Abstract
Background:
Osteoporosis is a major public health problem affecting the elderly population, particularly women. The objective of the study was to evaluate the effects of adding weight-bearing exercise as opposed to nonweight-bearing programs to the medical treatment of bone mineral density (BMD) and health-related quality of life (HRQoL) of elderly patients with osteoporosis.
Materials and Methods:
Participating in the study were 40 elderly osteoporotic patients (27 females and 13 males), with ages ranging from 60 to 67 years, who were receiving medical treatment for osteoporosis. They were assigned randomly into two groups: Group-I: Twenty patients practiced weight-bearing exercises. Group-II: Twenty patients did nonweight-bearing exercises. All patients trained for 45-60 min/session, two sessions/week for 6 months. BMD of the lumbar spine, right neck of femur, and right distal radial head of all patients were measured by dual-energy X-ray absorptiometry before and after both treatment programs. In addition, the QoL was measured by means of the HRQoL “ECOS-16” questionnaire.
Results:
T-tests proved that mean values of BMD of the lumbar spine, right neck of femur and right distal radial head were significantly increased in both groups with greater improvement in the weight-bearing group. The QoL was significantly improved in both groups, but the difference between them was not significant.
Conclusion:
Addition of weight-bearing exercise program to medical treatment increases BMD more than nonweight-bearing exercise in elderly subjects with osteoporosis. Furthermore, both weight-bearing and nonweight-bearing exercise programs significantly improved the QoL of patients with osteoporosis.
Keywords: Bone mineral density, geriatrics, nonweight-bearing exercises, osteoporosis, quality of life, weight-bearing exercises
INTRODUCTION
Osteoporosis is a major public health problem affecting the elderly population, particularly women.[1,2] It is a chronic disorder affecting one in every three women, and one in every five men over the age of 50 years.[3] Osteoporosis is a systemic skeletal disorder characterized by low-bone mass, deterioration of bone tissue, increased bone fragility, and its susceptibly to recurrent fractures.[4,5] It is a silent disease which means that there are no warning signs until a fracture occurs.[1,6] Osteoporosis takes a huge toll on the suffering and health care costs, with hip fractures as the most serious and costly result of multiple associated complications.[7]
Dual-energy X-ray absorptiometry (DEXA) is considered a valid and reliable method of diagnosing osteoporosis. This technique is commonly used to measure the central skeleton (spine, proximal femur) or some parts of the peripheral skeleton such as distal and proximal radius.[6,8,9] There is strong evidence that physical activity and exercise training contribute to an increase in bone mass.[10,11,12] They have a positive effect on growing bones[13] during childhood, adolescence, and adulthood,[2,14] particularly to overcome osteoporosis in elderly subjects.[12,15] Exercise training has many advantages such as improving mechanical properties of bone by changing its composition.[1,16,17] Public health programs should be designed to help,[18] prevent bone loss and promote osteogenesis,[19,20,21] improve body composition, muscle strength and balance, and reduce recurrent falls and associated risks of fractures.[12,22]
Different exercise techniques that benefit mechanical properties of bone are recommended.[17] Some are resistive exercises in the form of strength training programs which are used to increase muscular strength, enhance bone mass, improve balance, and mobility and in turn lead to improve quality of life (QoL).[12,17,19] There are also weight-bearing exercises which are most popular with children, adolescents, adults, and postmenopausal women,[11,20] because this type[18] generates the highest mechanical load on bones.[1,23] Weight-bearing exercises are applied with different modes such as walking, running[17] or jumping.[22,24]
Health-related QoL (HRQoL) assessment plays an important role in therapeutic intervention for patients with osteoporosis.[25] HRQoL is used as an outcome measure that complements bone mineral density (BMD) measurement in osteoporosis as imaging tests. It does not adequately reflect the extent to which the patient is affected in his or her daily activities.[26]
The objective of the study was to evaluate the effects of adding weight-bearing exercise in contrast to nonweight-bearing exercise programs to medical treatment of BMD and the QoL of elderly patients with osteoporosis.
MATERIALS AND METHODS
Study design
Experimental study.
