Abstract
Background:
Amyloid A (AA) amyloidosis is a multisystem, progressive and fatal disease. Renal involvement occurs early in the course of AA. We aimed to investigate the etiology, clinical and laboratory features, and outcome of patients with biopsy-proven renal AA amyloidosis.
Materials and Methods:
A total of 121 patients (male/female: 84/37, mean age 42.6 ± 14.4 years) were analyzed retrospectively between January of 2001 and May of 2013. Demographic, clinical and laboratory features and outcomes data were obtained from follow-up charts.
Results:
Familial Mediterranean fever (37.2%) and tuberculosis (24.8%) were the most frequent causes of amyloidosis. Mean serum creatinine and proteinuria at diagnosis were 2.3 ± 2.1 mg/dL and 6.7 ± 5.3 g/day, respectively. Sixty-eight (56.2%) patients were started dialysis treatment during the follow-up period. Mean duration of renal survival was 64.7 ± 6.3 months. Age, serum creatinine and albumin levels were found as predictors of end-stage renal disease. Fifty patients (%41.3) died during the follow-up period. The mean survival of patients was 88.7 ± 7.8 months (median: 63 ± 13.9). 1, 2 and 5 years survival rates of patients were 80.7%, 68.2% and 51.3%, respectively. Older age, male gender, lower levels of body mass index, estimated glomerular filtration rate, serum albumin, calcium, and higher levels of phosphor, intact parathyroid hormone and proteinuria were associated with a higher mortality. Higher serum creatinine, lower albumin, dialysis requirement and short time to dialysis were predictors of mortality.
Conclusion:
The outcome of patients with AA amyloidosis and renal involvement is poor, particularly in those who had massive proteinuria, severe hypoalbuminemia and dialysis requirement at the outset.
Keywords: Amyloid A amyloidosis, dialysis, end-stage renal disease, survival
INTRODUCTION
Amyloidosis defines a group of diseases characterized by extracellular deposition of protein precursorsas insoluble fibrillar aggregates, able to induce both organ dysfunction and/or cellular death. Amyloid fibrils can be identified in biopsy specimens both by their characteristic appearance on electron microscopy and by their ability to bind Congo red and thioflavine-T. They are randomly organized and are approximately 8-10 nanometers in diameter.[1,2] Despite a common beta fibrillar structure, the protein precursors and pathogenetic mechanisms of amyloid fibrils may vary according to the different type of amyloidosis; amyloid light-chain (AL) (primary or immunoglobulin light chain associated), amyloid A (AA) (secondary or reactive to chronic inflammation) and familial amyloidosis (AF) or heredofamilial.[3,4] AA amyloidosis occurs in the setting of longstanding inflammation. Familial Mediterranean fever (FMF), chronic infections (such as tuberculosis), chronic rheumatologic diseases and inflammatory bowel disease are the most frequentetiologies.[5]
Kidneys are the most common sites of amyloid deposition in AL, AA, and several of hereditary amyloidoses. The hallmarks of renal amyloidosis are severe proteinuria and progressive renal failure.[6] Without treatment, amyloidosis-associated kidney disease usually progresses to end-stage renal disease (ESRD).[7] In this study, we retrospectively analyzed clinical features, survival and prognostic markers in AA amyloidosis with renal involvement.
MATERIALS AND METHODS
All records of patients who were treated in our in/outpatient clinic of nephrology between January of 2001 and May of 2013 were reviewed for the presence of biopsy-proven AA amyloidosis with renal involvement. Other forms of amyloidosis were excluded. All patients had renal biopsy and AA amyloidosis was confirmed by hematoxylin-eosin, Congo red, crystal violet and anti-AA antibody stains. All specimens were examined by the same pathologist. Data were obtained from patients’ follow-up charts and electronic hospital records, and all patients had to have at least one clinical control after diagnosis. Diagnosis of FMF was confirmed according to criteria's described by Livneh et al.,[8] while other etiologies were reassessed by reviewing past medical records. The creatinine clearance was estimated by MDRD-4 formula. Stages of renal disease were classified according to K/DOQI-NKF guidelines. Date of onset and type of dialysis and causes of death were recorded, if identified. Deaths were recorded as unknown whenever the exact cause was not reported.
