Abstract
Rationale: HIV seropositivity has long been considered a contraindication to lung transplantation, primarily because of the potential risks of added immunosuppression. In the past decade, however, experience with kidney and liver transplantation in the setting of HIV infection, with achievement of satisfactory outcomes, has grown considerably. This promising development has created a need to reconsider this contraindication to lung transplantation.
Objectives: There is presently limited evidence upon which to base medical decision-making regarding lung transplantation in individuals with HIV infection. In our present study, we wished to extend the existing literature by reporting the outcomes of three individuals with HIV infection who underwent lung transplantation at two centers.
Methods: We compiled data for a case series of three HIV-infected subjects undergoing lung transplantation at two centers.
Measurements and Main Results: We reviewed medical records to investigate the effects of lung transplantation on the course of HIV infection, the development of HIV-related opportunistic infections or malignancies, the occurrence of lung transplant and HIV drug interactions, and the extent of acute rejection. Subject 1, who underwent transplantation for HIV-associated pulmonary arterial hypertension, experienced recalcitrant acute rejection requiring a lymphocyte-depleting agent with subsequent rapid development of bronchiolitis obliterans syndrome. Subjects 2 and 3, who underwent transplantation for idiopathic pulmonary fibrosis, experienced mild acute rejection but remain free from chronic rejection at 4 and 2 years after transplant, respectively.
Conclusions: Lung transplantation may be feasible for carefully selected patients in the setting of controlled HIV infection. On the basis of our experience with three patients, we caution that acute graft rejection may be more common in such patients.
Keywords: acute rejection, human immunodeficiency virus, idiopathic pulmonary fibrosis, lung transplantation, pulmonary arterial hypertension
With current therapeutics, HIV infection has evolved to become a chronic disease with an extended life expectancy, albeit marked by multiorgan complications that are unrelated to acute opportunistic infection (1–3). The burden of nonmalignant lung disease in HIV-infected individuals is growing. HIV infection is an independent risk factor for chronic obstructive pulmonary disease, lung cancer, asthma, interstitial lung disease, and, saliently, pulmonary arterial hypertension (4–7). The latter has an estimated prevalence of 0.5% among certain groups of persons with HIV infection (8). As a result, experience with solid organ transplantation involving the kidney, liver, and the heart among individuals with HIV is growing (9–13). To date, however, the literature contains only one published report of lung transplantation in a recipient infected with HIV, which was performed for cystic fibrosis. At the time the case was published, the recipient had experienced an excellent outcome at 2 years after receiving the lung transplant (14). Nonetheless, HIV seropositivity remains a contraindication to lung transplantation (15). Reluctance to provide lung transplantation as a treatment option for persons infected with HIV is driven by concerns that iatrogenic immunosuppression may increase the risk of infection and HIV reactivation. There is also trepidation that combining antiretroviral therapy with immunosuppressive medications may result in unmanageable drug–drug interactions. In addition, some have raised ethical concerns regarding the allocation of a scarce resource to persons with HIV infection (16).
To address some existing knowledge gaps, we report outcomes in three subjects with HIV infection who underwent lung transplantation. We aimed to address four main questions. (1) Does lung transplantation affect the course of HIV infection? (2) Are lung transplant recipients at increased risk for HIV-related opportunistic infections or malignancies? (3) Can drug–drug interactions between immunosuppressive and antiretroviral medications be managed? (4) Are lung transplant recipients with HIV infection at increased risk for acute rejection, consistent with the experience in liver and kidney transplant recipients with HIV infection (9–12)? Information gathered from this series has been reported previously in abstract form (17–19).
Methods
We compiled data from medical records of all persons with HIV infection undergoing lung transplantation at the UCSF Medical Center and Houston Methodist Hospital. Of 1,394 lung transplantations performed, 3 (0.2%) were in individuals with HIV infection. To determine whether lung transplantation affects the course of HIV, we evaluated cluster of differentiation 4 (CD4) counts and HIV viral loads before and after lung transplantation. We defined HIV-related opportunistic infections or malignancies as those unique to patients with HIV infection and not commonly observed in other lung transplant recipients (see online supplement). Drug–drug interactions were defined as drug interactions requiring a change or a significant dose adjustment of standard immunosuppressive, antimicrobial prophylactic, or antiretroviral drug regimens. Acute rejection was defined according to International Society for Heart and Lung Transplantation (ISHLT) Lung Rejection Study Group guidelines (20). The Committee for Human Research waived review of this study because it includes three or fewer subjects in a case series.
