Abstract
Background:
The obesity epidemic, which is among the most common nutritional disorders, is rising rapidly worldwide. It leads to several health problems such as metabolic disorders, stroke, and even cancer. Efforts to control obesity with exercise and diet have a limited value in obese patients and different approaches to do this have been tried. In this paper, we share our experience with bioenteric intragastric balloon (BIB) in treating obesity: Its safety, tolerability, and its efficacy in weight reduction.
Materials and Methods:
From January 2009 to September 2012, a total of 190 gastric balloons was inserted on patients at the endoscopy unit in King Fahd Hospital of the University, Al-Khobar. This is an evaluation of the first 100 patients. All the patients had a body mass index of over 30 kg/m2 and were within the age range of 17-55 with a mean age of 32 years. After consent, preballoon investigation tests and anesthesia evaluation, BIB was inserted under monitored anesthesia care sedation in the endoscopy suite. The balloon was filled with 500-700 mls of stained saline. All patients' were given an analgesic and antiemetic for a week and antisecretory proton pump inhibitor's for 6 months. Diet and the importance of the exercise were part of the preballoon insertion phase and protocol. The balloon was removed after 6-12 months.
Results:
The weight loss response to BIB in the 100 patients are classified into four groups: In the uncooperative, noncompliant patients - the maximum weight loss was 7 kg, while in the most compliant patients the weight loss reached up to 39 kg. In addition, there was significant improvement into diabetes mellitus, hypertension, dyslipidemia, and fatty liveras. Its safety and tolerability were extremely acceptable.
Conclusion:
Our data indicates that in well-selected patients, BIB is an effective device, which with minimum complications helps to achieve body weight loss and resolve many obesity related morbidities in cooperative and dedicated obese patients.
Keywords: Dedicated patients, gastric balloon, obesity
INTRODUCTION
Obesity has reached new epidemic proportions in the world. The World Health Organization reports that worldwide more than one billion people are overweight, at least 500 million of whom are severely obese. An estimated 115 million of these people will develop serious medical conditions, including diabetes mellitus (DM), cardiovascular diseases (CVD), stroke, liver problems, and cancer. Studies show that those potentially life threatening conditions can be improved with weight loss.[1,2] Overall, prevalence of obesity among Saudis is known to be 35.5%.[3] Effective weight management for individuals who are overweight is not possible, what with a trail of pharmacological approaches snot achieving any significant outcomes. Bariatric surgery has provided significant weight reduction and gained popularity, but there is a price to pay, for there could be complications in the procedure and its outcome, as well as associated major morbidities and even some mortality.
There is, therefore, a growing demand for the less invasive approaches. Among them endoscopic management of obesity has attracted much attention.
Endoluminal treatment of obesity involves:
Prosthesis such as the deployment of intragastric balloon to restrict oral intake and duodenojejunal bypass
Endoscopic suturing and stapling devices, which are still being perfected.
Since 1980, intragastric balloons have been used to induce satiety by decreasing the capacity of the gastric reservoir, thereby reducing food intake leading to weight loss in obese patients. Gastric balloons have been upgraded and improved and a number of papers on them with conflicting results have been published.[4,5,6] This paper, presents our experience of the safety, tolerance and weight reduction efficacy of the bioenteric intragastric balloon (BIB) in our obese patients.
MATERIALS AND METHODS
The first 100 patients with BIB were 41 males and 59 females in the age range of 17-55 with a mean age of 32. The criteria for inclusion were as follows: (1) Body mass index of more than 30 kg/m2 and (2) failure to achieve weight control by means of lifestyle modification with or without pharmacological agents.
Exclusion criteria were as follows: Endocrinological causes of obesity, pregnancy, or planning to get pregnant, presence of gastric lesions such as active peptic ulcer disease, varices, large hiatus hernia, and previous gastric surgery, patients on anticoagulants, alcoholics, and drug abusers.
Diet control of 1200 kcal and the value of regular exercise was discussed and their importance explained. Patients gave their consent, and the need for the first 3 days of adaptation was emphasized. Basic laboratory tests, that is, full blood count, biochemical profile, glycated hemoglobin, insulin level, thyroid function tests, lipid profile, electrocardiogram, and abdominal ultrasound were done. Anesthesia evaluation was done, and the procedure performed under monitored anesthesia care (MAC) sedation in the endoscopy suite (BIB; Inamed, Santa Barbara, CA, USA) was used.
