Abstract
Background:
Obesity is a major health problem worldwide for which conventional therapy efficacy is limited. Traditional Chinese medicine, particularly body acupoint stimulation, provides an alternative, effective, and safe therapy for this medical challenge. The present study was designed to compare the effects of distinct methods to stimulate the same set of acupoints, on anthropometric and biochemical parameters in obese women.
Materials and Methods:
Ninety-nine obese women were randomly assigned to six groups of treatment: Acupuncture with moxibustion, long needle acupuncture with moxibustion, electroacupuncture (EA), EA with moxibustion, embedded catgut with moxibustion (CGM) and sham acupuncture as control. Obesity-related parameters, including body weight, body mass index (BMI), waist and hip circumferences, waist/hip ratio, biochemical parameters (triglycerides, cholesterol, glucose, insulin) and homeostasis model of assessment - insulin resistance (HOMA-IR) index, were determined before and after each treatment.
Results:
Body weight and BMI were significantly reduced in response to all treatments. Interestingly, acupoint catgut embedding therapy combined with moxibustion was the only treatment that produced a significant reduction in body weight (3.1 ± 0.2 kg, P < 0.001), BMI (1.3 ± 0.1 kg/m2, P < 0.001), insulin (3.5 ± 0.8 mcU/ml, P < 0.1) and HOMA-IR (1.4 ± 0.2 units, P < 0.01) in comparison with sham group. Furthermore, this treatment was able to bring back obese women to a state of insulin sensitivity, indicating that acupoint catgut embedding therapy combined with moxibustion could be useful as a complementary therapy to reduce the risk of diabetes associated to obesity in women.
Conclusion:
Overall, our results confirmed the effectiveness of acupoints stimulation to assist in the control of body weight in women. They also highlighted the more favorable effects of embedded catgut-moxibustion combination that may be due to the extended and consistent stimulation of acupoints.
Keywords: Acupuncture therapy, complementary therapies, insulin resistance, moxibustion, obesity
INTRODUCTION
Obesity is a worldwide public health problem. In the United States, more than 30% of the adult population is obese;[1] in Europe similarly, adult obesity incidence is between 10% and 30%.[2,3] Due to the coexistence of malnutrition problems with an overconsumption of energy-dense diets and low physical activity, obesity has also become a serious disease concern in some developing countries, including Mexico.[4,5] Obesity is related to multiple metabolic abnormalities of the metabolic syndrome, which represent risk factors for cardiovascular diseases that are the leading causes of death worldwide.[6,7] Therefore, it is fundamental to implement health strategies to control obesity and its associated comorbidities.
Treatment of obesity requires a balanced diet and physical exercise. Pharmacological therapy and bariatric surgery represent an option in selected cases. In the last decade, many people have turned to complementary and alternative medicine to aid with weight loss. The traditional Chinese medicine (TCM) offers a complete medical system that has been used to diagnose, treat, and prevent illnesses for >2,000 years. One of the most commonly used therapies of TCM is acupuncture, which consists in stimulating specific points on the body (acupoints), by inserting thin metal needles into superficial structures such as skin, subcutaneous tissue, or muscles, in order to remove blockages in the flow of vital energy or life force called “qi” that circulates throughout the body through a system of pathways called channels.[8] Acupoint stimulation can also be performed by moxibustion that involves burning mugwort (moxa) near the skin,[9] or catgut embedding in which an implanted surgical chromic thread extend the stimulation for 15-18 days.[10]
Several reports showed the beneficial effects of acupoint stimulation for obesity control. Auricular acupressure (i.e., stimulation of acupoints in or behind the ear) with a low-calorie diet produced a significant reduction of body fat mass and leptin levels in obese patients.[11] Weight loss in obese women treated with electroacupuncture (EA) (i.e., stimulation of acupoints by a small electric current) has been associated with changes in lipid profile, lipoproteins, leptin, insulin, glucose, neurotransmitters, as well as in inflammatory and immunological markers.[12,13,14,15,16,17,18] Acupoint catgut embedding therapy also produced a decrease in lipids, and a significant reduction of insulin and leptin resistance. Moreover, insulin sensitivity remained for a 1-year period, demonstrating the sustained effects of this method.[19,20] All these studies stimulate distinct sets of acupoints, and the number and duration of the sessions vary, which makes difficult to compare the efficiency of the different treatments. Here, we aimed to compare the effects of the stimulation of the same set of acupoints by distinct methods, namely, acupuncture, EA, moxibustion and catgut embedding, in obese women. Specifically, we evaluated changes in anthropometric and biochemical parameters related to obesity in response to each treatment.