Statistical analysis
SPSS 20.0 software (SPSS Inc., Chicago, USA) was used for statistical analysis. Parametric data were presented as mean ± standard error. Student's t-test was used for parametric variables and Mann-Whitney U-test was used for nonparametric variables. Spearman and Pearson tests were used for correlation analysis. Yates correction Chi-square test and Fisher's exact test were used for comparison of qualitative data. Multivariate analyses were performed by logistic regression analysis. Survival was estimated by Kaplan-Meier and Log Rank (Mantel-Cox) test. P < 0.05 was considered to be significant.
RESULTS
Data of 121 patients (34 females) were available to review. Mean age at diagnosis was 42.6 ± 14.4 (range: 17-74) years. Mean follow-up period was 38.2 ± 37.2 (1-167) months. Demographic features and laboratory findings at diagnosis are given in Table 1. FMF (37.1%) and tuberculosis (24.7%) were the most frequent causes, followed by unknown etiology (19.8%), chronic rheumatologic diseases (8.2%), chronic obstructive pulmonary diseases (6.6%) and others (3.3%). All FMF patients received 1-2 mg colchicine for an average of 8.7 ± 6.4 years prior to renal biopsy. Compared with FMF, patients who had underlying tuberculosis were older (P = 0.007) and had significantly lower levels of albumin (P = 0.005), higher levels of proteinuria (P = 0.007) and ferritin (P = 0.035) [Table 1]. Amyloid deposition was mainly located in the glomerulus showing a mesangial nodular pattern and extraglomerular vessels were involved in 65% of cases. Nephrotic syndrome was the presentation of 47 (38.5%) patients, whereas nonnephrotic proteinuria with and without renal failure was present in 41.5% and 20% of patients, respectively.
Table 1.
The demographic features and laboratory findings at the time of diagnosis

Dialysis
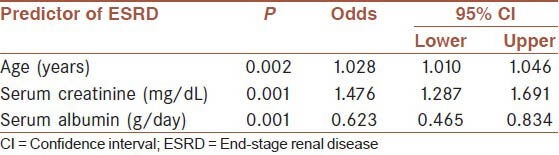
Sixty-eight (56.2%) needed dialysis treatment during follow-up (62 hemodialysis [HD], 6 peritoneal dialysis [PD]). Mean renal survival was 64.7 ± 6.3 months (median: 52 ± 11). Renal survival rates at 1, 2 and 5 years were 81.7%, 67.3% and 46.1%, respectively [Table 2]. Patients who needed dialysis were significantly older, had higher serum urea, creatinine, phosphorus, intact parathormone, proteinuria and lower estimated glomerular filtration rate (eGFR), serum albumin, hemoglobin levels at presentation [Table 3]. A multivariate logistic regression analysis showed that age, serum creatinine and albumin levels were predictors of ESRD [Table 4]. Etiology was not a significant predictor of requirement for dialysis. During the first 3 months follow-up period 26 patients (21.4%) were started on dialysis and14 patients died (nine patients were receiving dialysis). Renal and patient survivals were not significantly different between FMF and tuberculosis groups, except for the first 3 months where patients with tuberculosis significantly more frequently required dialysis (8% vs. 23%, P = 0.004); and had a higher mortality rate (2.2% vs. 16.6%, P = 0.018).
Table 2.
Renal survival time according to etiology and stage of renal failure
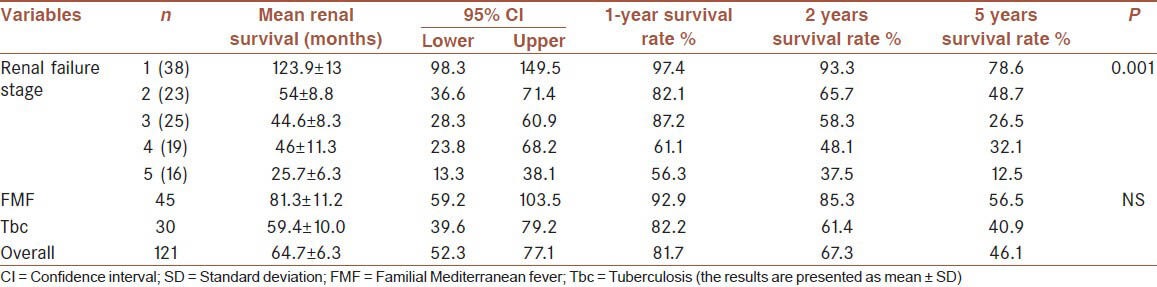
Table 3.