As a first step, esophagogastroduodenoscopy was performed to rule out abnormalities that would preclude balloon insertion in the patients. After the removal of the scope came the assembly of the collapsed balloon. A sheet, a collapsed balloon and the balloon filling tube were inserted into the stomach. The location of the collapsed balloon was controlled by re-inserting the scope, and under direct observation the balloon was filled, near the antral region, with the amount of 500-700 mls of saline (stained with 1O mls of methylene blue) using a 20 ml syringe.
After the balloon had been filled, the scope and the syringe attached to the balloon with the filling tube were taken out. Gentle suction led to the sealing of the valve and the balloon, that is, “filling tube”. The filling tube was gently detached from the balloon and with minimum pulling pressure removed. The balloon was, therefore, left in a semi-floating condition in the stomach [Figures 1–4]. The mean procedure time was 25 min. The patients were kept for 2 h for postprocedure care and discharged home. They were allowed to start with small amounts of liquid after 6 h, 1.5 l in the first 24 h, and to start on a soft diet the 2nd day.
Figure 1.
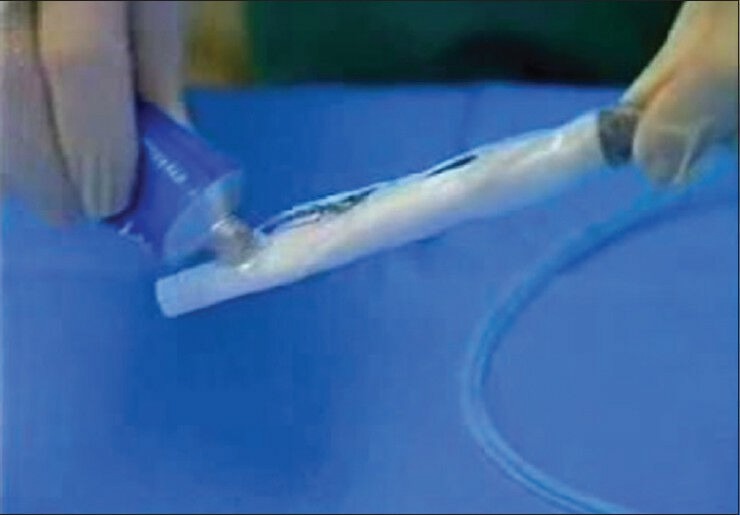
(Bioenteric intragastric balloon) Balloon before insertion
Figure 4.
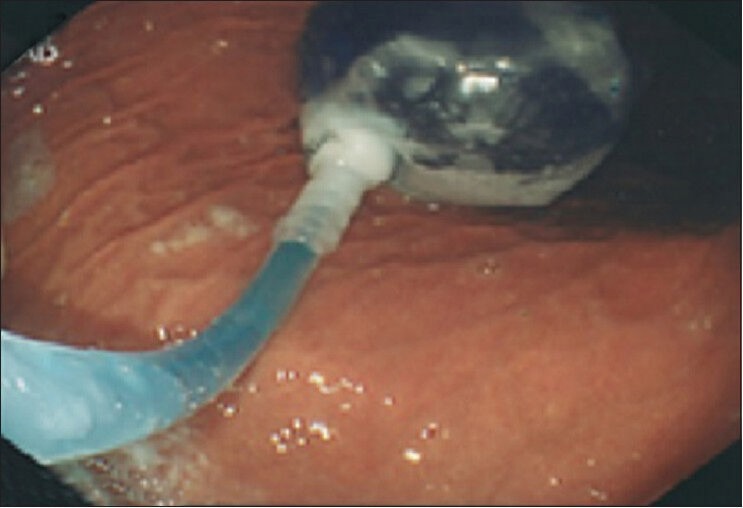
Completely deflated balloon, ready for removal
Figure 2.
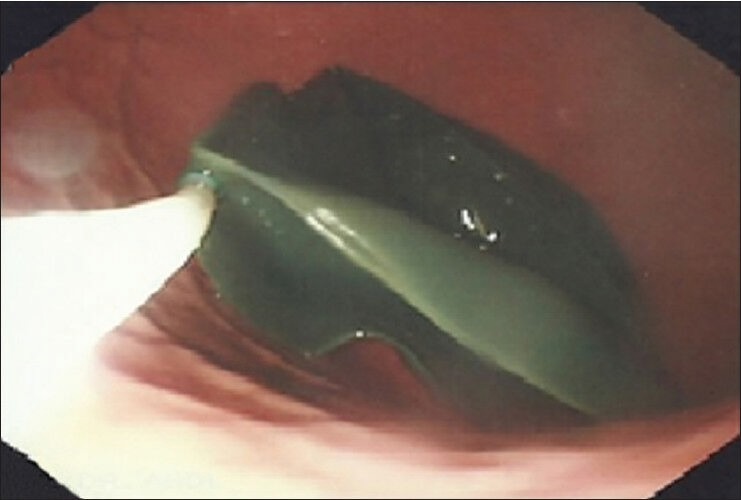
Early stage of filling of bioenteric intragastric balloon
Figure 3.