MATERIALS AND METHODS
Subjects
The current study was designed as a randomized, placebo-controlled, clinical study. Patients were recruited at the Acupuncture Clinic of the National School of Medicine and Homeopathy of the National Polytechnic Institute in Mexico City, Mexico, according to the following eligibility criteria: Women between the ages of 18 and 45 with body mass index (BMI) ≥25 kg/m2, without a known metabolic syndrome. Smokers or alcoholic participants, pregnant or breast-feeding women, and patients with previous acupuncture or drug treatment for obesity in the last 6 months, were excluded. Patients who failed to attend at least one session were eliminated. The Ethics Committee of the National School of Medicine and Homeopathy of the National Polytechnic Institute approved the protocol. The research was conducted in accordance with the last update of Helsinki declaration. All selected participants signed the informed consent.
Treatments
Women (n = 138) that were selected according to eligibility and exclusion criteria, were randomly assigned into six groups of treatment following an adaptive biased-coin randomization method, namely urn randomization: Acupuncture with moxibustion (AM), long needle acupuncture with moxibustion (LNAM), EA, EA with moxibustion (EAM), catgut embedding with moxibustion (CGM) and sham acupuncture (placebo) as control. The study was single blind, i. e. participants did not know if they were assigned to a treatment group or the placebo group. Acupuncture points were selected to cover the main syndromes related to obesity according to TCM as follows: CV6 (Qihai), CV12 (Zhongwan), and bilateral ST25 (Tianshu), ST36 (Zusanli) and SP6 (Sanyinjiao) for all groups, as well as BL20 (Pishu) and BL23 (Shenshu) for moxibustion stimulation. For the AM group, sterile stainless-steel acupuncture needles (Natural) (1.5 cun length at ST36 and SP6 acupoints; 3 cun length at CV6, CV12 and ST25 acupoints) were inserted to a depth of approximately 1-2 cm after skin sterilization. For LNAM protocol, 1.5 cun length needles were used to stimulate ST36 and SP6 acupoints, while 6 cun length needles were inserted at CV6, CV12 and ST25 acupoints to a depth of approximately 10-12 cm. BL20 and BL23 points were also stimulated with moxibustion in both AM and LNAM groups. For EA and EAM protocols, 3 cun acupuncture needles (at CV6, CV12 and ST25 acupoints), and 1.5 cun needles (at ST36 and SP6 acupoints) were connected to an acupuncture stimulator (KWD-808 li, GreatWall Brand) using dispersed-dense wave at 4 Hz high density frequency. The main advantage of EA is that the insertion of needles does not require extreme precision because the current delivered through the needle stimulates a larger area than the needle itself. In addition, BL20 and BL23 points were stimulated with moxibustion in the EAM group. For CGM protocol, a hypodermic needle (21 G × 32 mm) was used to introduce a chromic catgut strand 00 (Catgut acupuncture kit, Shuangyi) at CV6, CV12, ST25, ST36 and SP6 acupoints. The catgut thread was later completely absorbed by the body (about 18-21 days). BL20 and BL23 points were also stimulated with moxibustion. Finally, sham acupuncture involved the use of stainless-steel needles covered with a plastic film and a cap to avoid needle insertion, as previously described by Takakura and Yajima.[21] Subjects from AM, LNAM, EA, EAM and sham groups received two treatments per week for a total of 6-week (acupuncture for 20 min with or without moxibustion for 5 min in each session). For CGM group, catgut was implanted each 3-week, for a total of 6-week, whereas moxibustion was applied twice a week. Patients were asked to keep their usual lifestyle, including their diet and physical activity. These recommendations were reinforced at each session all over the protocol.