Results
Subject 1
Subject 1 was a 40-year-old woman who was diagnosed with HIV infection at age 30 (Table 1). Prior to undergoing transplantation, she had maintained a CD4+ T cell count >1,000 cells/mm3 and experienced no AIDS-defining illness, including opportunistic infections. Her antiretroviral regimen consisted of lamivudine/zidovudine and efavirenz. At age 33, she was diagnosed with HIV-related pulmonary arterial hypertension. Despite receiving therapy with continuous intravenous treprostinil, oral bosentan, and sildenafil, her pulmonary arterial hypertension progressed to New York Heart Association Class III symptoms. Right heart catheterization showed a mean pulmonary artery pressure of 57 mm Hg and a Fick cardiac index of 1.48 L/min/m2.
Table 1.
Baseline demographics and clinical features of three HIV-positive patients prior to undergoing lung transplantation
| Characteristics | Subject 1 | Subject 2 | Subject 3 |
|---|---|---|---|
| Indication for transplant | HIV-associated PAH | IPF | IPF |
| Sex | Female | Male | Male |
| Age at transplant (yr) | 40 | 65 | 60 |
| Race/ethnicity | Caucasian | Caucasian | Caucasian |
| Hepatitis B serologies | +Surface ab, −core ab, −surface ag | +Core ab, +surface ab, −surface ag, −DNA | +Core ab, +surface ab, −surface ag, −DNA |
| Hepatitis C serologies | Negative | +HCV ab, −RNA | Negative |
| CMV | +IgG | +IgG | +IgG |
| EBV | +IgG | Not performed | +IgG |
| Malignancy history | None | Basal cell carcinoma of nose, local excision 2 yr prior | Hodgkin’s lymphoma >5 yr ago, currently in remission |
| HIV viral load | Undetectable | Undetectable | Undetectable |
| CD4 (cells/mm3) | 1,053 | 302 | 407 |
| Pretransplantation opportunistic infection history | None | None | None |
| cART regimen | Lamivudine, zidovudine, efavirenz | Emtricitabine, tenofovir, atazanavir | Lamivudine, abacavir, atazanivir, ritonavir |
| FEV1 (% predicted | 74 | 38 | 54 |
| FVC (% predicted) | 82 | 48 | 50 |
| TLC (% predicted) | 95 | 48 | 66 |
| DLCO (% predicted) | 75 | 23 | 25 |
| 6-MWD (ft) | 964 | 400 | 500 |
| RA pressure (mm Hg) | 9 | 6 | 6 |
| PA pressure (mm Hg) | 81/41 (57) | 40/24 (30) | 46/26 (32) |
| Wedge (mm Hg) | 14 | 14 | 8 |
| Baseline Cr (mg/dL) | 0.66 | 0.7 | 1.1 |
| Peak PRAs | HLA Class I = 3 HLA Cass II = 5 Repeat PRAs were negative in each of the 10 subsequent months they were measured. |
Negative | Negative |
Definition of abbreviations: 6-MWD = 6-minute-walk distance; ab, antibody; ag = antigen; cART = combined antiretroviral therapy; CD4 = cluster of differentiation 4; Cr = serum creatinine; DlCO = diffusing capacity of the lung for carbon monoxide; FEV1 = forced expiratory volume in 1 second; FVC = forced expiratory volume; HCV = hepatitis C virus; IPF = idiopathic pulmonary fibrosis; PA = pulmonary arterial; PAH = pulmonary arterial hypertension; PRAs = panel reactive antibodies; RA = right atrial; TLC = total lung capacity.
In 2009, she underwent bilateral lung transplantation (Table 2). Induction immunosuppressive therapy included basilixumab, intravenous mycophenolate mofetil (1,000 mg), and intravenous methylprednisolone (1,000 mg). The perioperative period was complicated by ISHLT Grade 3 primary graft dysfunction at 24 and 72 hours, which required a tracheostomy and mechanical ventilation for 10 days. She was discharged on room air on postoperative Day 23. Her antiretroviral regimen was unchanged, although the efavirenz dosage was reduced while she was on voriconazole.
Table 2.