Subjects
A total of 40 elderly patients (27 females and 13 males) participated in this study. They were selected from nursing homes for the elderly in Cairo, Egypt. Their ages ranged from 60 to 67 years. Three males in Group-I and two in Group-II had a history of smoking. The patients were randomly put into two groups by a blinded and independent research assistant who opened sealed envelopes that contained a computer-generated randomization card. A total of 20 patients (14 females and 6 males) with a mean age of 64.5 ± 3.36 years participated in a weight-bearing exercise program (Group-I), whereas 20 patients (13 females and 7 males) with a mean age of 63.8 ± 3.11 years participated in nonweight-bearing exercise program (Group-II).
Inclusion criteria
Elderly subjects aged between 60 and 67 years were recruited for this study if their “T-score,” were < −2.5, they were receiving medical treatment that included oral calcium supplementation (1000 mg/day), alendronate sodium (one 10 mg tablet/day or 70 mg tablet/week) with Vitamin-D (400 international unit/day), and achieved at least 41 score of Berge balance scale,[27,28] (indicating sufficient balance performance and low risk of falls to enable them to perform exercise training programs).[1,2,27]
Exclusion criteria
Elderly subjects were excluded from the study if they had unstable cardiopulmonary conditions, uncontrolled hypertension, diabetes mellitus; severe renal or hepatic diseases, progressive neurological disease, chronic disabling arthritis, significant dementia, anemia and marked obesity or were receiving hormone replacement therapy or any medications that interfered with the balance.
Ethical considerations
The approval of this study was obtained from the Institutional Reviewed Board of Faculty of Physical therapy, Cairo University, Egypt, before the assessment and treatment of patients was started. All participants were informed that the data collected would be submitted for publication, and they signed an informed consent form prior to their participation in the study.
Evaluative instrumentation
Dual-energy X-ray absorptiometry: (GE Medical Systems, USA), bone densitometer was used to measure BMD at the neck of the femur, lumbar spine (L3-L5) and right distal radial head for each patient of the respective groups before and after physical therapy intervention.[6,8,9,10] DEXA was used to detect “T-score,” which is the number of standard deviations above or below the mean BMD for normal young adults. Osteoporosis is defined as a “T-score” < −2.5.[6,8,10]
ECOS-16 HRQoL questionnaire was used to evaluate the effects of both programs on the QoL of the elderly subjects.[22] ECOS-16 questionnaire is a self-administered questionnaire is consisting of 16 items: 12 items from the QoL Questionnaire of the European Foundation for Osteoporosis and 4 items from the osteoporosis QoL Questionnaire. ECOS-16 questionnaire includes four dimensions dealing with physical function, pain, fear of illness and psychosocial function. The score for each item ranges from 1 (best HRQoL) to 5 (worst HRQoL). The time frame for completing the questionnaire was 1 week. All items had the same weight on the overall questionnaire score which was, calculated as the mean score of all response items.[26]
Assessment procedure
Dual-energy X-ray absorptiometry was used to measure BMD at the neck of the femur, lumbar spine (L3-L5) and right distal radial head for every patient in the two groups before and after physical therapy interventions. ECOS-16 (HRQoL) questionnaire was used to evaluate effects of the two programs on the QoL of the subjects.[26] Each participant answered and completed the four dimensions of ECOS-16 questionnaire before and after both physical therapy programs. The total score of each item was summated, and the mean score of all responses was calculated. This study was conducted at the out-clinic of Faculty of Physical Therapy, Cairo University, Egypt.
Treatment procedure
Every patient in the two groups was trained for 45-60 min/session, two sessions/week for 6 months. In addition, he or she had the same medical treatment. The patients had a warming up routine of a brisk walk and gentle stretch of arms, knee flexors, calf muscles, lower back muscles for 10 min,[18] finishing with cooling down in the form of a brisk walk for 5 min.[29]
Group-I: Weight-bearing exercise program[1,19,24] included bench press and double leg press,[3] quarter squats up to right angle knee flexion,[12] wide stance mini-squat,[3] quadruped position and step-up exercises,[3] wall slides with upper limb,[10] and standing on one limb with arm support.[18]
Group-II: Nonweight-bearing exercise program[1,19] included hip exercises (extension and abduction),[29] leg extension exercise,[23] arm exercises, biceps curl, triceps curl,[18] quadriceps and hamstrings curl,[3] wrist curl in (flexion, extension, rotation,[18] and back extension exercises from standing.[30] These exercises were performed in three sets, repeated 8 times. Resistance was determined to be 25% of 1 repetition maximum.[3]
Statistical analysis
The collected data were analyzed by using SPSS (version 20.0) Paired t-test was used to compare the effects of each exercise training program in each group. Independent t-test was used to compare the differences between weight-bearing and nonweight-bearing exercise programs. Statistical significance was determined at a P <.05 and a confidence interval of 0.95.