Demographic features and laboratory findings of the patients according to dialysis requirement
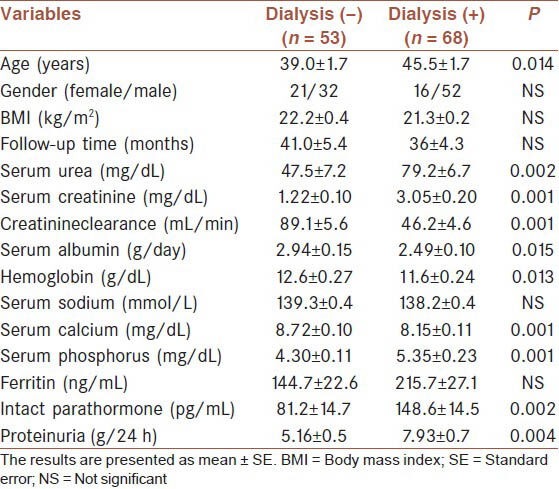
Table 4.
Multivariate logistic regression analysis of the factors that predicts ESRD
Survival
Fifty patients (41.3%) died during follow-up period. Mean overall survival was 88.7 ± 7.8 months. Survival rates at 1, 2 and 5 years were 80.7%, 68.2% and 51.3%, respectively [Table 5]. Older age, male gender, lower body mass index, eGFR, serum albumin and Ca levels, shorter time to dialysis, and higher levels of serum phosphorus, intact parathyroid hormone (iPTH), ferritin and protein uria were associated with lower odds of patient survival [Table 6]. A multivariate logistic regression analysis indicated that higher serum creatinine (for creatinine >1.3 mg/dL, hazard ratio [HR] 1.97, P = 0.019; 95% confidence interval [CI] 1.1-3.5), lower albumin level (for albumin <2.6 g/dL, HR 1.87, P = 0.028; 95% CI 1.06-3.30), short time to dialysis, dialysis requirement and stage of renal disease were predictors of death [Table 7, Figures 1 and 2]. Analysis of survival characteristics according to age groups revealed a dramatically lower life expectancy of patients older than 60 years [Table 8].
Table 5.
Patient survival time according to etiology, dialysis requirement and stage of renal failure
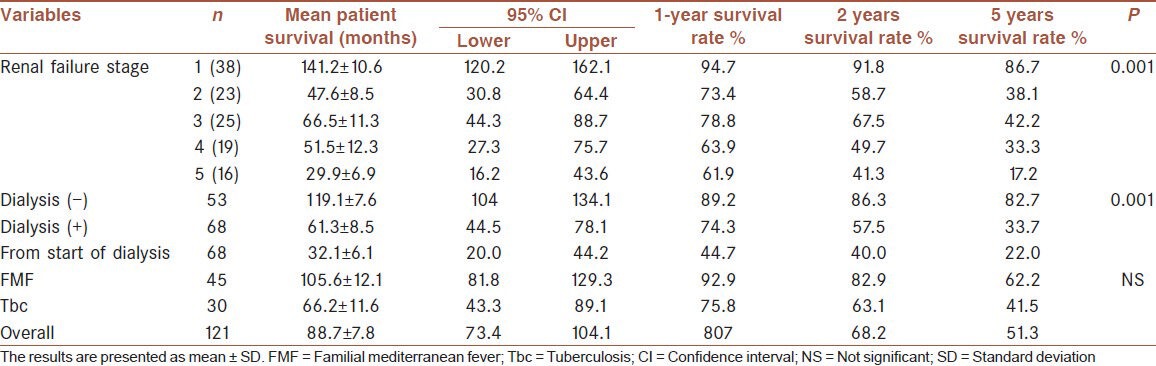
Table 6.
Demographic features, laboratory findings of died and alive patients
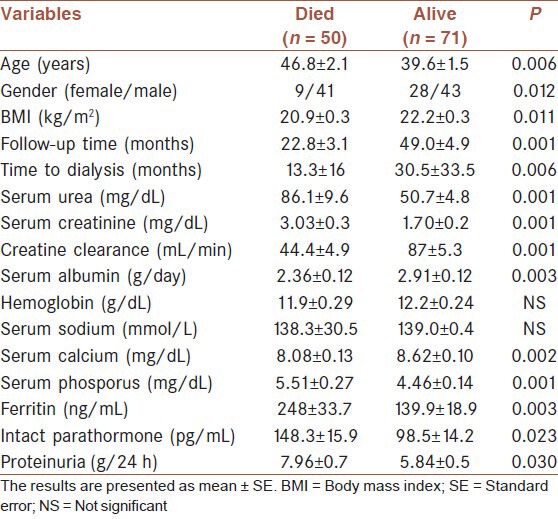
Table 7.