Clinical and biochemical evaluation
A standard health questionnaire with a complete medical history was administered by a physician at the beginning of the protocol; age and anthropometric values were also assessed. Patients’ body weight (kg) was measured in their underwear to the nearest 0.1 kg using a calibrated balance scale. Height (m) was measured to the nearest centimeter using a rigid stadiometer. BMI was calculated and expressed in kilogram per meter square following World Health Organization recommendations. Waist circumference (cm) was measured midway between the lateral lower rib margin and the iliac crest, to the nearest 0.1 cm; hip circumference (cm) was measured at the level of major trochanters through the pubic symphysis; the waist-hip ratio (WHR) index was also determined. A venous blood sample was taken from the left cubital vein at 07:00 to 08:00 am after an overnight fast in order to determine total cholesterol, triglycerides glucose, and insulin concentrations using standard protocols. Normal values for cholesterol and triglycerides were defined as <150 mg/dl and <200 mg/dl, respectively. Normal values for glucose and insulin were defined as 80-110 mg/dl and 2-20 mcU/ml, respectively. Insulin resistance was determined by the homeostasis model of assessment - insulin resistance (HOMA-IR), using the following formula: HOMA-IR = (fasting insulin [mcU/ml] × fasting glucose [mg/dl])/405. A HOMA-IR index of 3.8 was chosen as the cut-off point to define insulin resistance.[22] At the end of the 6-week trial, anthropometric and biochemical data were obtained again for each patient.
Statistical analyses
Prism 5 (GraphPad Software, Inc.) was used to conduct statistical analyses. Descriptive characteristics of the variables (weight, BMI, waist circumference, hip circumference, WHR, triglycerides, cholesterol, glucose, insulin and HOMA-IR index) were expressed as mean ± standard error of the mean. The paired Student's t-test was used to compare initial and final data in each group. ANOVA-Tukey's multiple comparison test was used to compare mean age, baseline anthropometric and biochemical data, as well as mean changes of each parameter between groups. The level of significance was set as P ≤ 0.05.
RESULTS
Tolerance and general effects
All treatments were very well-tolerated, and none of the obese women suspended the therapy due to adverse side-effects. Most patients reported a reduction of appetite and anxiety; some of them also reported an increase in energy. Unfortunately, these data were not rigorously documented by the physician.
Effects on anthropometric data
A total of 200 women that complied with all the eligibility criteria described above were enrolled into the study, but 62 were excluded. During the treatment period, several women were eliminated because they failed to attend at least one session and only 99 women completed the protocol [Figure 1]. Mean age was 35.9 ± 7.8 years, with the following distribution: 18-20 years, 3%; 21-25 years: 10%; 26-30 years, 10%; 31-35 years, 17%; 36-40 years, 17% and 41-45 years, 43%. Body weight range was 66.6-138.5 kg (87.06 ± 15.9 kg). Most patients (62/99) were classified as obesity I (30 < BMI ≤ 34.9 kg/m2); the others presented obesity II (16/99: 35 < BMI ≤ 39.9 kg/m2) and III (18/99; BMI ≥ 40 kg/m2), and only three patients were overweight (25 < BMI ≤ 29.9 kg/m2).
Figure 1.
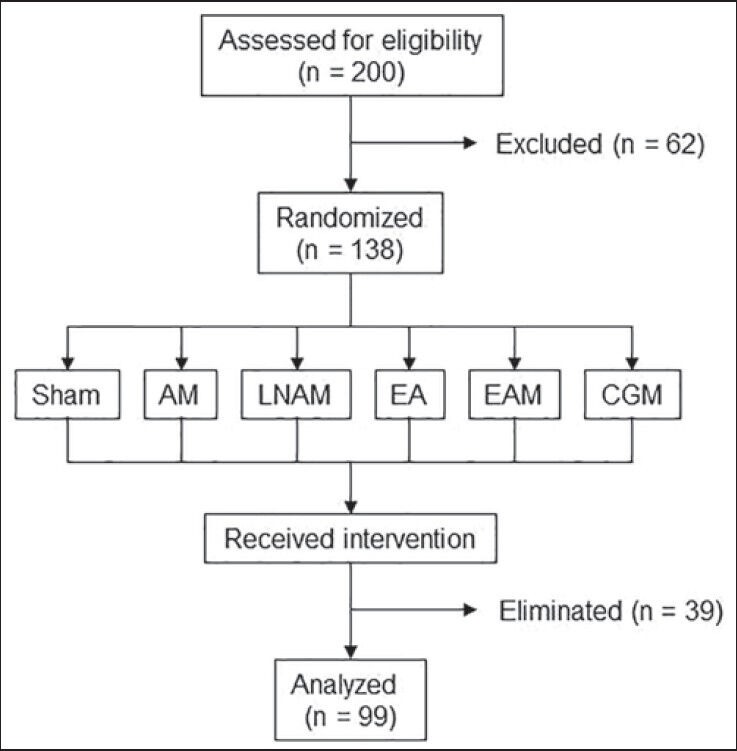
Study design. Participants were recruited at the Acupuncture Clinic of the National School of Medicine and Homeopathy of the National Polytechnic Institute in Mexico City, Mexico. Eligibility criteria were: Women between the ages of 18-45, with body mass index ≥25 kg/m2, without a known metabolic syndrome. Smokers or alcoholic women, pregnant or breast feeding women, and women with previous acupuncture or drug treatment for obesity in the last 6 months, were excluded. The study was designed as a randomized, placebo-controlled and single blind study
The cohort available for final statistical analysis included 22 patients in AM, 10 patients in LNAM, 10 patients in EA, 20 patients in EAM, 25 patients in CGM, 12 patients in sham group. Mean age and baseline anthropometric data are described in Table 1. Mean age, body weight, BMI, waist and hip circumferences, as well as WHR, were not significantly different among groups according to ANOVA test (data not shown). Mean BMI was between 33.4 ± 1.3 kg/m2 and 38.6 ± 2.6 kg/m2, and mean WHR was higher than 0.83 in all groups, confirming that women presented obesity I and II, with a cardiovascular risk.
