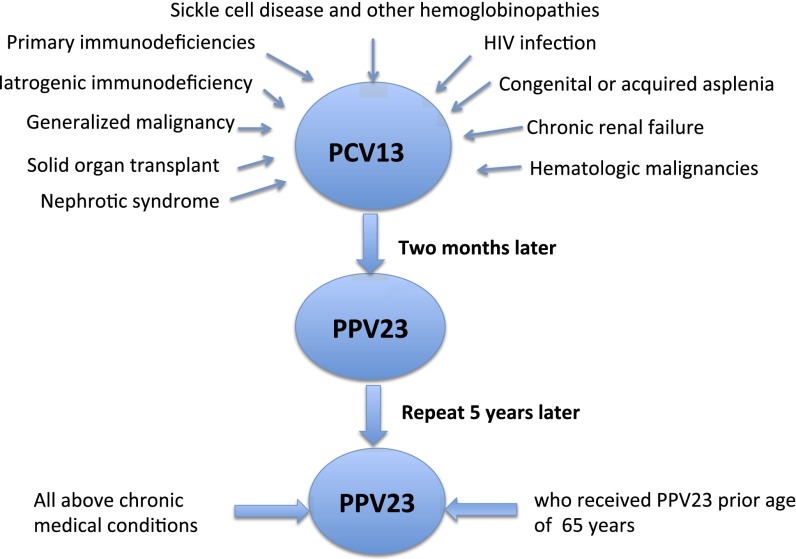
As discussed earlier, the type-specific antibody levels after pneumococcal vaccination decline over time. Therefore, revaccination helps boost antibody levels and maintain protection. The ACIP recommends one repeat PPV23 vaccination for all individuals at age 65 years provided that at least 5 years has passed from the previous PPV23 vaccination. The ACIP does not currently recommend more than one revaccination, due to lack of sufficient data on safety, immunogenicity, and cost effectiveness (45) (Figure 1).
Figure 1.
Diagram showing indications for PCV13 and PPV23 revaccination. PCV13 = 13-valent pneumococcal conjugate vaccine; PPV23 = 23-valent pneumococcal polysaccharide vaccine.
Influenza and Pneumococcal Dual Vaccination
Influenza and pneumococcal disease are associated with considerable mortality each year. The two vaccines can be given at the same time, ideally at the recommended time of year for influenza vaccination. It has been shown that dual vaccination with influenza and pneumococcal vaccines significantly decreases mortality in elderly people (46, 47).
Vaccination Protocol
Persons receiving the pneumococcal vaccine should be in their best achievable immunogenic state. Accordingly, the vaccines should be given before immunosuppressive therapy whenever possible.
Immunosuppressed persons who have never received a pneumococcal vaccine should receive a single dose of PCV13 first, followed by a dose of PPV23 at least 8 weeks later. A second dose of PPV23 is recommended for patients 5 years after the first PPV23 vaccine (Tables 1 and 2).
Patients who previously received PPV23 and are immunosuppressed should receive a single dose of PCV13 at least 1 year after the last dose of PPV23.
The efficacy of PPV23, PCV13, and dual vaccination among immunocompromised individuals should be further investigated. The pneumococcal antibody levels in immunocompromised individuals are reported as persistently lower than with normal immunity. Therefore, repeat vaccination with conjugated vaccine may be a consideration. The role of cell-mediated immunity after conjugated vaccine needs further study.