Multivariate logistic regression analysis of the factors that predicts mortality

Figure 1.
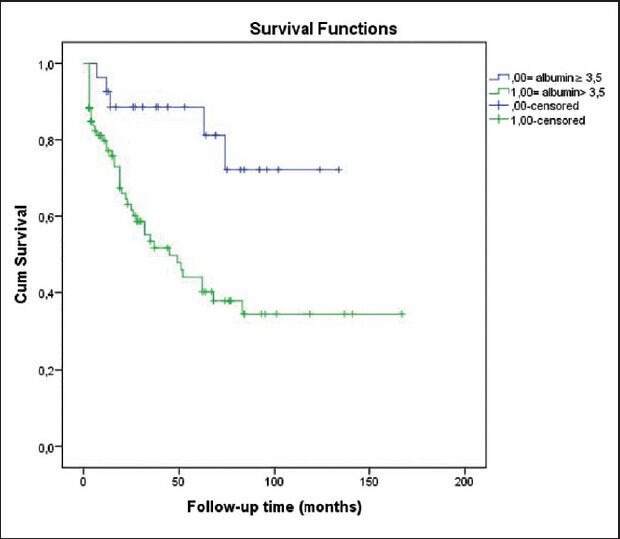
Patient survival according to serum albumin level
Figure 2.
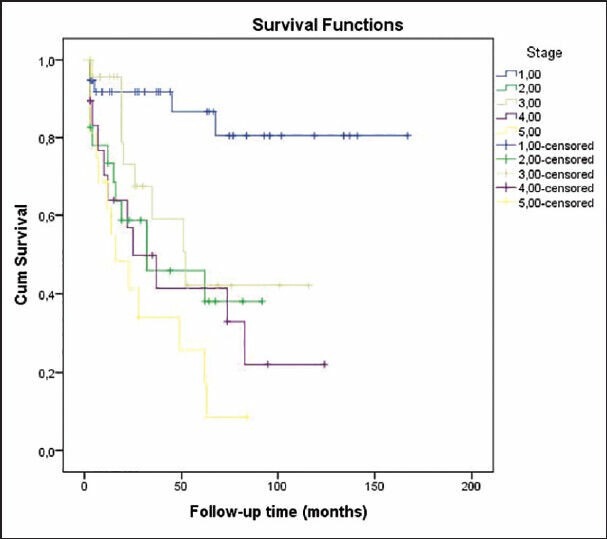
Patient survival according to stage of renal disease
Table 8.
Patients survival time according to age groups

Mortality of patients who reached to ESRD was significantly higher than those who did not (P = 0.001). Forty-three (43/68, 63.2%) of all dialyzed patients died, while only 7 (13.2%) of the 53 patients who did not reach to ESRD. Mean survival of patients who required dialysis versus who did not were 61.3 ± 8.5 versus 119.1 ± 7.6 months, respectively. 1, 2 and 5 years survival rates from the start of dialysis were 44.7%, 40.0% and 22.0% compared to 89.2%, 86.3% and 82.7% for patients who did not need dialysis, respectively [Table 5].
Mean survival of patients with FMF and tuberculosis were 105.6 ± 12.1 and 66.2 ± 11.6 months, respectively. 1, 2 and 5 years survival rates of patients with FMF were 92.9%, 82.9% and 62.2%, as compared to 75.8%, 63.1% and 41.5% for patients with tuberculosis, respectively [Table 5]. Overall mortality was not significantly different between patients with FMF and tuberculosis, except for greater mortality rate of tuberculosis patients within the first 3 months of follow-up (P = 0.018).
Causes of death
Of 50 patients who died, 9 (18%) were due to chronic nutritional debility, which led to cachexia, malnutrition and infection, 26 (52%) were due to hypotension that led to complications of HD insufficiency such as uremia, hyperkalemia and hypervolemia, and 15 (30%) were due to unidentified causes.