Table 1.
Initial and final values of anthropometric parameters in the different groups of treatment
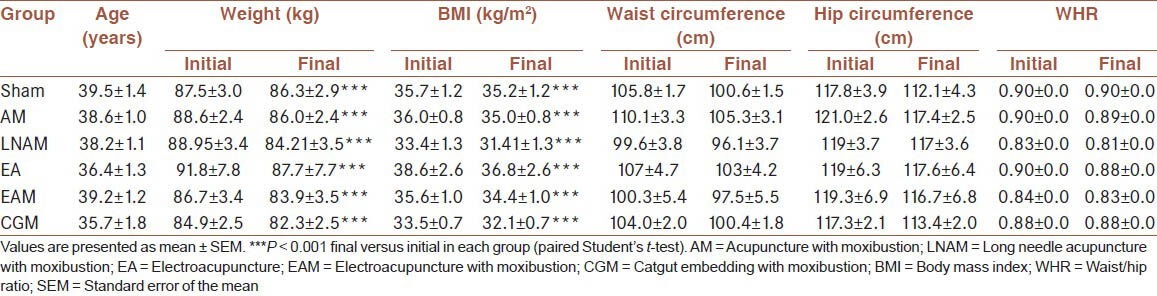
At the end of the study, body weight and BMI were significantly reduced in all groups (P < 0.001), although final BMI values still corresponded to obesity I and II [Table 1]. A comparative analysis between groups revealed that body weight was significantly reduced in response to all treatments in comparison with the sham group, according to ANOVA-Tukey's test. Consequently, BMI was also significantly reduced in all groups when compared with sham group. In addition, body weight reduction was higher in LNAM group when compared with EAM group (P < 0.01); BMI reduction was higher in LNAM when compared with EAM (P < 0.001) and CGM (P < 0.05) groups, whereas BMI reduction was higher in EA group in comparison with EAM group (P < 0.01) [Figure 2]. In contrast, no changes were observed in waist circumference, hip circumference, or WHR in patients from all groups at the end of the protocol [Table 1].
Figure 2.