Clinical course after lung transplantation
| Subject 1 | Subject 2 | Subject 3 | |
|---|---|---|---|
| Transplantation date | October 2009 | April 2010 | May 2012 |
| Transplant type | Bilateral | Bilateral | Single, right |
| Ischemic time (min), right/left* | 283/329 | 163/206 | 336 |
| Cardiopulmonary bypass (yes/no) | Yes | Yes | No |
| CMV status: donor/recipient | −/+ | +/+ | −/+ |
| Immunosuppression induction | Basilixumab, methylprednisolone 1,000 mg intravenous, MMF 1,000 mg intravenous | Basilixumab, methylprednisolone 500 mg intravenous; MMF 1,000 mg intravenous | Basilixumab, methylprednisolone 500 mg intravenous; MMF 1,000 intravenous |
| PGD at 24 h | 3 | 0 | 0 |
| PGD at 72 h | 3 | 0 | 0 |
| Calcineurin inhibitor | Tacrolimus POD 1, initial trough goal 10–14 ng/dL for 6 mo | Tacrolimus POD 2, initial trough goal 10–15 ng/dL for 6 mo | Tacrolimus POD 1, initial trough goal 10–15 ng/dL for 6 mo |
| Maintenance immunosuppressive therapy | Tacrolimus (trough 8–10 ng/dL), MMF 750 mg twice daily, and prednisone 10 mg daily | Tacrolimus (trough 8–10 ng/dL), MMF 1,000 mg twice daily, and prednisone 10 mg daily | Tacrolimus (trough 8–10 ng/dL),MMF 1,000 mg twice daily, prednisone 10 mg daily |
| Prophylaxis after transplant | Valganciclovir indefinitely, TMP-SMX, inhaled amphotericin, voriconazole 400 mg by mouth twice daily | Valganciclovir for 6 mo, TMP-SMX, inhaled amphotericin, voriconazole 200 twice daily | Valganciclovir for 6 mo, TMP-SMX, itraconazole |
| Explant | Plexogenic arteriopathy with pulmonary atherosclerosis consistent with pulmonary arterial hypertension. | UIP and severe medial hypertrophy of smooth muscles of pulmonary arterioles | UIP and severe medial hypertrophy of smooth muscles of pulmonary arterioles |
| Major perioperative complication | PGD requiring tracheostomy | None | None |
| Airway issues | None | Bilateral anastomotic strictures: covered AERO stent (left) and uncovered Ultraflex stent (right) | None |
| cART regimen | Started POD 5: Lamivudine, zidovudine, efavirenz | Started POD 4; no change; emtricitabine, tenofovir, atazanavir initially; Added raltegravir POD 14 for newly +viral load <20 copies/mL) | Started POD 1; lamivudine, abacavir, atazanavir, ritonavir |
| Serum creatinine 1 yr after transplant | 0.6 mg/dL | 1.1 mg/dL | 1.1 mg/dL |
| Infectious issues | RSV pneumonia 15 mo after transplant requiring admission | Pseudomonas colonization of stents, pseudomonas pneumonia | None |
| CD4 nadir | 183 | 68 | 211 |
| Current CD4 | 400 | 588 | 746 |
| Acute cellular rejection | A2, A2, A1, A2 (4, 9, 10, 11 months posttransplantation, respectively) | A1 (POD 42) | A1 (POD 8) |
| Bronchiolitis obliterans syndrome | ISHLT Grade 3 | ISHLT Grade 0 | ISHLT Grade 0 |
| Recurrence of other disease (hepatitis C, lymphoma) | N/A | No | No |
| Skin cancer | No | No | No |
| CMV, EBV, HBV, HCV, HHV-8 titers | Negative | Negative | Negative |
| Anti-HLA antibodies | Negative | Negative | Negative |
| Other major events | Pulmonary embolus 2 mo postoperatively | Pulmonary vein isolation/ablation for atrial fibrillation/flutter | Massive hemoptysis from posttransplantation surveillance bronchoscopy at 6 mo, admitted and observed |
| Functional status | Severely limited due to respiratory impairment | Excellent, actively employed | Excellent, actively employed |
| Last follow-up | 52 mo after transplantation | 48 mo after transplantation | 24 mo after transplantation |
Definition of abbreviations: cART = combined antiretroviral therapy; CD4 = cluster of differentiation 4; CMV = cytomegalovirus; EBV = Epstein-Barr virus; HBV = hepatitis B virus; HCV = hepatitis C virus; HHV-8 = human herpesvirus 8 ISHLT = International Society for Heart and Lung Transplantation; MMF = mycophenolate mofetil; PGD = primary graft dysfunction; POD = postoperative day; RSV = respiratory syncytial virus; TMP-SMX = trimethoprim-sulfamethoxazole; UIP = usual interstitial pneumonia.
Ischemic time and surgical duration was average for all cases.