RESULTS
Demographical data of patients
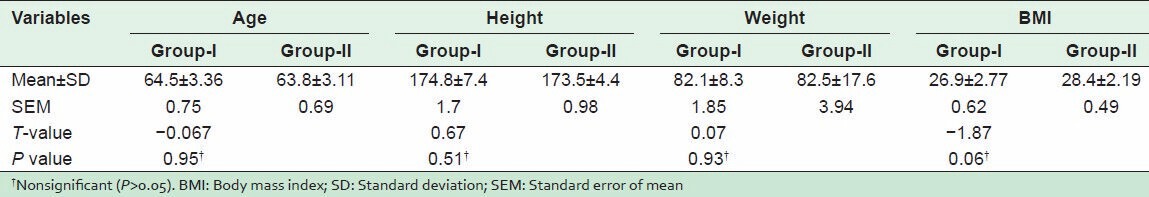
There were nonsignificant differences between Group-I and Group-II in demographic data including age, weight, height, and body mass index P > 0.05 [Table 1].
Table 1.
Demographical data of patients of Group-I (weight-bearing) and Group-II (nonweight-bearing) exercise programs
Bone mineral density
This was measured after weight-bearing and nonweight-bearing exercise programs. There was a significant difference between mean values of “T-score” of the lumbar spine, neck of femur and right distal radial head after weight-bearing and nonweight-bearing exercise programs (P < 0.05) as shown in [Figures 1 and 2]. The results of the comparison between Group-I and Group-II before the start of the study, showed that there were nonsignificant differences between “T-score” of the lumbar spine, neck of femur and right distal radial head between the two groups (P > 0.05) [Table 2]. However, when after interventions Group-I (weight-bearing exercises) was compared with Group-II (nonweight-bearing exercise), there were significant differences in “T-score” of the lumbar spine, neck of femur and right distal radial head (P < 0.05) [Table 3] with higher percentages of improvements in Group-I than in Group-II [Figure 3].
Figure 1.
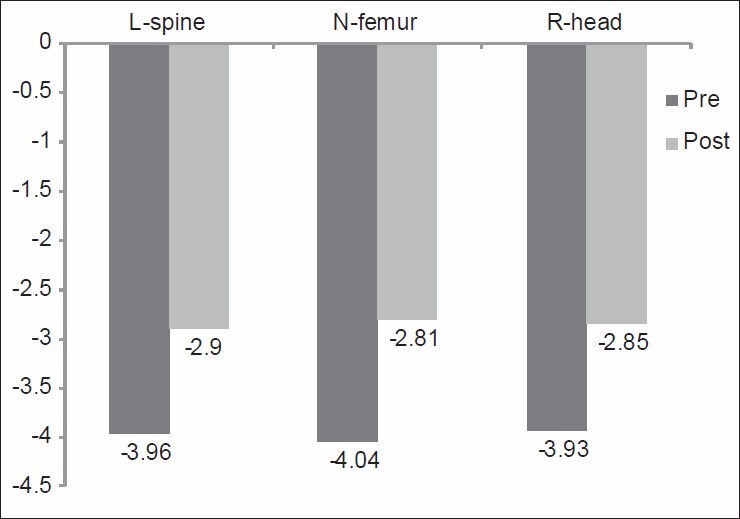
Mean values of “T-score” of the L-spine, N-femur, and R-head of patients before and after weight-bearing exercise program (Group-I). L-spine: Lumbar spine; N-femur: Neck of femur; R-head: Right distal radial head. P = 0.02, 0.00, 0.01 of the L-spine, N-femur and R-head respectively
Figure 2.