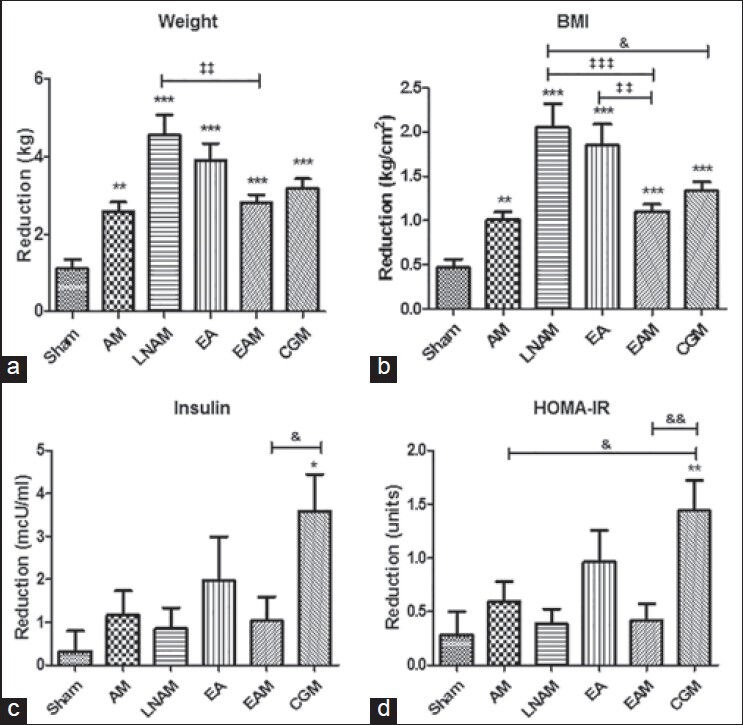
Comparative analyses of changes in weight (a), body mass index (b), insulin (c) and homeostasis model of assessment - insulin resistance (d) between groups of treatment. Data were compared using ANOVA-Tukey's multiple comparison test. ***P < 0.001, **P < 0.01, *P < 0.05 versus sham. &&P < 0.01, &P < 0.05 versus catgut embedding with moxibustion. ‡‡‡P < 0.001, ‡‡P < 0.01 versus electroacupuncture with moxibustion
Effects on biochemical parameters
Mean baseline biochemical data are described in Table 2. Concentrations of triglycerides, cholesterol, glucose and insulin age, as well as HOMA-IR, were not significantly different among groups according to ANOVA test (data not shown). Patients in all groups have high triglycerides values, while cholesterol concentrations were in normal range (except in EA group). Glucose and insulin concentrations were in normal range, indicating that subjects were not diabetic. However, HOMA-IR values were equal or higher than 3.8 in sham, AM, LNAM, EA and CGM groups, showing that these obese women had insulin resistance at the beginning of the protocol.
Table 2.
Initial and final values of biochemical parameters in the different groups of treatment
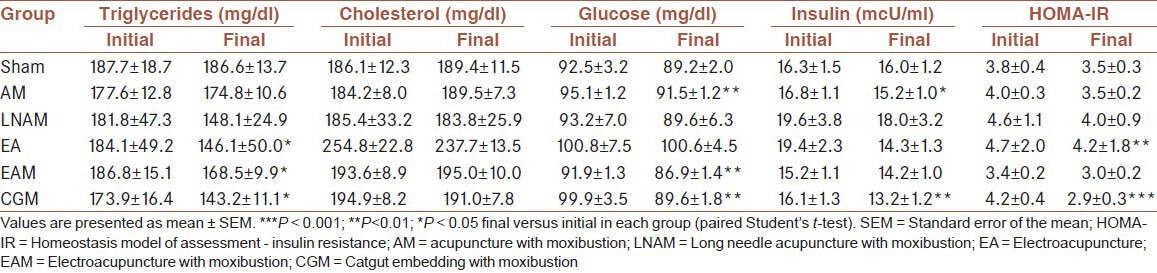
At the end of the study, we observed a significant reduction of triglycerides in EA, EAM (P < 0.05) and CGM (P < 0.01) groups [Table 2]. But these changes were not significant in comparison with sham and other groups, when data were compared using ANOVA test (data not shown). In contrast, cholesterol was not affected [Table 2]. Additionally, AM, EAM, and CGM treatments led to a reduction of glucose (P < 0.01), but there was no significant difference with sham group according to ANOVA test (data not shown). Interestingly, insulin was reduced in response to CGM treatment (P < 0.01), while HOMA-IR was reduced in EA (P < 0.01) and CGM (P < 0.001) groups [Table 2]. Multiple comparison analysis revealed that changes in triglycerides and glucose were not significant in comparison with sham and other groups, when data were compared using ANOVA test (data not shown). In contrast, the reduction of insulin (P < 0.05) and HOMA-IR (P < 0.01) observed in CGM group was significant in comparison with sham group (ANOVA-Tukey's test) [Figure 2].
