Abstract
Rationale: Persons with cystic fibrosis are at high risk of pulmonary nontuberculous mycobacterial infection, with a national prevalence estimated at 13%. The risk of nontuberculous mycobacteria associated with specific environmental exposures, and the correlation with climatic conditions in this population has not been described.
Objectives: To describe the association of pulmonary nontuberculous mycobacteria with individual exposures to water and soil aerosols, and the population associations of these infections with climatic factors.
Methods: We conducted a nested case–control study within a cohort study of pulmonary nontuberculous mycobacteria prevalence at 21 geographically diverse national cystic fibrosis centers. Incident nontuberculous mycobacterial infection cases (at least one prior negative culture followed by one positive culture) were age- and sex-matched to culture-negative controls. Exposures to water and soil were assessed by administering a standardized questionnaire. Cohort prevalence at each of the 21 centers was correlated with climatic conditions in the same area through linear regression modeling.
Measurements and Main Results: Overall, 48 cases and 85 control subjects were enrolled. Indoor swimming was associated with incident infection (adjusted odds ratio, 5.9, 95% confidence interval, 1.3–26.1), although only nine cases (19%) and five control subjects (6%) reported indoor swimming in the 4 months prior to infection. Exposure to showering and municipal water supply was common among both cases and control subjects: 77% of cases and 76% of control subjects reported showering at least daily. In linear regression, average annual atmospheric water vapor content was significantly predictive of center prevalence (P = 0.0019), with R2 = 0.40.
Conclusions: Atmospheric conditions explain more of the variation in disease prevalence than individual behaviors. The risk of specific exposures may vary by geographic region due to differences in conditions favoring mycobacterial growth and survival. However, because exposure to these organisms is ubiquitous and behaviors are similar among persons with and without pulmonary nontuberculous mycobacteria, genetic susceptibility beyond cystic fibrosis is likely to be important for disease development. Common individual risk factors in high-risk populations remain to be identified.
Keywords: nontuberculous mycobacteria, cystic fibrosis, epidemiology, environmental
The prevalence of pulmonary nontuberculous mycobacterial (PNTM) disease is increasing in the United States (1, 2), with marked regional variation in disease prevalence (1). Recent studies have identified both host and environmental factors associated with disease or disease clustering in populations (1, 3). Host features predisposing individuals to disease include chronic obstructive pulmonary disease and, among women, rheumatoid arthritis (1, 3) and thoracic skeletal abnormalities (4). Steroid use and immunomodulatory drug use have also been identified as risk factors for prevalent disease (3).
Persons with cystic fibrosis (CF) are known to be at increased risk for PNTM infection, with a prevalence estimated at approximately 13% in the United States (5). The role of individual behaviors that may increase the risk of exposures to nontuberculous mycobacterial infection (NTM) in the environment in this highly susceptible population has not been evaluated. Recent studies showing the frequent identification of mycobacteria in household showerheads (6), as well as studies linking household water system mycobacterial isolates to identical patient isolates (7), have raised considerable concern among persons susceptible to this infection. In a recent population-based case–control study of older adults (3), researchers examined the role of host and environmental risk factors in disease susceptibility and found very few water or soil exposures associated with prevalent disease. In contrast, several host factors, including immunosuppressive therapy, rheumatoid arthritis, and skeletal abnormalities (3), were significantly associated with an increased disease prevalence. To better understand the role of individual behaviors that might increase the risk of NTM infection through increased exposure to mycobacteria in water or soil, we designed a case–control study among persons with CF. In addition, we assessed the association of climatic factors on the prevalence of PNTM infection in CF populations. The geographic prevalence of NTM infection by CF center has been reported previously (5), but the association with climatic factors has not. Preliminary results from this study were previously reported in the form of an abstract (8).
Methods
Study Population
We conducted a nested case–control study to assess the risk of environmental factors on incident NTM infection as part of a larger case–cohort study of this disease among patients with CF at 21 geographically diverse U.S. cystic fibrosis centers. The findings from the case–cohort study have been described previously (5). As part of the initial study, incident NTM infection cases were defined as patients who had at least one prior negative culture followed by one positive culture. Cases were matched to either one or two culture-negative control subjects by age, sex, FEV1, and state. Cases and control subjects were followed for 15 months with a sputum test at each 3-month visit. Control subjects who became sputum-positive during the course of this follow-up period were reclassified as cases for purposes of the original analysis and were excluded from this case–control study of environmental risk factors.