Footnotes
CME will be available for this article at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization Pneumococcal vaccines Geneva, Switzerland: World Health Organization; 2013. Available from: http://www.who.int/biologicals/areas/vaccines/pneumo/en/ [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Yu H, Rubin J, Dunning S, Li S, Sato R. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J Am Geriatr Soc. 2012;60:2137–2143. doi: 10.1111/j.1532-5415.2012.04208.x. [DOI] [PubMed] [Google Scholar]
- 4.Aliberti S, Kaye KS. The changing microbiologic epidemiology of community-acquired pneumonia. Postgrad Med. 2013;125:31–42. doi: 10.3810/pgm.2013.11.2710. [DOI] [PubMed] [Google Scholar]
- 5.Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, et al. Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human ServicesHealthy people 2010[CDC website]. Available from: http://wonder.cdc.gov/data2010/
- 7.High KP. Overcoming barriers to adult immunization. J Am Osteopath Assoc. 2009;109(6, Suppl 2):S25–S28. [PubMed] [Google Scholar]
- 8.Appel A. Improving adult immunization rates: overcoming barriers. Am Fam Physician. 2011;84:977–978. [PubMed] [Google Scholar]
- 9.Fedson DS, Nicolas-Spony L, Klemets P, van der Linden M, Marques A, Salleras L, Samson SI. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines. 2011;10:1143–1167. doi: 10.1586/erv.11.99. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000;30:122–140. doi: 10.1086/313609. [DOI] [PubMed] [Google Scholar]
- 11.Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias LA, Phan HM, Solomon E. Bacteremic and nonbacteremic pneumococcal pneumonia: a prospective study. Medicine (Baltimore) 2000;79:210–221. doi: 10.1097/00005792-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Plevneshi A, Svoboda T, Armstrong I, Tyrrell GJ, Miranda A, Green K, Low D, McGeer A Toronto Invasive Bacterial Diseases Network. Population-based surveillance for invasive pneumococcal disease in homeless adults in Toronto. PLoS One. 2009;4:e7255. doi: 10.1371/journal.pone.0007255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croney CM, Coats MT, Nahm MH, Briles DE, Crain MJ. PspA family distribution, unlike capsular serotype, remains unaltered following introduction of the heptavalent pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2012;19:891–896. doi: 10.1128/CVI.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licciardi PV, Toh ZQ, Dunne E, Wong SS, Mulholland EK, Tang M, Robins-Browne RM, Satzke C. Protecting against pneumococcal disease: critical interactions between probiotics and the airway microbiome. PLoS Pathog. 2012;8:e1002652. doi: 10.1371/journal.ppat.1002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JM, Khandavilli S, Camberlein E, Hyams C, Baxendale HE, Brown JS. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS One. 2011;6:e25558. doi: 10.1371/journal.pone.0025558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradstedt H, Iovino F, Bijlsma JJ. Streptococcus pneumoniae invades endothelial host cells via multiple pathways and is killed in a lysosome dependent manner. PLoS One. 2013;8:e65626. doi: 10.1371/journal.pone.0065626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmann S, Hammerschmidt S. Versatility of pneumococcal surface proteins. Microbiology. 2006;152:295–303. doi: 10.1099/mic.0.28610-0. [DOI] [PubMed] [Google Scholar]
- 18.Pracht D, Elm C, Gerber J, Bergmann S, Rohde M, Seiler M, Kim KS, Jenkinson HF, Nau R, Hammerschmidt S. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect Immun. 2005;73:2680–2689. doi: 10.1128/IAI.73.5.2680-2689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, Oldfield NJ, Self T, Ala’Aldeen DA, Tuomanen EI. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royle SJ. The cellular functions of clathrin. Cell Mol Life Sci. 2006;63:1823–1832. doi: 10.1007/s00018-005-5587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austrian R. A brief history of pneumococcal vaccines. Drugs Aging. 1999;15:1–10. doi: 10.2165/00002512-199915001-00001. [DOI] [PubMed] [Google Scholar]
- 22.Smillie WG, Warnock GH, White HJ. A study of a type I Pneumococcus epidemic at the State Hospital of Worcester, Mass. Am J Public Health Nations Health. 1938;28:293–302. doi: 10.2105/ajph.28.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macleod CMH, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82:445–465. [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258–261. [PubMed] [Google Scholar]
- 25.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein–polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 26.Sankilampi U, Honkanen PO, Bloigu A, Leinonen M. Persistence of antibodies to pneumococcal capsular polysaccharide vaccine in the elderly. J Infect Dis. 1997;176:1100–1104. doi: 10.1086/516521. [DOI] [PubMed] [Google Scholar]
- 27.Konradsen HB. Quantity and avidity of pneumococcal antibodies before and up to five years after pneumococcal vaccination of elderly persons. Clin Infect Dis. 1995;21:616–620. doi: 10.1093/clinids/21.3.616. [DOI] [PubMed] [Google Scholar]