DISCUSSION
In the last decade, there is an increasing interest for alternative medicine, such as the TCM, to help for obesity control, which represents a major public health problem worldwide. Notably, different acupuncture related methods involving the stimulation of body acupoints, have been reported as safe and efficient therapies to reduce body weight and associated parameters. The results of the present study confirm that 6-week treatments based on the stimulation of the same set of acupoints by AM, LNAM, EA, EAM, and embedded catgut with moxibustion (CGM) were able to significantly reduce body weight and BMI in obese women, as it has been previously reported by different groups. Moreover, our data validate that CV6, CV12, ST25, ST36, and SP6, as well as BL20 and BL23 acupuncture points, are relevant for regulating anthropometric parameters, whatever the method used for stimulation, probably because these acupoints cover most syndromes that are related to obesity according to TCM. Tang et al. also reported a decrease in body weight and BMI by stimulating almost the same set of acupoints through EA and catgut embedding therapy.[23] In addition, no adverse effects of acupuncture treatments were seen in the present study.
The fact that body weight and BMI were slightly reduced in sham group suggests a placebo effect, since patients claimed they did not modify their diet and physical activity. Importantly, these slight changes were not associated with changes in biochemical parameters in this control group. In contrast, in most groups of treatment, body weight and BMI reduction was associated with a significant modulation of biochemical parameters, mainly triglycerides, glucose, and insulin, but not cholesterol. Particularly, triglyceride reduction observed in patients from EA and EAM groups is in agreement with other reports that showed the effect of strong voltage (5-20 V) 30-40 Hz and dense-disperse wave EA to efficiently reduce triglycerides.[12,17,24] We did not find any reports about the modulation of biochemical parameters by EA associated with moxibustion, probably because these methods are not commonly used together. However, our results evidence that their combination could be an interesting complementary strategy to control body weight.
Interestingly, acupoint catgut embedding therapy with moxibustion was the only treatment that produced a significant reduction of triglycerides as previously reported,[20,25,26] with a decrease of glucose, insulin and HOMA-IR index. All treatments involved the stimulation of the same set of acupoints (CV6, CV12, ST25, ST36, and SP6, as well as BL20 and BL23), during the same number of weeks, which strongly suggests that the better effects observed in response to acupoint catgut embedding therapy with moxibustion were directly related to the method used to stimulate these acupoints. Interestingly, the reduction of HOMA-IR index from 4.2 ± 0.4 to normal values (2.9 ± 0.3) shows that obese women who were insulin resistant had become insulin sensitive at the end of the protocol. In contrast, HOMA-IR index was also significantly reduced in EA group, but women remained insulin resistant. Chen et al. also reported that embedded catgut allowed control of body weight, insulin and glucose levels, as well as insulin resistance in obese patients.[27] In obese rats, Yan et al. reported that catgut embedding allows control of insulin resistance. Moreover, results evidenced a decrease in both serum and hypothalamic levels of leptin, an adipokine that suppresses appetite and promotes energy metabolism.[20] Therefore, we propose that acupoint catgut embedding therapy with moxibustion may be used as a complementary treatment to reduce the risk of diabetes associated to obesity in women.
Although acupoint catgut embedding therapy can be used alone to control obesity, it seems that its combination with another TCM method enhances the effects. Indeed, Tang et al. reported that EA with catgut embedding produced a higher reduction in body weight, BMI, and WH index in comparison with EA alone.[23] Similarly, Shi et al. showed that EA together with catgut embedding and acupuncture-cupping significantly reduced body weight, BMI, and WHR in comparison with EA alone.[28] In the present work, we also demonstrate the better efficiency of catgut embedding with moxibustion for the control of anthropometric and biochemical parameters, as well as insulin resistance in obese women.
The better effects of acupoint catgut embedding therapy may be related to the persistent stimulation produced by the suture at the acupoints. It has been reported that the combined effects of proteolytic enzymes and macrophage action against the absorbable surgical thread may improve and extend the acupoint stimulation during 18-21 days as a consequence of the mild irritation in subcutaneous tissue.[20] It has also been shown that catgut embedding in specific acupoints can activate the satiety center and inhibit the hunger center through regulation of norepinephrine, dopamine, 5-hydroxytryptamine (serotonin) and 5-hydroxyindoleacetic acid in the feeding center in rats; this may contribute to enhance the effects observed on anthropometric and biochemical parameters.[29]
CONCLUSION
Our comparative analysis confirmed the efficiency of acupoint stimulation to significantly reduce body weight, BMI, and several biochemical parameters in obese women, without producing adverse side-effects. Most importantly, our results highlighted that acupoint catgut embedding therapy with moxibustion was also able to reverse insulin resistance, which indicates that acupoint catgut embedding therapy may represent an interesting health strategy to control obesity and its associated comorbidities. However, this study has some limitations, such as the small sample size of each group of treatment, the short duration of the treatment (only 6-week), the use of acupoints without considering the TCM syndromes of each patient, the lack of a systematic control of dietary and physical activity records, and the absence of an appropriate follow-up of possible body weight rebound. Further studies including larger sample sizes, the stimulation of acupoints according to TCM syndromes of each patients, and a longer protocol are needed to demonstrate that acupoint catgut embedding therapy with moxibustion is able to control body weight and insulin resistance in obese women.