Her first year after transplantation was complicated by recurrent episodes of ISHLT Grade A2 rejection that occurred at 4, 9, and 11 months and by Grade A1 rejection at 10 months. The first two episodes of ISHLT Grade A2 rejection were treated with intravenous methylprednisolone followed by oral prednisone taper. The third Grade A2 episode was treated with antithymocyte globulin (ATG). The dose of ATG (6 mg/kg intravenous injection divided into four doses) was not modified for her HIV status (Table 3). She was hospitalized with severe respiratory syncytial virus (RSV) infection 15 months after transplantation and treated with intravenous immunoglobulin and inhaled ribavirin. Notably, at the time of RSV infection, she had already developed ISHLT Grade 2 bronchiolitis obliterans syndrome (21). Eighteen months after transplantation, and one month after RSV infection, donor-specific antibody testing identified an antibody against HLA Class II locus DQ7 (mean fluorescence index = 2,822). All samples prior to this date had been negative (see Table E1 in the online supplement), and thus this antibody was not temporally associated with her development of bronchiolitis obliterans syndrome. For bronchiolitis obliterans syndrome prophylaxis, she had been maintained on azithromycin 250 mg thrice weekly since 2 months after transplantation. Aspiration was not suspected to be a significant clinical concern. Nevertheless, she had been maintained on a proton pump inhibitor since transplantation.
Table 3.
Timeline transbronchial biopsies and interventions after lung transplant in Subject 1 (HIV-associated pulmonary arterial hypertension)
| Biopsy date | Result | Intervention |
|---|---|---|
| 2 wk | A0/B0 | |
| 4 wk | A0/B0 | |
| 2 mo | N/A | Pulmonary emboli fortuitously discovered on abdominal CT scan; patient started on anticoagulation. This biopsy deferred. |
| 4 mo | A2/B0 | Pulsed with 500 mg intravenous methylprednisolone for 3 d with prednisone taper per standard protocol. |
| 5 mo | A0/B0 | |
| 9 mo | A2/B0 | Pulsed with 500 mg intravenous methylprednisolone for 3 d with prednisone taper |
| 10 mo | A1/B0 | |
| 11 mo | A2/B0 | ATG for refractory rejection* |
Definition of abbreviations: ATG = antithymocyte globulin. The “A” acute rejection score indicates degree of perivascular and interstitial mononuclear infiltrates. The “B” acute rejection score indicates the degree of lymphocytic bronchiolitis.
ATG dose (6 mg/kg intravenous injection divided into four doses).
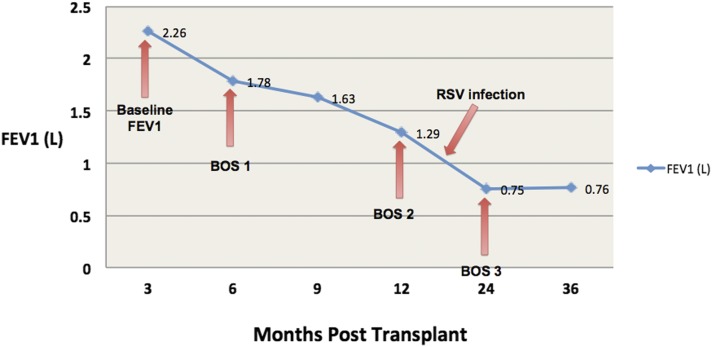
Four years after transplantation, she has ISHLT Grade 3 bronchiolitis obliterans syndrome with a forced expiratory volume in 1 second of 0.8 L, which is reduced from a peak value of 2.3 L after transplantation, and she has limited functional status (Figure 1). Her CD4+ cell count decreased from 1,053 to 183 cells/mm3 after ATG and subsequently increased to >400 cells/mm3. Her HIV viral load remains undetectable.
Figure 1.
Rapid development of bronchiolitis obliterans syndrome. Graph depicting the rapid decrease in forced expiratory volume in 1 second (FEV1) over the first 2 years after lung transplantation in Subject 1 (HIV-associated pulmonary arterial hypertension). An obstructive pattern developed, which was visualized by spirometry. The FEV1/FVC ratios fell in parallel with the changes noted in the graph, with the most recent FEV1/FVC ratio being 35%. CT chest imaging demonstrated severe air trapping on expiratory images, without evidence of fibrosis, including in the periphery. BOS = bronchiolitis obliterans syndrome, International Society for Heart and Lung Transplantation Grades 1–3; RSV = respiratory syncytial virus.
Subject 2
Subject 2 was a 65-year-old man with HIV infection (Table 1) who underwent bilateral lung transplantation in 2010 for idiopathic pulmonary fibrosis (Table 2). Before receiving the transplants, he had an undetectable viral load, a CD4 nadir of 302 cells/mm3, and no history of AIDS-defining illness. He had hepatitis C virus (HCV) antibody seropositivity without cirrhosis and undetectable HCV RNA, and he had previously been treated for basal cell carcinoma. His antiretroviral regimen included emtricitabine, tenofivir, and atazanivir. Right heart catheterization revealed that his mean pulmonary arterial pressure was 30 mm Hg.