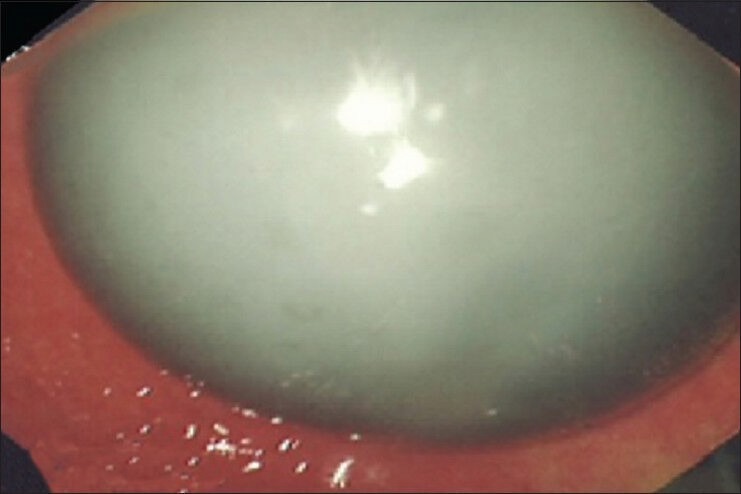
Properly placed filled intragastric balloon, before deflation
Antiemetic and analgesic medication (Kytril BD granisetron hydrochloride + Tramodol 50 mg twice daily) to last 3 days were also given. Proton pump inhibitor (PPI) 20 mg once daily was to be taken for the whole period. The balloon was removed in 6-12 months under MAC sedation in the endoscopy suite. The average time for the removal of the balloon was 20 min, and patients were discharged 2 h after the procedure, with the advice to continue the diet and exercise protocol.
RESULTS
Owing to our protocol, early removal without a definite indication was not allowed. On account of intolerance, that is, pressure symptoms, nausea, and abdominal cramps, three patients had the balloon removed early, 2-7 days after the initial insertion in other centers. Otherwise, with 3 days of antiemetic and analgesics it was generally well tolerated and the balloon remained for the scheduled period.
Episodes of minimal hypoxia (temporally drop of oxygen to 70%) during the MAC sedation with the usage of propofol were seen in 10% of patients. In two morbidly obese patients, because of significant drop in oxygen saturation the procedure was discontinued.
In two patients, evidence of balloon leakage was noticed and one of them noticed a change in urine color due to the absorption of methylene blue. In one patient, who did not come for follow-up at 18 months, the balloon deflated spontaneously and migrated into the intestine and was passed uneventfully per rectum. He gave a history of 2 days of mild abdominal cramp prior its passing per rectum. The balloons were removed under MAC sedation in the endoscopy room. The removal was not associated with any bleeding, that is, injury, no aspiration. For six patients, the scheduled day for removal had to be cancelled and rebooked for next day despite 12 h of fasting because of incomplete emptying of the stomach.
The outcome in our patients was classified into four groups. Group 1 accounted for 30% of patients whose maximum weight loss was up to 7 kg in a period of 6-12 months. Group 2 represented 10% of the patients, who had had minimal nutritional change with little exercise and had lost 8-13 kg. Group 3 represented 30% of the patients. They had been purposeful on the protocol and had done their best but had been unable to achieve perfection and had lost 14-25 kg. Group 4 comprised the remaining 30% of patients who resolutely followed the advice and protocol and lost 26-39 kg of body weight in a period of 6-12 months. There was also significant improvement in different aspects of metabolic syndrome such as DM, hypertension, dyslipidemia, and fatty liver in all compliant patients.
DISCUSSION
Over the years, several researchers have tried different balloon devices. The most recently introduced BIB is spherical in shape with a high volume capacity (700 mls) and is filled with saline instead of air. Studies have shown that complication rates are very low.[7,8,9]
The balloon itself and technique for positioning it is safe. In our study, there was no deaths, perforations, ulcer or obstruction. The rate for spontaneous deflation was 2%. Episodic and temporary hypoxemia in short neck morbidly obese patient was observed. Hence, we suggest that intubation for this type of patient is safe and less stressful. We found that preparation for the removal of the balloon required 2 days of intake of pure liquids with no fiber-containing nutrient.