AUTHORS’ CONTRIBUTIONS
JMGV collected and processed anthropometric and biochemical data, performed statistical analyses, participated in manuscript preparation. CGH provided assistance in design of the study, data processing and manuscript preparation. FBC provided assistance in recruitment and treatment of patients, data collection and manuscript preparation. FLR provided assistance in the design of the study, analyzed data, and participated in manuscript preparation. AZC provided assistance for data collection and statistical analyses, and participated in manuscript preparation. CLC provided assistance in the design of the study, and participated in manuscript preparation. LAM carried out the design of the study, coordinated the study and wrote the manuscript. All authors have read and approved the content of the final version. In addition, they all agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Source of Support: This study is funded by Mexican grants from COFAA-IPN, SIP-IPN (20131116) and CONACyT (113143).
Conflict of Interest: None declared.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, et al. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–76. [PubMed] [Google Scholar]
- 3.European Commission. Brussels: European Commission; 2010. Strategy for Europe on Nutrition, overweight and obesity related health issues – Implementation Progress Report 2010. Available from: http://ec.europa.eu/health/nutrition_physical_activity/docs/implementation_report_en.pdf . [Google Scholar]
- 4.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ENSANUT. 2012. Available from: http://ensanut.insp.mx/doctos/ENSANUT2012_Nutricion.pdf .
- 6.Ren J, Kelley RO. Cardiac health in women with metabolic syndrome: Clinical aspects and pathophysiology. Obesity (Silver Spring) 2009;17:1114–23. doi: 10.1038/oby.2009.8. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan PW, Ghushchyan V, Wyatt HR, Wu EQ, Hill JO. Impact of cardiometabolic risk factor clusters on health-related quality of life in the U.S. Obesity (Silver Spring) 2007;15:511–21. doi: 10.1038/oby.2007.580. [DOI] [PubMed] [Google Scholar]
- 8.Traditional Chinese Medicine, National Center for Complementary and Alternative Medicine, Traditional Chinese Medicine: An Introduction (NCCAM Backgrounder) 2009. NCCAM Pub No.: D428. Available from: http://nccam.nih.gov/sites/nccam.nih.gov/files/ Backgrounder_Traditional_Chinese_Medicine_10-25-2013.pdf .
- 9.Acupuncture today. The acupuncture and oriental medicine news sources. Available from: http://www.acupuncturetoday.com/abc/moxibustion.php .
- 10.Xia Z. Catgut embedding of modern clinical improvement and weight loss technology promotion. Traditional Chinese Folk Medicine Research and Development Association. 2010 Dec 7; [Google Scholar]
- 11.Darbandi M, Darbandi S, Mobarhan MG, Owji AA, Zhao B, Iraji K, et al. Effects of auricular acupressure combined with low-calorie diet on the leptin hormone in obese and overweight Iranian individuals. Acupunct Med. 2012;30:208–13. doi: 10.1136/acupmed-2011-010121. [DOI] [PubMed] [Google Scholar]
- 12.Cabioglu MT, Ergene N. Electroacupuncture therapy for weight loss reduces serum total cholesterol, triglycerides, and LDL cholesterol levels in obese women. Am J Chin Med. 2005;33:525–33. doi: 10.1142/S0192415X05003132. [DOI] [PubMed] [Google Scholar]
- 13.Cabioglu MT, Ergene N. Changes in serum leptin and beta endorphin levels with weight loss by electroacupuncture and diet restriction in obesity treatment. Am J Chin Med. 2006;34:1–11. doi: 10.1142/S0192415X06003588. [DOI] [PubMed] [Google Scholar]