DISCUSSION
As a complication of chronic inflammatory diseases, chronic infections and FMF, AA amyloidosis is relatively a rare disease in the western world, whereas it is more common in Turkey due to the high prevalence of FMF and tuberculosis.[5,6,7] In developed countries, rheumatic diseases comprise up to 75% of all causes of secondary amyloidosis; whereas FMF and tuberculosis were the most common underlying diseases both in our and in another large report from Turkey.[7] This difference is supported by a review of 64 patients with AA amyloidosis from the Mayo Clinic reporting that chronic rheumatologic disorders were responsible for 65% of cases, while 17% were due to chronic infections.[9]
Renal involvement, typically manifested by nephrotic range proteinuria and renal failure, occurs in 80-90% of cases with amyloidosis and portends poor prognosis and high morbidity.[10] Long standing inflammation forms the basis of development of AA amyloidosis with a mean duration of latency was found 17 years in a UK series, though only a minority of these patients ends up with amyloidosis.[5] The reasons of this is not clear, but certain genetic isoforms of severe aplastic anemia (SAA) were found to increase the risk and severity of secondary amyloidosis.[11,12] This field of research will provide practical methods to discriminate between high and low risk patients. The progressive nature of the disease may be predicted very early by the levels of serum AA protein in serum, where levels well above the reference range were shown be indicative of increased amyloid load and organ dysfunction.[13] As with more conventional methods, Gertz and Kyle analyzed 64 patients with AA amyloidosis and demonstrated that renal dysfunction (serum creatinine value of >2 mg/dL) at presentation was strongly associated with shorter survival.[9] Other studies also confirmed that serum creatinine concentration is an important predictive factor for survival of patients with AA amyloidosis.[14,15,16,17] In our study, patients with serum creatinine of >2 mg/dL and albumin <3.5 mg/dL had shorter both renal and overall survival. In addition, we also found that older age, male gender, higher levels of phosphorus, iPTH, albuminuria and lower levels of serum albumin, calcium, hemoglobin were associated with poor prognosis. This is supported by the fact that serum creatinine level, stage of renal disease, short time to dialysis, dialysis requirement and low serum albumin were found as predictors of mortality in logistic regression analysis.
Reports on survival vary depending on whether they are early or new, with older studies had a median survival ranging from 24 to 31 months.[9,18,19] Median survival of patients with creatinine levels of at least 1.69 mg/dL (150 μmol/L) was found 18 months in are port from 1989.[20] However, overall survival in our study was better than older reports (mean: 88.7 ± 7.8 months). Furthermore, mean survival reached to nearly 10 years for patients who did not need dialysis.
Amyloidosis was associated with poor patient survival following dialysis and/or renal transplantation, reduced renal allograft survival and a significant incidence of disease recurrence in the allograft.[21] Survival rates on dialysis were slightly lower in our patients when compared to 1, 2 and 6 years survival rates of 72%, 62% and 44% reported by Martinez, 1 and 5 year survival rates of 68% and 30% reported by Moroni et al., respectively.[22,23] However, a study by Torregrosa showed that 1-year survival rate in dialysis was 42.7%, similar to our results.[24] Another report by Estevereveal even worse survival rates at 1 and 2 years, 30% and 5%, respectively.[25] However, in the analysis of studies by Moroni and Esteve the majority of patients had AA amyloidosis; they had also patients with primary (AL) amyloidosis included which could have altered the survival rates. The differences in survivals could also be due to socioeconomic factors, dialysis practices, extrarenal organ involvement, etc. Furthermore, underlying causes of AA amyloidosis may have an effect on survival, as our study showed that the difference in mortality between patients with FMF and tuberculosis was obvious in favor of FMF, although it did not reach a statistical significance due to a low event rate. We were not able to assess the effect of normalizing inflammation/high serum SAA, but both from a mechanistic standpoint and outcomes of a large series; one can understand that such an attempt should have a major role in prognosis.[5]
None of the patients in our study received renal transplantation as a mean of renal replacement therapy. Although experience with renal transplantation is limited, studies suggest that patient and graft survivals are similar in amyloidosis and matched control groups with other disorders.[26,27] In addition, posttransplant recurrence of amyloidosis was also low (1 in 23 patients) with colchicine treatment.[26] These findings should encourage clinicians to provide appropriate candidates with cadaveric or living donor transplantation, as morbidity and mortality with dialysis appear to be worse.