Three days of analgesics and antiemetic's with regular long-term 20 mg PPI was found to be extremely helpful for early abdominal distention, colicky pain, and vomiting. PPI was extremely effective for the prevention of ulcer and balloon damage from the effect of gastric acid. Weight loss traditionally is the main objective. Weight-loss in our dedicated group of patients was impressive, with the average weight loss of 32.5 kg. However, in 1-year follow-up in this group of patients, 15% of the lost weight had been regained. In group 3, patients' average weight loss was 19.5 kg. In group 2 patients' average weight loss was 10.5 kg and in the group that was the least compliant, that is, group 1, was 4.5 kg.
Obviously, this outcome is not comparable with the results obtained in bariatric surgery, but there were noteworthy resolution of co-morbidities. The risk of death from CVD and other co-morbidities increase with a range of moderate and severe obesity.[10,11] The benefit of 10 kg weight loss in terms of co-morbidities (DM, HTN, and dyslipidemia) are well documented.[12,13]
CONCLUSION
Our data documents that in well-selected patients the BIB is an effective device that can be used to achieve a body weight loss and resolve many obesity-related morbidities with minimum complications. We believe that patients' motivation and compliance, as well as respect for dietary and behavioral rules are mandatory in order to arrive at the expected results.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i. [PubMed] [Google Scholar]
- 2.Genco A, Cipriano M, Bacci V, Cuzzolaro M, Materia A, Raparelli L, et al. BioEnterics Intragastric Balloon (BIB): A short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes (Lond) 2006;30:129–33. doi: 10.1038/sj.ijo.0803094. [DOI] [PubMed] [Google Scholar]
- 3.Al-Nozha MM, Al-Mazrou YY, Al-Maatouq MA, Arafah MR, Khalil MZ, Khan NB, et al. Obesity in Saudi Arabia. Saudi Med J. 2005;26:824–9. [PubMed] [Google Scholar]
- 4.Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013;9:290–5. doi: 10.1016/j.soard.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Giuricin M, Nagliati C, Palmisano S, Simeth C, Urban F, Buri L, et al. Short-and long-term efficacy of intragastric air-filled balloon (Heliosphere® BAG) among obese patients. Obes Surg. 2012;22:1686–9. doi: 10.1007/s11695-012-0700-6. [DOI] [PubMed] [Google Scholar]
- 6.Ganesh R, Rao AD, Baladas HG, Leese T. The Bioenteric Intragastric Balloon (BIB) as a treatment for obesity: Poor results in Asian patients. Singapore Med J. 2007;48:227–31. [PubMed] [Google Scholar]
- 7.Totté E, Hendrickx L, Pauwels M, Van Hee R. Weight reduction by means of intragastric device: Experience with the bioenterics intragastric balloon. Obes Surg. 2001;11:519–23. doi: 10.1381/096089201321209459. [DOI] [PubMed] [Google Scholar]
- 8.Roman S, Napoléon B, Mion F, Bory RM, Guyot P, D'Orazio H, et al. Intragastric balloon for “non-morbid” obesity: A retrospective evaluation of tolerance and efficacy. Obes Surg. 2004;14:539–44. doi: 10.1381/096089204323013587. [DOI] [PubMed] [Google Scholar]
- 9.Sallet JA, Marchesini JB, Paiva DS, Komoto K, Pizani CE, Ribeiro ML, et al. Brazilian multicenter study of the intragastric balloon. Obes Surg. 2004;14:991–8. doi: 10.1381/0960892041719671. [DOI] [PubMed] [Google Scholar]
- 10.Polymeris A. The pluses and minuses of bariatric surgery for morbid obesity: An endocrinological perspective. Hormones (Athens) 2012;11:233–40. doi: 10.14310/horm.2002.1353. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 12.Deitel M. How much weight loss is sufficient to overcome major co-morbidities? Obes Surg. 2001;11:659. doi: 10.1381/09608920160558524. [DOI] [PubMed] [Google Scholar]
- 13.Pinkney JH, Sjöström CD, Gale EA. Should surgeons treat diabetes in severely obese people? Lancet. 2001;357:1357–9. doi: 10.1016/S0140-6736(00)04524-4. [DOI] [PubMed] [Google Scholar]