- 28.McDonald E, Jarrett MP, Schiffman G, Grayzel AI. Persistence of pneumococcal antibodies after immunization in patients with systemic lupus erythematosus. J Rheumatol. 1984;11:306–308. [PubMed] [Google Scholar]
- 29.Kumar D, Welsh B, Siegal D, Chen MH, Humar A. Immunogenicity of pneumococcal vaccine in renal transplant recipients—three year follow-up of a randomized trial. Am J Transplant. 2007;7:633–638. doi: 10.1111/j.1600-6143.2007.01668.x. [DOI] [PubMed] [Google Scholar]
- 30.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Antibodies against pneumococcal polysaccharides after vaccination in HIV-infected individuals: 5-year follow-up of antibody concentrations. Vaccine. 1999;18:524–530. doi: 10.1016/s0264-410x(99)00240-6. [DOI] [PubMed] [Google Scholar]
- 31.Chen M, Hisatomi Y, Furumoto A, Kawakami K, Masaki H, Nagatake T, Sueyasu Y, Iwanaga T, Aizawa H, Oishi K. Comparative immune responses of patients with chronic pulmonary diseases during the 2-year period after pneumococcal vaccination. Clin Vaccine Immunol. 2007;14:139–145. doi: 10.1128/CVI.00336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–524. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 33.Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, Lewis LS, Carlone G, DeStefano F, Holder P, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA. 1999;281:243–248. doi: 10.1001/jama.281.3.243. [DOI] [PubMed] [Google Scholar]
- 34.Grabenstein JD, Manoff SB. Pneumococcal polysaccharide 23-valent vaccine: long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adults. Vaccine. 2012;30:4435–4444. doi: 10.1016/j.vaccine.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 35.Jackson LA, Janoff EN. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis. 2008;47:1328–1338. doi: 10.1086/592691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnstone J, Eurich DT, Minhas JK, Marrie TJ, Majumdar SR. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis. 2010;51:15–22. doi: 10.1086/653114. [DOI] [PubMed] [Google Scholar]
- 37.Kumar D, Chen MH, Welsh B, Siegal D, Cobos I, Messner HA, Lipton J, Humar A. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis. 2007;45:1576–1582. doi: 10.1086/523583. [DOI] [PubMed] [Google Scholar]
- 38.Hung CC, Chang SY, Su CT, Chen YY, Chang SF, Yang CY, Liu WC, Wu CH, Chang SC. A 5-year longitudinal follow-up study of serological responses to 23-valent pneumococcal polysaccharide vaccination among patients with HIV infection who received highly active antiretroviral therapy. HIV Med. 2010;11:54–63. doi: 10.1111/j.1468-1293.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6–18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2013;62:521–524. [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–819. [PubMed] [Google Scholar]
- 41.Luján M, Burgos J, Gallego M, Falcó V, Bermudo G, Planes A, Fontanals D, Peghin M, Monsó E, Rello J. Effects of immunocompromise and comorbidities on pneumococcal serotypes causing invasive respiratory infection in adults: implications for vaccine strategies. Clin Infect Dis. 2013;57:1722–1730. doi: 10.1093/cid/cit640. [DOI] [PubMed] [Google Scholar]
- 42.Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31:3950–3956. doi: 10.1016/j.vaccine.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong A, Marrie TJ, Garg S, Kellner JD, Tyrrell GJ, Group S SPAT (Streptococcus pneumoniae Alberta Team) Group. Increased risk of invasive pneumococcal disease in haematological and solid-organ malignancies. Epidemiol Infect. 2010;138:1804–1810. doi: 10.1017/S0950268810000919. [DOI] [PubMed] [Google Scholar]
- 44.Debbache K, Varon E, Hicheri Y, Legrand P, Donay JL, Ribaud P, Cordonnier C. The epidemiology of invasive Streptococcus pneumoniae infections in onco-haematology and haematopoietic stem cell transplant patients in France: are the serotypes covered by the available anti-pneumococcal vaccines? Clin Microbiol Infect. 2009;15:865–868. doi: 10.1111/j.1469-0691.2009.02810.x. [DOI] [PubMed] [Google Scholar]
- 45.Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, Zanis C, Whaley M, Romero-Steiner S, Butler JC, et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55–74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine. 2011;29:2287–2295. doi: 10.1016/j.vaccine.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 46.Mahamat A, Daurès JP, de Wzieres B. Additive preventive effect of influenza and pneumococcal vaccines in the elderly: results of a large cohort study. Hum Vaccin Immunother. 2013;9:128–135. doi: 10.4161/hv.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan TC, Hung IF, Luk JK, Shea YF, Chan FH, Woo PC, Chu LW. Prevention of mortality and pneumonia among nursing home older adults by dual pneumococcal and seasonal influenza vaccination during a pandemic caused by novel pandemic influenza A (H1N1) J Am Med Dir Assoc. 2012;13:698–703. doi: 10.1016/j.jamda.2012.05.009. [DOI] [PubMed] [Google Scholar]