Inductive immunosuppression therapy included basilixumab, intravenous mycophenolate mofetil (1,000 mg), and intravenous methylprednisolone (500 mg). His antiretroviral regimen was resumed on postoperative Day 4. On postoperative Day 10, tacrolimus had to be reduced to 0.5 mg every Monday, Wednesday, and Friday because of elevated troughs. This elevation may have been driven by reinitiating atazanavir, a protease inhibitor, on postoperative Day 4. On postoperative Day 14, raltegravir, an integrase inhibitor, was added for a newly detectable HIV viral load (<20 copies/ml), which was attributed to the interruption of therapy from postoperative Days 0 through 4. He was discharged on postoperative Day 18. The explanted lungs showed histopathological findings consistent with idiopathic pulmonary fibrosis as well as severe medial hypertrophy of small pulmonary arteries and arterioles. Surprisingly, the arteriopathy was similar to that seen in pulmonary arterial hypertension (Figure E1), but not traditionally seen in idiopathic pulmonary fibrosis.
On postoperative Day 42, he had Grade A1 acute rejection, which was treated with intravenous methylprednisolone. His first postoperative year was also complicated by granulation tissue at both bronchial anastomotic sites. He underwent placements of a covered AERO stent (Alveolus, Inc., Charlotte, NC) and an uncovered Ultraflex stent (Boston Scientific, Natick, MA) in the left and right mainstem bronchi, respectively. These stents were complicated by Pseudomonas aeruginosa colonization. To date, the stents remain patent and the patient is free of bronchiolitis obliterans syndrome. His CD4 nadir was 68 cells/mm3. We did not add or adjust prophylaxis for opportunistic infections based on this CD4 level. At last measure, his CD4 count was 588 cells/mm3. His viral load has remained undetectable since raltegravir was added. He has had two episodes of pseudomonas pneumonia in the third year after transplantation, which were treated successfully with antibiotics. He has not developed anti-HLA antibodies. He has not had a recurrence of skin malignancy or reactivation of HCV. Now, 4 years after lung transplantation, he enjoys an excellent functional status and has returned to active employment.
Subject 3
Subject 3 was a 60-year-old man with HIV infection (Table 1) who underwent single right lung transplantation in 2012 for idiopathic pulmonary fibrosis (Table 2). Prior to lung transplantation, his viral load had been undetectable. His CD4 count was 407 cells/mm3, and he had no AIDS-defining illness. His antiretroviral regimen included lamivudine, abacavir, atazanavir, and ritonavir. He had a history of Hodgkin’s lymphoma, which had been in remission since treatment in 2002.
Induction immunosuppressive treatment included basilixumab, intravenous mycophenolate mofetil (1,000 mg), and intravenous methylprednisolone (500 mg). His antiretroviral regimen was resumed on postoperative Day 1. Because of ritonavir-induced cytochrome P450 3A4 (CYP3A4) suppression, a lower dose and a longer dose interval for tacrolimus were anticipated. A graph illustrating tacrolimus trough levels over time underscores this relationship (Figure E2). On postoperative Day 8, he had Grade A1 acute rejection, which was treated with intravenous methylprednisolone. He was discharged on postoperative Day 11.
Now, 24 months after lung transplantation, he remains free of bronchiolitis obliterans syndrome, has an excellent functional status, and has returned to active employment. He remains on the same antiretroviral regimen. His tacrolimus dosing is 0.5 mg orally every 12 days. He has grown Mycobacterium avium-intracellulare in a bronchial washing without clinical or radiographic evidence of disease. His CD4 count most recently has ranged from 211 to 746 cells/mm3, and his HIV viral load remains undetectable.
Discussion
The current ISHLT Pulmonary Scientific Council consensus guidelines designate HIV seropositivity an absolute contraindication to lung transplantation (15). Absent a formal reassessment of these guidelines, some centers are considering persons with HIV infection for transplantation on a case-by-case basis. Such evaluations are hindered by an evidence base that, until now, has been limited to one publication in which the authors reported excellent medium-term outcomes (14). Our experience provides additional data and insights into important clinical questions. HIV is now a chronic disease. A 20-year-old adult with HIV infection who is being treated with antiretroviral therapy is currently expected to live into the eighth decade of life (2). However, individuals with HIV infection are still at increased risk for chronic obstructive pulmonary disease, lung cancer, pulmonary arterial hypertension, and pulmonary fibrosis; therefore, the number of persons with HIV presenting for lung transplant evaluation is anticipated to grow (4–7, 22). As experience in solid organ transplantation for persons with HIV grows, the lung transplantation community may need to cautiously reassess this contraindication to treatment.