Similar to the features of our 31 patients with early mortality, Joss et al. reported that patients who commenced renal replacement therapy or died within 3 months of presentation were significantly older, suffered from more advanced renal disease, had more severe albuminuria and lower serum albumin levels.[28] In addition, we also found that these patients had higher phosphorus and iPTH, and lower calcium and hemoglobin levels.
Most common causes of mortality were renal failure (35%) and infections (8.5-46.6%) in previous series.[24,29,30] Either HD or PD can be used effectively for the treatment of ESRD due to amyloidosis, with the limiting factors being the degree of extra renal amyloid deposition, hypotension with HD, and peritonitis with continuous ambulatory peritoneal dialysis.[18] In the present study, most of the patients received HD treatment.
CONCLUSION REMARKS
Familial Mediterranean fever and tuberculosis are the most frequent underlying causes of AA amyloidosis. AA amyloidosis with renal involvement is progressive and carries a high risk of morbidity and mortality, particularly in those who had massive proteinuria, severe hypoalbuminemia and dialysis requirement at the outset. We suggest that careful evaluation of manifestations of amyloidosis may give the possibility of making earlier diagnosis, thereby prognosis could be improved.
AUTHORS’ CONTRIBUTIONS
EA: Data collection, literature review, design of the study, patient follow-up. EK: Design of the study, literature review, statistical analysis, patient follow-up.TS: Design of the study, literature review, review of manuscript, patient follow-up.TB: Patient follow-up, review of manuscript. YK: Patient follow-up. TS: Data collection, patient follow-up. MS: Data collection, patient follow-up. CA: Data collection, patient follow-up. ZAU: Data collection, patient follow-up. AOK: Data collection, patient follow-up. FB: Data collection, patient follow-up. AAO: Pathological assessment of all renal biopsies. AU: Responsible from the project and correcting author.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 2.George G. Glenner. Amyloid deposits and amyloidosis. N Engl J Med. 1980;302:1333–43. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Gertz MA. Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 4.Gertz MA, Lacy MQ, Dispenzieri A. Immunoglobulin light chain amyloidosis and the kidney. Kidney Int. 2002;61:1–9. doi: 10.1046/j.1523-1755.2002.00085.x. [DOI] [PubMed] [Google Scholar]
- 5.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361–71. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 6.Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17:3458–71. doi: 10.1681/ASN.2006050460. [DOI] [PubMed] [Google Scholar]
- 7.Tuglular S, Yalcinkaya F, Paydas S, Oner A, Utas C, Bozfakioglu S, et al. A retrospective analysis for aetiology and clinical findings of 287 secondary amyloidosis cases in Turkey. Nephrol Dial Transplant. 2002;17:2003–5. doi: 10.1093/ndt/17.11.2003. [DOI] [PubMed] [Google Scholar]
- 8.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40:1879–85. doi: 10.1002/art.1780401023. [DOI] [PubMed] [Google Scholar]
- 9.Gertz MA, Kyle RA. Secondary systemic amyloidosis: Response and survival in 64 patients. Medicine (Baltimore) 1991;70:246–56. [PubMed] [Google Scholar]
- 10.Obici L, Merlini G. AA amyloidosis: Basic knowledge, unmet needs and future treatments. Swiss Med Wkly. 2012;142:w13580. doi: 10.4414/smw.2012.13580. [DOI] [PubMed] [Google Scholar]
- 11.van der Hilst JC. Recent insights into the pathogenesis of type AA amyloidosis. Scientific World Journal. 2011;11:641–50. doi: 10.1100/tsw.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T, Higashi S, Tomoda K, Tsukano M, Baba S, Shono M. Significance of SAA1.3 allele genotype in Japanese patients with amyloidosis secondary to rheumatoid arthritis. Rheumatology (Oxford) 2006;45:43–9. doi: 10.1093/rheumatology/kei112. [DOI] [PubMed] [Google Scholar]