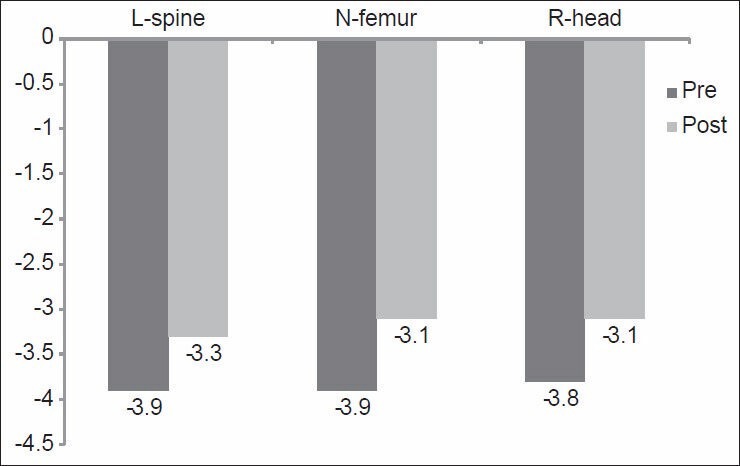
Mean values of “T-score” of the L-spine, N-femur, and R-head of patients before and after nonweight-bearing exercise program (Group-II). L-spine: Lumbar spine; N-femur: Neck of femur; R-head: Right distal radial head. P = 0.00, 0.018, 0.017 of the L-spine, N-femur and R-head respectively
Table 2.
Mean values of “T-score” of the lumbar spine, neck of femur and right distal radial head before both weight-bearing (Group-I) and nonweight-bearing (Group-II) exercise programs
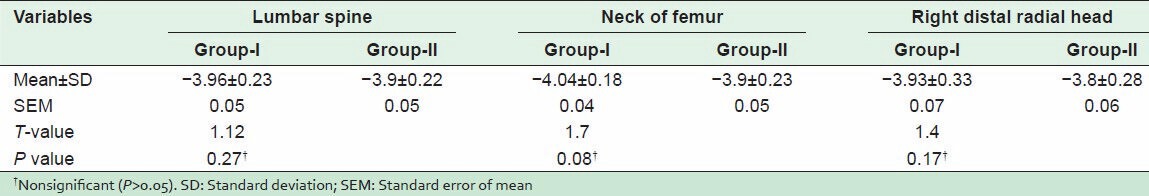
Table 3.
The effect of weight-bearing (Group-I) and nonweight-bearing (Group-II) exercise programs on BMD of the lumbar spine, neck of femur and right distal radial head
Figure 3.
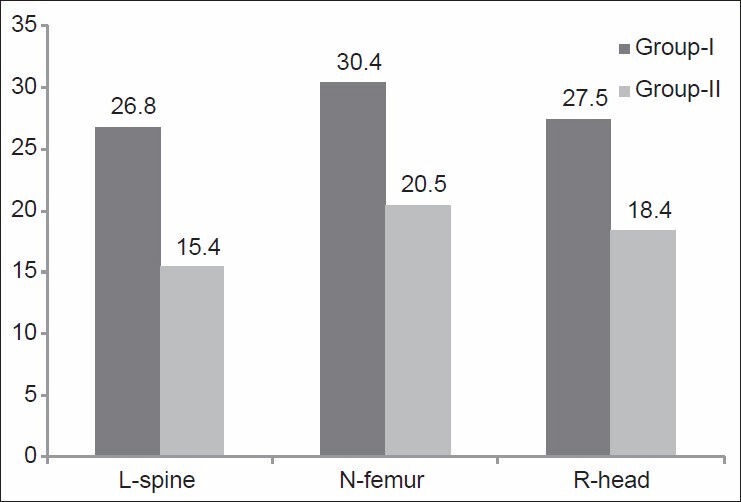
Percentages of improvements after weight-bearing (Group-I) and nonweight-bearing (Group-II) exercise training programs. L-spine: Lumbar spine; N-femur: Neck of femur; R-head: Right distal radial head
Quality of life
This was measured after weight-bearing and nonweight-bearing exercise programs. The mean values of the QoL were significantly improved after both treatment programs. Good results and improvements are indicated by reduced scores on the questionnaire. Scores on the questionnaire on HRQoL was reduced significantly from (47.3 ± 4.7) to (34.8 ± 3.3) in Group-I (P < 0.05), and from (41.1 ± 4.3) to (34 ± 3.2) in Group-II (P < 0.05), [Figure 4]. There were nonsignificant differences between the two treatment programs (P > 0.05).
Figure 4.