- 14.Cabioglu MT, Ergene N. Changes in levels of serum insulin, C-Peptide and glucose after electroacupuncture and diet therapy in obese women. Am J Chin Med. 2006;34:367–76. doi: 10.1142/S0192415X06003904. [DOI] [PubMed] [Google Scholar]
- 15.Cabioglu MT, Ergene N, Surucu HS, Celik HH, Findik D. Serum IgG, IgA, IgM, and IgE levels after electroacupuncture and diet therapy in obese women. Am J Chin Med. 2007;35:955–65. doi: 10.1142/S0192415X07005429. [DOI] [PubMed] [Google Scholar]
- 16.Cabioglu MT, Gündogan N, Ergene N. The efficacy of electroacupuncture therapy for weight loss changes plasma lipoprotein A, apolipoprotein A and apolipoprotein B levels in obese women. Am J Chin Med. 2008;36:1029–39. doi: 10.1142/S0192415X08006430. [DOI] [PubMed] [Google Scholar]
- 17.Abdi H, Zhao B, Darbandi M, Ghayour-Mobarhan M, Tavallaie S, Rahsepar AA, et al. The effects of body acupuncture on obesity: Anthropometric parameters, lipid profile, and inflammatory and immunologic markers. ScientificWorldJournal 2012. 2012:603539. doi: 10.1100/2012/603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Güçel F, Bahar B, Demirtas C, Mit S, Cevik C. Influence of acupuncture on leptin, ghrelin, insulin and cholecystokinin in obese women: A randomised, sham-controlled preliminary trial. Acupunct Med. 2012;30:203–7. doi: 10.1136/acupmed-2012-010127. [DOI] [PubMed] [Google Scholar]
- 19.Yan R, Liu X, Bai J, Yu J, Gu J. Influence of catgut implantation at acupoints on leptin and insulin resistance in simple obesity rats. J Tradit Chin Med. 2012;32:477–81. doi: 10.1016/s0254-6272(13)60058-8. [DOI] [PubMed] [Google Scholar]
- 20.Yan RH, Liu XM, Bai J, Hou BB, Yu J, Gu JS. Clinical efficacy of simple obesity treated by catgut implantation at acupoints. Chin J Integr Med. 2012;3:1215–7. doi: 10.1007/s11655-012-1215-7. [DOI] [PubMed] [Google Scholar]
- 21.Takakura N, Yajima H. A double-blind placebo needle for acupuncture research. BMC Complement Altern Med. 2007;7:31. doi: 10.1186/1472-6882-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu HQ, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS One. 2011;6:e21041. doi: 10.1371/journal.pone.0021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang CL, Dai DC, Zhao GF, Zhu WF, Mei LF. Clinical observation on electroacupuncture combined with catgut implantation at acupoints for treatment of simple obesity of heart and spleen deficiency type. Zhongguo Zhen Jiu. 2009;29:703–7. [PubMed] [Google Scholar]
- 24.Yu M, Xiao XQ, Tang CL, Liu ZL, Hou YX, Gao J, et al. Effect of different intensities of electroacupuncture on expression of monocyte chemoattractant protein-1 and TNF-alpha in adipose tissue in obesity rats. Zhen Ci Yan Jiu. 2011;36:79–84. [PubMed] [Google Scholar]
- 25.Wang SX, Li YH. Effects of catgut-embedding at acupoints on contents of leptin and blood fat in obese rats (article in Chinese) J Trad Chin Med. 2009;26:63–5. [Google Scholar]
- 26.Gao L, Kong XJ, Shi X. Effects of electroacupuncture and acupoint catgut-embedding on mRNA expression of lipid metabolism gene PPAR-gamma and related lipase of rats with simple obesity. Zhongguo Zhen Jiu. 2011;31:535–8. [PubMed] [Google Scholar]
- 27.Chen F, Wu S, Zhang Y. Effect of acupoint catgut embedding on TNF-alpha and insulin resistance in simple obesity patients. Zhen Ci Yan Jiu. 2007;32:49–52. [PubMed] [Google Scholar]
- 28.Shi Y, Zhang LS, Zhao C, He CQ. Comparison of therapeutic effects of acupuncture-cupping plus acupoint catgut embedding and electroacupuncture on simple obesity of stomach and intestine excess-heat type. Zhongguo Zhen Jiu. 2006;26:547–50. [PubMed] [Google Scholar]
- 29.Zhao M, Liu ZC, Su J. Acupuncture effects on satiety of hypothalamus of rats with experimental obesity (article in Chinese) Zhen Ci Yan Jiu. 1999;24:98–100. [Google Scholar]