It appears that lung transplantation may impact the course of HIV early after transplant surgery. In all three of our subjects, we observed decreases in CD4 levels early after transplantation. In general, IL-2 receptor antagonists, such as basilixumab, do not lead to significant T cell depletion (23). The drop in CD4+ T cells may be due in part to mycophenolate mofetil (24). Although the role of any induction therapy for persons with HIV undergoing lung transplantation remains unknown, given the low incidence of infection observed in our cases, we cautiously feel that the risks of rejection outweigh the risks of CD4+ T cell depletion early after transplantation. Despite CD4 decreases, two of our three subjects never had detectable viral loads, and the third subject had transient viremia in the setting of a brief hiatus from antiretroviral therapy from postoperative Days 0–4. Further, no instances of AIDS-related opportunistic infections or malignancies were observed during the study follow-up period.
Drug–drug interactions were challenging but manageable. Standard tacrolimus trough targets and the duration of fungal prophylaxis were not adjusted. Efavirenz, a non-nucleoside reverse transcriptase inhibitor, was given to Subject 1. Efavirenz increases the metabolism of calcineurin inhibitors, but its own metabolism is decreased by voriconazole. Using voriconazole for aspergillosis prophylaxis did pose challenges in efavirenz dosing, necessitating a 50% dose reduction with a concomitant >100% increase in the prophylactic dose of voriconazole. Subject 3 was given the protease inhibitor ritonavir as part of his antiretroviral regimen. Ritonavir, a potent CYP3A4 and P-glycoprotein inhibitor, is usually administered as a booster to increase the effects of other protease inhibitors (25, 26). Our use of ritonavir required a dramatic reduction in tacrolimus dosing. The use of protease inhibitors, especially ritonavir, requires heightened vigilance for drug toxicities. If feasible, an antiretroviral regimen absent efavirenz or ritonavir could simplify immunosuppressive drug management after lung transplantation. For example, integrase inhibitors and chemokine C-C motif receptor antagonists have fewer drug interactions and may be considered in consultation with HIV experts prior to transplantation (25).
Of concern is that all three subjects experienced acute cellular rejection. Acute rejection was recurrent and refractory in Subject 1, in whom it was followed by severe bronchiolitis obliterans syndrome. Although acute rejection occurs in approximately one-third of lung transplant recipients within the first year after transplantation (27), most episodes are successfully treated with corticosteroids. Treatment of glucocorticoid-recalcitrant acute rejection in patients with HIV infection is challenging. The potential benefits of treating acute rejection with a lymphocyte-depleting agent need to be weighed against the risks of CD4+ reduction in an already immunocompromised host. Although ATG increases the risk of infection in recipients with and without HIV infection (9, 28), it is unclear if those with HIV infection are at heightened risk. Investigators of a case series evaluated the effects of ATG on 20 kidney transplant recipients with HIV. Although treatment of acute rejection with ATG was successful, it was also associated with profound and long-lasting suppression of CD4+ T cell counts and increased risk of serious infection requiring hospitalization. Most were routinely observed infections after transplantation; only one was an AIDS-defining illness (Candida esophagitis) (29). In Subject 1, ATG treatment for acute rejection was successful, but it also resulted in CD4+ depletion to an AIDS-defining nadir (183 cells/mm3). Her subsequent rebound was also to a plateau substantially lower than her level prior to lung transplantation.
In several studies of liver and kidney transplantation in individuals who are HIV positive (9, 10, 12, 30), HIV infection appears to be an independent risk factor for acute rejection. In a kidney transplantation study, subjects with HIV infection were found to have a two- to threefold increased risk of acute rejection, and glucocorticoid-resistant acute rejection was common (9). In a cohort of kidney transplant recipients with HIV infection (N = 92), the 1-year incidence of acute rejection was 55% (12), which is substantially higher than the national registry reports of 12.3% (9). Another cohort of liver transplant recipients coinfected with HIV and HCV (N = 89) experienced 3-year acute rejection rates that were 1.6-fold higher than liver transplant recipients infected with HCV only. The cumulative incidence of acute rejection by Year 3 requiring treatment was significantly higher for patients coinfected with HCV and HIV (39%) versus those with HCV infection alone (24%) (P = 0.01) (10). A U.S. national registry analysis of 516 kidney transplant recipients with HIV infection identified a twofold increased risk of acute rejection and an increased risk of graft loss compared with recipients not infected with HIV. Notably, these differences were not observed among subjects who received ATG induction therapy (31).