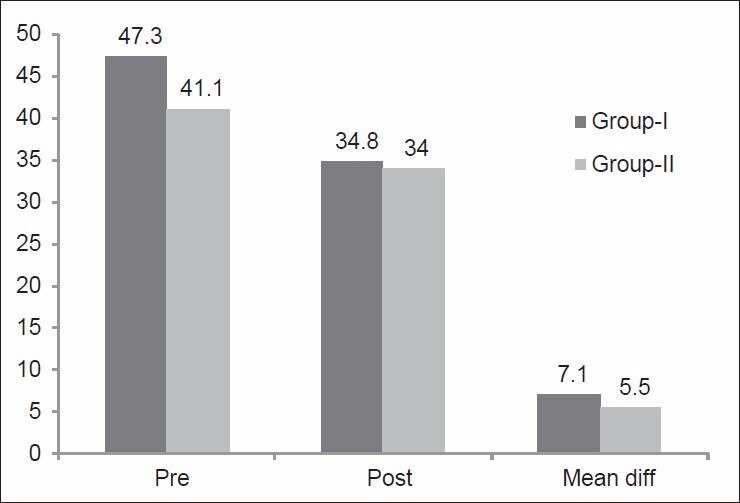
Mean values of scores for health-related quality of life of patients before and after weight-bearing (Group-I) and nonweight-bearing (Group-II) exercise programs. P = 0.00, and 0.01, for pre and post); Mean diff: The mean for differences between pre and post in each group, P = 0.08. Pre: Before both exercise programs; Post: Post both exercise programs; Mean diff: Mean difference between pre and post of both groups
DISCUSSION
The results of this study suggest that there is a significant increase of “T-score” of the lumbar spine, right neck of femur, and right distal radial head after either weight-bearing- and nonweight-bearing exercise programs. “T-score” is calculated by DEXA, the most common radiological method used in diagnosing osteoporosis, that also reflects direct improvements of BMD in subjects with osteoporosis.[8,9,19]
The results of the current study are in agreement with a study that reported that patients with low BMD would benefit a great deal from specific exercise programs.[19,30] This can be explained by the fact that muscular strength is the main output of exercises enhancing BMD.[18,31,32] It is a predictor of BMD in elderly subjects,[33] because the force exerted by muscle to pull bones while muscular contraction has a strong osteogenic stimulus.[34] In support of this idea, it was postulated that a combination of progressive impact jumping and resistance training can increase BMD in premenopausal women.[12] In a recent study,[19] it was found that “T-score” of the neck of the femur, significantly increased after the application of combined weight- and nonweight-bearing exercise programs, suggesting that exercises can be effective in decreasing bone loss and maintaining good bone quality after menopause.
In contrast to our results, another study found that strength training had very little effect on BMD at the femoral neck, lumbar spine, distal and proximal radial heads. This may be because the exercise program used in that study,[35] was designed to maximize stress on wrist bones only while the exercises used in the current study covered all major bones of the body.
The significant positive changes of “T-score” in Group-I (weight-bearing exercises) than Group-II (nonweight-bearing exercises), indicated that weight-bearing had more influence on BMD especially on weight-bearing bones. The mechanical loads of weight-bearing activities are transmitted to the skeleton by muscle pull and gravitational forces where the bone cells selectively respond to different mechanical stresses to increase or decrease BMD.[6] It also suggests that weight-bearing activities have more osteogenic effect on bone than nonweight-bearing activities.[1,14] For example, running and jumping have been useful in increasing bone strength, mass, and morphometric in middle age osteopenia[20] especially at weight-bearing sites.[16] In a previous study of the whole body vibration training, the authors found that there was a significant increase in BMD of proximal hip in postmenopausal women.[36] It has also been reported that the effect of training programs on bone is site specific[19,28] and load dependent.[17,18] Exercise training usually benefits intrinsic and extrinsic bone mechanical properties.[17]
The results of the current study suggest that HRQoL measured by “ECOS-16 questionnaire” significantly reduced after both weight-bearing and nonweight-bearing exercise programs with no significant differences between them. One of the limitations of this study was that the four dimensions (physical function, pain, fear of illness and psychosocial function) of HRQoL questionnaire were assessed as one outcome measure and not as separate items.