Exposures to water and soil were assessed by administering a standardized questionnaire, with the 4 months prior to entry into the case cohort study used as the reference period. The 4-month period allowed us to ascertain exposures for case during the period in which their culture converted from negative to positive. In addition, this short window allowed ascertainment of usual exposures with a minimal recall bias. Detailed information on various types of exposures to water and soil was recorded. In addition, calculated NTM infection prevalence data and climatic data were obtained for each CF center. Climatic data were obtained from the weather station for the ZIP code nearest to the residence of the patient attending a given CF center. These data included humidity, vapor pressure, temperature, and precipitation. For a given center, climate data were averaged over the climate data corresponding to the ZIP codes of all patients attending that center. The protocol was approved by the institutional review boards at the participating sites (5).
Statistical Analysis
Conditional logistic regression in which we controlled for the matched case–control set was used to perform univariate analyses with all potential environmental exposures. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each exposure type. For variables with varying levels of exposure (e.g., bathing never, less than once per day, at least once per day), a model was fit using an ordinal variable to represent the levels of exposure. Any significant (P < 0.05) exposures were included in a multivariate model that was built using backward stepwise selection methods. The adjusted OR (ORa) was calculated for significant variables. For the climatic analysis, a linear model was fit to assess the correlation of climatic factors with center-level prevalence, as well as the significance of each climatic factor in predicting center prevalence. All statistical analyses were conducted using Statistical Analysis System (SAS) 9.1 software (SAS Institute, Cary, NC).
Results
Overall, 48 cases (36%) were studied together with 85 matched controls (64%). These case numbers included 10 “control” subjects who ultimately had a nontuberculous mycobacteria–positive sputum test during the follow-up period. These patients were reenrolled as “cases,” and their data as control subjects were excluded. The detailed types of exposures assessed are shown in Table 1. In univariate conditional logistic regression models, the only factors significantly associated with incident NTM infection were residence in a single-family home, tap water that appeared rusty or unclear, and ever swimming in an indoor pool. Jacuzzi use was associated with an elevated but nonsignificant risk (OR, 1.9; 95% CI, 0.9–4.3) (Table 1). After controlling for all of these factors in a multivariate conditional logistic regression model, the three named factors all remained significant. Cases were 5.9 times as likely as control subjects to report that they swam in an indoor pool (ORa, 5.9; 95% CI, 1.3–26.1) in the 4 months prior to sample collection. The rarity of this exposure (9% of cases and 5% of control subjects reported ever swimming) limited the power to assess a dose–response effect for this factor. Cases were also more likely to report residence in a single-family home versus other types of residences (ORa = 4.2; 95% CI = 1.4–13.4), as well as using tap water at home that was rusty or unclear (ORa, 3.5; 95% CI, 1.02–11.7). Most types of water exposures, such as showering or drinking tap water, were common and showed little variation between groups: 84–89% of cases and control subjects used water from a municipal supply, and 76–77% showered at least daily. Drinking tap water was also common, with >80% of both cases and control subjects reporting this behavior.
Table 1.
Individual exposures to potential sources of nontuberculous mycobacteria and their association with incident NTM infections
| Factor | Cases (N = 48), n (%) | Controls (N = 85), n (%) | Odds Ratio (95% CI)* | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Demographics | ||||