The reasons for increased rejection in kidney and liver transplantation are under active investigation, but may include immunologic, virologic, and pharmacologic factors. Canaud and colleagues examined 19 kidney transplant recipients with HIV infection who did not have detectable levels of plasma HIV RNA at the time of transplantation. Despite this presentation, these investigators discovered, by using electron microscopy and molecular techniques, that HIV infected the allograft in 13 subjects (68%) (32). They proposed that acute rejection might be related to HIV infection of the allograft. In a different pharmacologic study of kidney and liver transplant recipients with HIV infection, researchers identified a poor correlation between cyclosporine trough levels and area under the curve (AUC) levels. These authors proposed that inadequate cyclosporine AUC levels might be responsible for the increased risk of rejection. Tacrolimus trough and AUC levels, however, exhibited a strong correlation (33). Because their study included only four cases, any discussion of the specific causes or mechanisms for a possible increased risk of rejection in their lung transplant recipients with HIV infection would be speculative.
Interestingly, all three of our patients had smooth muscle hypertrophy in the smaller pulmonary arteries and arterioles. Two of them had idiopathic pulmonary fibrosis, which is frequently associated with World Health Organization Group III pulmonary hypertension. However, their histopathology more closely resembled that seen in HIV-associated pulmonary arterial hypertension. Thus, absent more data, heightened awareness of potential concomitant pulmonary arterial hypertension in lung transplant candidates with HIV infection, regardless of disease indication, is recommended with the caveat that particular attention be paid to preoperative echocardiogram and right hearth catheterization results.
The major limitation of this study is its size. We emphasize that, until a more robust evidence base is available, lung transplantation for persons with HIV infection should be considered only on a case-by-case basis by a multidisciplinary team that includes specialists in HIV medicine. It is noteworthy that three of the four subjects now reported in the literature experienced good medium-term (14) or long-term outcomes, thus demonstrating that good outcomes are at least feasible.
As the population with well-controlled HIV infection grows, so, too, will the number of persons with advanced lung disease and HIV infection who are potential candidates for lung transplantation. Our case series demonstrates that lung transplantation in the setting of HIV infection may be reasonable. In this limited case series, AIDS-related opportunistic infections and persistent reactivation of HIV did not prove to be problematic. Drug–drug interactions were challenging, but could be overcome. Notably, heightened vigilance for acute rejection is warranted. The relationship between HIV seropositivity and acute rejection in solid organ transplantation requires further study. Further systematic data collection and outcomes assessments are needed as experience accumulates in lung transplantation for individuals with HIV infection.
Acknowledgments
Acknowledgments
The authors express their gratitude to the Houston Methodist Hospital lung transplant research group and to the University of California, San Francisco, lung transplant team.
Footnotes
Supported by the National Heart Lung and Blood Institute, National Institutes of Health (5T32HL007185-37 [R.M.K.] and K23 HL111115 [J.P.S.]).
Author Contributions: R.M.K.: co–first author, conception of study, data abstraction, manuscript preparation. H.S.: co–first author, conception of study, data abstraction, manuscript preparation. P.D.B.: manuscript preparation. N.S.: manuscript preparation, data abstraction. M.L.: manuscript preparation. J.G.: manuscript preparation. J.K.: manuscript preparation. S.S.: manuscript preparation. S.H.: manuscript preparation. M.E.K.: manuscript preparation. L.L.: manuscript preparation. C.H.: manuscript preparation. J.P.S.: study conception, manuscript preparation.
This article has a data supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 4.Gingo MR, Morris A. Pathogenesis of HIV and the lung. Curr HIV/AIDS Rep. 2013;10:42–50. doi: 10.1007/s11904-012-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gingo MR, Balasubramani GK, Kingsley L, Rinaldo CR, Jr, Alden CB, Detels R, Greenblatt RM, Hessol NA, Holman S, Huang L, et al. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS ONE. 2013;8:e58812. doi: 10.1371/journal.pone.0058812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doffman SR, Miller RF. Interstitial lung disease in HIV. Clin Chest Med. 2013;34:293–306. doi: 10.1016/j.ccm.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S, Wallace MR Triservice AIDS Clinical Consortium. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 8.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 9.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, Davis C, Blumberg E, Simon D, Subramanian A, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363:2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terrault NA, Roland ME, Schiano T, Dove L, Wong MT, Poordad F, Ragni MV, Barin B, Simon D, Olthoff KM, et al. Solid Organ Transplantation in HIV: Multi-Site Study Investigators. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18:716–726. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taege A. Organ transplantation and HIV progress or success? A review of current status. Curr Infect Dis Rep. 2013;15:67–76. doi: 10.1007/s11908-012-0309-x. [DOI] [PubMed] [Google Scholar]