- 13.Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001;358:24–9. doi: 10.1016/S0140-6736(00)05252-1. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka F, Migita K, Honda S, Fukuda T, Mine M, Nakamura T, et al. Clinical outcome and survival of secondary (AA) amyloidosis. Clin Exp Rheumatol. 2003;21:343–6. [PubMed] [Google Scholar]
- 15.Bergesio F, Ciciani AM, Manganaro M, Palladini G, Santostefano M, Brugnano R, et al. Renal involvement in systemic amyloidosis: An Italian collaborative study on survival and renal outcome. Nephrol Dial Transplant. 2008;23:941–51. doi: 10.1093/ndt/gfm684. [DOI] [PubMed] [Google Scholar]
- 16.Sasatomi Y, Kiyoshi Y, Uesugi N, Hisano S, Takebayashi S. Prognosis of renal amyloidosis: A clinicopathological study using cluster analysis. Nephron. 2001;87:42–9. doi: 10.1159/000045883. [DOI] [PubMed] [Google Scholar]
- 17.Tiitinen S, Myllykangas R, Helin H, Kaarela K. Modern trends in the diagnosis of secondary amyloidosis. Scand J Rheumatol Suppl. 1987;67:30–1. doi: 10.3109/03009748809105291. [DOI] [PubMed] [Google Scholar]
- 18.Triger DR, Joekes AM. Renal amyloidosis — A fourteen-year follow-up. Q J Med. 1973;42:15–40. [PubMed] [Google Scholar]
- 19.Browning MJ, Banks RA, Tribe CR, Hollingworth P, Kingswood C, Mackenzie JC, et al. Ten years’ experience of an amyloid clinic — A clinicopathological survey. Q J Med. 1985;54:213–27. [PubMed] [Google Scholar]
- 20.Lindqvist B, Andersen S, Isacsson B, Lundberg E. The course of renal amyloidosis with uraemia. Int Urol Nephrol. 1989;21:555–9. doi: 10.1007/BF02549595. [DOI] [PubMed] [Google Scholar]
- 21.Tang W, McDonald SP, Hawley CM, Badve SV, Boudville N, Brown FG, et al. End-stage renal failure due to amyloidosis: Outcomes in 490 ANZDATA registry cases. Nephrol Dial Transplant. 2013;28:455–61. doi: 10.1093/ndt/gfs492. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Vea A, García C, Carreras M, Revert L, Oliver JA. End-stage renal disease in systemic amyloidosis: Clinical course and outcome on dialysis. Am J Nephrol. 1990;10:283–9. doi: 10.1159/000168121. [DOI] [PubMed] [Google Scholar]
- 23.Moroni G, Banfi G, Montoli A, Bucci A, Bertani T, Ravelli M, et al. Chronic dialysis in patients with systemic amyloidosis: The experience in northern Italy. Clin Nephrol. 1992;38:81–5. [PubMed] [Google Scholar]
- 24.Torregrosa E, Hernández-Jaras J, Calvo C, Ríus A, García-Pérez H, Maduell F, Vera JM. Amiloidosis secundaria (AA) y afectación renal. NEFROLOGÍ. 2003:XXIII. Número 4. [PubMed] [Google Scholar]
- 25.Esteve V, Almirall J, Ponz E, García N, Ribera L, Larrosa M, et al. Renal involvement in amyloidosis. Clinical outcomes, evolution and survival. Nefrologia. 2006;26:212–7. [PubMed] [Google Scholar]
- 26.Sherif AM, Refaie AF, Sobh MA, Mohamed NA, Sheashaa HA, Ghoneim MA. Long-term outcome of live donor kidney transplantation for renal amyloidosis. Am J Kidney Dis. 2003;42:370–5. doi: 10.1016/s0272-6386(03)00676-0. [DOI] [PubMed] [Google Scholar]
- 27.Pasternack A, Ahonen J, Kuhlbäck B. Renal transplantation in 45 patients with amyloidosis. Transplantation. 1986;42:598–601. doi: 10.1097/00007890-198612000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Joss N, McLaughlin K, Simpson K, Boulton-Jones JM. Presentation, survival and prognostic markers in AA amyloidosis. QJM. 2000;93:535–42. doi: 10.1093/qjmed/93.8.535. [DOI] [PubMed] [Google Scholar]
- 29.David J, Vouyiouka O, Ansell BM, Hall A, Woo P. Amyloidosis in juvenile chronic arthritis: A morbidity and mortality study. Clin Exp Rheumatol. 1993;11:85–90. [PubMed] [Google Scholar]
- 30.Ahlmen M, Ahlmen J, Svalander C, Bucht H. Cytotoxic drug treatment of reactive amyloidosis in rheumatoid arthritis with special reference to renal insufficiency. Clin Rheumatol. 1987;6:27–38. doi: 10.1007/BF02200997. [DOI] [PubMed] [Google Scholar]