The improvements of HRQoL seen in the current study are supported by some previous investigations which found that strengthening exercises may prevent loss of physical independence and thereby improve the QoL in the elderly.[15,19] The well-designed supervised exercise program may have psychosocial advantages such as the alleviation of depression, loneliness, and isolation and improvements in cognitive function, the main parts of the “ECOS-16 questionnaire,” which improved in both groups after exercise program.[15,19] All patients in the current study received medical treatment (calcium and Vitamin-D), as part of good nutrition, which is still considered essential to good bone health.[22] Calcium is the most important nutrient for obtaining peak bone mass. Vitamin-D is required for optimal calcium absorption and is, therefore, also important for bone health.[10,11,12]
Limitation of study
This study had some limitations, e.g. the number of patients was relatively small. Thus, a larger sample size would provide a much better insight into the efficacy of the both training programs. The measurements were performed only before and after treatment without further follow-up assessment. Hence, it would be preferable to have follow-up of the patients involved for longer periods. The HRQoL was measured by “ECOS-16 questionnaire as a total score not individual sub-items. It would have been preferable to measure improvements in the four sections of the questionnaire in order to determine more accurately which component of the QoL had particularly improved in the patients.
Recommendations
Several risk factors for osteoporosis such as a, gender, sedentary lifestyle and smoking and body type have been reported in literature.[4,33] These risk factors need further studies. Physical therapy programs for osteoporosis should include some weight-bearing exercises combined with nonweight-bearing exercise programs in addition to medical treatment. Educational health programs including early change of lifestyle from sedentary to an active lifestyle, and calcium-rich dietary interventions should be established in our community to prevent the risks factors. In addition, programs should be designed for the elderly and measures taken to prevent and reduce the incidence of osteoporosis as much as possible.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. CMAJ. 2010;182:1864–73. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebeling PR, Daly RM, Kerr DA, Kimlin MG. Building healthy bones throughout life. An evidence-informed strategy to prevent osteoporosis in Australia. Med J Aust. 2013;2:1–9. doi: 10.5694/j.1326-5377.2013.tb04225.x. [DOI] [PubMed] [Google Scholar]
- 3.Body JJ, Bergmann P, Boonen S, Boutsen Y, Bruyere O, Devogelaer JP, et al. Non-pharmacological management of osteoporosis: A consensus of the Belgian Bone Club. Osteoporos Int. 2011;22:2769–88. doi: 10.1007/s00198-011-1545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Rauda R, Martinez-Garcia S. Osteoporosis-related life habits and knowledge about osteoporosis among women in El Salvador: A cross-sectional study. BMC Musculoskelet Disord. 2004;5:29. doi: 10.1186/1471-2474-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadat-Ali M, AlElq A. Osteoporosis among male Saudi Arabs: A pilot study. Ann Saudi Med. 2006;26:450–4. doi: 10.5144/0256-4947.2006.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafeez F, Zulfiqar S. An assessment of osteoporosis and low bone density in postmenopausal women. Pak J Physiol. 2009;5:41–4. [Google Scholar]
- 8.Kanis JA, Glüer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11:192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 9.Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP, et al. Evaluation of decision rules for referring women for bone densitometry by dual-energy X-ray absorptiometry. JAMA. 2001;286:57–63. doi: 10.1001/jama.286.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Vondracek SF, Linnebur SA. Diagnosis and management of osteoporosis in the older senior. Clin Interv Aging. 2009;4:121–36. doi: 10.2147/cia.s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: A randomized controlled trial. J Bone Miner Res. 2001;16:148–56. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 12.Winters KM, Snow CM. Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res. 2000;15:2495–503. doi: 10.1359/jbmr.2000.15.12.2495. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs RK, Snow CM. Gains in hip bone mass from high-impact training are maintained: A randomized controlled trial in children. J Pediatr. 2002;141:357–62. doi: 10.1067/mpd.2002.127275. [DOI] [PubMed] [Google Scholar]