- 12.Malat GE, Ranganna KM, Sikalas N, Liu L, Jindal RM, Doyle A. High frequency of rejections in HIV-positive recipients of kidney transplantation: a single center prospective trial. Transplantation. 2012;94:1020–1024. doi: 10.1097/TP.0b013e31826c3947. [DOI] [PubMed] [Google Scholar]
- 13.Uriel N, Jorde UP, Cotarlan V, Colombo PC, Farr M, Restaino SW, Lietz K, Naka Y, Deng MC, Mancini D. Heart transplantation in human immunodeficiency virus-positive patients. J Heart Lung Transplant. 2009;28:667–669. doi: 10.1016/j.healun.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Bertani A, Grossi P, Vitulo P, D’Ancona G, Arcadipane A, Nanni Costa A, Gridelli B. Successful lung transplantation in an HIV- and HBV-positive patient with cystic fibrosis. Am J Transplant. 2009;9:2190–2196. doi: 10.1111/j.1600-6143.2009.02779.x. [DOI] [PubMed] [Google Scholar]
- 15.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, et al. Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Boyd AS. Organ transplantation in HIV-positive patients. N Engl J Med. 1990;323:1492. doi: 10.1056/NEJM199011223232120. [DOI] [PubMed] [Google Scholar]
- 17.Hussain R, Seethamraju H. Lung transplantation in human immunodeficiency virus (HIV): expanding the horizons. Chest. 2011;140(4 Meeting Abstracts):172A. [Google Scholar]
- 18.Kern RM, Blanc PD, Hays S, Leard L, Kleinhenz ME, Kukreja J, Golden J, Singer JP. Lung transplantation for pulmonary hypertension secondary to HIV. Am J Respir Crit Care Med. 2013;187:A2485. [Google Scholar]
- 19.Kern R, Seethamraju H, Blanc P, Sinha N, Loebe M, Golden J, Kukreja J, Scheinin S, Hays S, Kleinhenz ME, et al. Lung transplantation in HIV seropositive patients. Chest. 2014;145(3 Meeting Abstracts issue):642A. doi: 10.1513/AnnalsATS.201402-083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 22.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, Oursler KK, Rimland D, Gibert CL, Butt AA, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweet SC. Induction therapy in lung transplantation. Transpl Int. 2013;26:696–703. doi: 10.1111/tri.12115. [DOI] [PubMed] [Google Scholar]
- 24.Bravo Soto JA, Esteban de la Rosa RJ, Luna del Castillo JD, Cerezo Morales S, García Olivares E, Osuna Ortega A, Asensio Peinado C. Effect of mycophenolate mofetil regimen on peripheral blood lymphocyte subsets in kidney transplant recipients. Transplant Proc. 2003;35:1355–1359. doi: 10.1016/s0041-1345(03)00364-6. [DOI] [PubMed] [Google Scholar]
- 25.van Maarseveen EM, Rogers CC, Trofe-Clark J, van Zuilen AD, Mudrikova T. Drug-drug interactions between antiretroviral and immunosuppressive agents in HIV-infected patients after solid organ transplantation: a review. AIDS Patient Care STDS. 2012;26:568–581. doi: 10.1089/apc.2012.0169. [DOI] [PubMed] [Google Scholar]
- 26.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Günthard HF, Hammer SM, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 27.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report 2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 29.Carter JT, Melcher ML, Carlson LL, Roland ME, Stock PG. Thymoglobulin-associated CD4+ T-cell depletion and infection risk in HIV-infected renal transplant recipients. Am J Transplant. 2006;6:753–760. doi: 10.1111/j.1600-6143.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 30.Roland ME, Barin B, Carlson L, Frassetto LA, Terrault NA, Hirose R, Freise CE, Benet LZ, Ascher NL, Roberts JP, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008;8:355–365. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 31.Locke JE, James NT, Mannon RB, Mehta SG, Pappas PG, Baddley JW, Desai NM, Montgomery RA, Segev DL. Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation. 2014;97:446–450. doi: 10.1097/01.TP.0000436905.54640.8c. [DOI] [PubMed] [Google Scholar]
- 32.Canaud G, Dejucq-Rainsford N, Avettand-Fenoël V, Viard JP, Anglicheau D, Bienaimé F, Muorah M, Galmiche L, Gribouval O, Noël LH, et al. The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol. 2014;25:407–419. doi: 10.1681/ASN.2013050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frassetto LA, Tan-Tam CC, Barin B, Browne M, Wolfe AR, Stock PG, Roland M, Benet LZ. Best single time point correlations with AUC for cyclosporine and tacrolimus in HIV-infected kidney and liver transplant recipients. Transplantation. 2014;97:702–707. doi: 10.1097/01.TP.0000441097.30094.31. [DOI] [PMC free article] [PubMed] [Google Scholar]