- 14.Gustavsson A, Olsson T, Nordström P. Rapid loss of bone mineral density of the femoral neck after cessation of ice hockey training: A 6-year longitudinal study in males. J Bone Miner Res. 2003;18:1964–9. doi: 10.1359/jbmr.2003.18.11.1964. [DOI] [PubMed] [Google Scholar]
- 15.Muncie HL, Jr, LeBlanc LL. Monitoring osteoporosis treatment: DXA should not be routinely repeated. Am Fam Physician. 2010;82:749–54. [PubMed] [Google Scholar]
- 16.Iwamoto J, Takeda T, Sato Y. Effect of treadmill exercise on bone mass in female rats. Exp Anim. 2005;54:1–6. doi: 10.1538/expanim.54.1. [DOI] [PubMed] [Google Scholar]
- 17.Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS. Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J Appl Physiol (1985) 2003;95:300–7. doi: 10.1152/japplphysiol.01076.2002. [DOI] [PubMed] [Google Scholar]
- 18.Kerr D, Ackland T, Maslen B, Morton A, Prince R. Resistance training over 2 years increases bone mass in calcium-replete postmenopausal women. J Bone Miner Res. 2001;16:175–81. doi: 10.1359/jbmr.2001.16.1.175. [DOI] [PubMed] [Google Scholar]
- 19.Tolomio S, Lalli A, Travain G, Zaccaria M. Effects of a combined weight-bearing and non-weight-bearing (warm water) exercise program on bone mass and quality in postmenopausal women with low bone-mineral density. Clin Ter. 2009;160:105–9. [PubMed] [Google Scholar]
- 20.Englund U, Littbrand H, Sondell A, Pettersson U, Bucht G. A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos Int. 2005;16:1117–23. doi: 10.1007/s00198-004-1821-0. [DOI] [PubMed] [Google Scholar]
- 21.Gnudi S, Malavolta N, Testi D, Viceconti M. Differences in proximal femur geometry distinguish vertebral from femoral neck fractures in osteoporotic women. Br J Radiol. 2004;77:219–23. doi: 10.1259/bjr/79551075. [DOI] [PubMed] [Google Scholar]
- 22.Brown JP, Josse RG. Scientific Advisory Council of the Osteoporosis Society of Canada. 2002. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. Can Med Assoc J. 2002;167(suppl 1):S1–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Korpelainen R, Keinänen-Kiukaanniemi S, Heikkinen J, Väänänen K, Korpelainen J. Effect of impact exercise on bone mineral density in elderly women with low BMD: A population-based randomized controlled 30-month intervention. Osteoporos Int. 2006;17:109–18. doi: 10.1007/s00198-005-1924-2. [DOI] [PubMed] [Google Scholar]
- 24.Mishra N, Mishra VN, Devanshi Exercise beyond menopause: Dos and Don'ts. J Midlife Health. 2011;2:51–6. doi: 10.4103/0976-7800.92524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15:1384–92. doi: 10.1359/jbmr.2000.15.7.1384. [DOI] [PubMed] [Google Scholar]
- 26.Badia X, Díez-Pérez A, Lahoz R, Lizán L, Nogués X, Iborra J. The ECOS-16 questionnaire for the evaluation of health related quality of life in post-menopausal women with osteoporosis. Health Qual Life Outcomes. 2004;2:41. doi: 10.1186/1477-7525-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46:239–48. [PMC free article] [PubMed] [Google Scholar]
- 28.Neuls PD, Clark TL, Van Heuklon NC, Proctor JE, Kilker BJ, Bieber ME, et al. Usefulness of the Berg Balance Scale to predict falls in the elderly. J Geriatr Phys Ther. 2011;34:3–10. doi: 10.1097/JPT.0b013e3181ff2b0e. [DOI] [PubMed] [Google Scholar]
- 29.Shanb AA, Youssef EF, El-Barkouky MG, Kamal R, Tawfick AM. The effect of magnetic therapy and active exercise on bone mineral density in elderly women with osteoporosis. J Musculoskelet Res. 2012;15:3–10. [Google Scholar]
- 30.Pfeifer M, Sinaki M, Geusens P, Boonen S, Preisinger E, Minne HW, et al. Musculoskeletal rehabilitation in osteoporosis: A review. J Bone Miner Res. 2004;19:1208–14. doi: 10.1359/JBMR.040507. [DOI] [PubMed] [Google Scholar]
- 31.Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C. Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology. 2001;47:207–12. doi: 10.1159/000052800. [DOI] [PubMed] [Google Scholar]
- 32.Vincent KR, Braith RW. Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc. 2002;34:17–23. doi: 10.1097/00005768-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: The Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–52. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 34.Burrows M, Nevill AM, Bird S, Simpson D. Physiological factors associated with low bone mineral density in female endurance runners. Br J Sports Med. 2003;37:67–71. doi: 10.1136/bjsm.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adami S, Gatti D, Braga V, Bianchini D, Rossini M. Site-specific effects of strength training on bone structure and geometry of ultradistal radius in postmenopausal women. J Bone Miner Res. 1999;14:120–4. doi: 10.1359/jbmr.1999.14.1.120. [DOI] [PubMed] [Google Scholar]
- 36.Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: A systematic review and meta-analysis. Osteoporos Int. 2010;21:1969–80. doi: 10.1007/s00198-010-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