| Females | 28 (58) | 49 (58) | 1.6 (0.5–5.7) | |
| Residential exposure | ||||
| Single-family house | 39 (81) | 54 (64) | 2.7 (1.0–7.1) | 4.2 (1.4–13.4) |
| Mobile home | 0 (0) | 5 (6) | NA | |
| Multiple-unit dwelling (apartment, condominium, or dormitory) | 9 (19) | 24 (28) | 0.7 (0.3–2.0) | |
| Moved to a new residence | 6 (13) | 19 (22) | 0.5 (0.2–1.6) | |
| Water exposure | ||||
| Residence water supply | ||||
| Municipal supply | 40 (89) | 70 (84) | 1.4 (0.4–5.1) | |
| Artesian well water | 0 (0) | 2 (2) | NA | |
| Closed/pumped well water | 5 (11) | 11 (13) | 0.9 (0.2–3.7) | |
| Filtered home drinking water | 15 (31) | 16 (19) | 1.7 (0.8–3.9) | |
| Showering | 0.6 (0.2–1.9) | |||
| Never showered | 0 (0) | 0 (0) | ||
| Showered <1 time/d | 11 (23) | 19 (24) | ||
| Showered ≥1 time/d | 36 (77) | 61 (76) | ||
| Bathing | 1.2 (0.6–2.5) | |||
| Never took baths | 28 (58) | 57 (67) | ||
| Bathed <1 time/d | 17 (35) | 21 (25) | ||
| Bathed ≥1 time/d | 3 (6) | 7 (8) | ||
| Drinking water type | ||||
| Usually drank tap water | 30 (63) | 56 (67) | 1.0 (0.4–2.2) | |
| Usually drank bottled water | 17 (35) | 22 (26) | 1.4 (0.6–3.5) | |
| Drinking tap water | ||||
| Tap at home | 1.1 (0.6–1.8) | |||
| Never drank tap water at home | 9 (19) | 17 (20) | ||
| Drank tap water at home <3 times/d | 17 (35) | 26 (31) | ||
| Drank tap water at home ≥3 times/d | 22 (46) | 42 (49) | ||
| Tap outside home | 1.4 (0.8–2.4) | |||
| Never drank tap water outside home | 24 (50) | 47 (55) | ||
| Drank tap water outside home <3 times/d | 17 (35) | 28 (33) | ||
| Drank tap water outside home ≥3 times/d | 7 (15) | 10 (12) | ||
| Drinking bottled water | 1.2 (0.7–2.1) | |||
| Never drank bottled water | 27 (56) | 54 (64) | ||
| Drank <3 glasses/d bottled water | 9 (19) | 15 (18) | ||
| Drank ≥3 glasses/d bottled water | 12 (25) | 16 (19) | ||
| Brushing teeth | 0.5 (0.2–1.4) | |||
| Brushed teeth with warm tap water | 9 (19) | 22 (26) | ||
| Brushed teeth with cold tap water | 39 (81) | 63 (74) | ||
| Gargled with tap water | 18 (38) | 27 (32) | 1.1 (0.5–2.5) | |
| Plumbing work done at home | 6 (13) | 3 (4) | 4.1 (0.8–21.0) | |
| Tap water at home appeared rusty/unclear | 12 (25) | 13 (15) | 2.9 (1.0–8.9) | 3.5 (1.02–11.7) |
| Ingested water from pond, stream, or lake | 7 (15) | 9 (10) | 2.2 (0.5–8.9) | |
| Swimming | ||||
| Outdoor pools | 1.3 (0.7–2.4) | |||
| Never swam in outdoor pools | 32 (67) | 60 (71) | ||
| Swam in outdoor pool <1 time/wk | 10 (21) | 15 (18) | ||
| Swam in outdoor pool ≥1 time/wk | 6 (13) | 9 (11) | ||
| Ever swam in outdoor pools | 16 (33) | 24 (29) | 1.6 (0.7–3.7) | |
| Indoor pools | 1.8 (0.8–4.1) | |||
| Never swam in indoor pools | 39 (81) | 80 (94) | ||
| Swam in indoor pool <1 time/wk | 7 (15) | 2 (2) | ||
| Swam in indoor pool ≥1 time/week | 2 (4) | 3 (4) | ||
| Ever swam in indoor pools | 9 (19) | 5 (6) | 4.2 (1.1–16.1) | 5.9 (1.3–26.1) |
| Jacuzzis | 1.9 (0.9–4.3) | |||
| Never used Jacuzzis | 32 (71) | 67 (82) | ||
| Used Jacuzzis <1 time/wk | 8 (18) | 14 (17) | ||
| Swam in Jacuzzis ≥1 time/wk | 5 (11) | 1 (1) | ||
| Ever used Jacuzzis | 13 (29) | 15 (18) | 1.7 (0.6–4.5) | |
| Ponds, streams, lakes | 1.6 (0.7–3.4) | |||
| Never swam in ponds, streams, or lakes | 35 (80) | 70 (89) | ||
| Swam in ponds, streams, or lakes <1 time/wk | 6 (14) | 5 (6) | ||
| Swam in ponds, streams, or lakes ≥1 time/wk | 3 (7) | 4 (5) | ||
| Gulf, ocean | 1.2 (0.6–2.5) | |||
| Never swam in gulf or ocean | 35 (76) | 69 (83) | ||
| Swam in gulf or ocean <1 time/wk | 10 (22) | 10 (12) | ||
| Swam in gulf or ocean ≥1 time/wk | 1 (2) | 4 (5) | ||
| Soil exposure | ||||
| Planting or digging in soil | 11 (23) | 19 (23) | 0.7 (0.3–1.7) | |
| Watering | 14 (29) | 19 (23) | 1.4 (0.6–3.5) | |
| Weeding | 12 (25) | 12 (14) | 1.5 (0.6–4.0) | |
| Composting | 1 (2) | 2 (2) | 0.8 (0.1–8.9) | |
| Mowing grass, cutting hay | 1 (25) | 6 (60) | 1.0 (0.1–16.0) | |
| Vapor exposure | ||||
| Air conditioner type | ||||
| Central air | 19 (76) | 38 (78) | 1.1 (0.2–5.8) | |
| Casement (window) unit | 12 (44) | 19 (34) | 2.6 (0.5–14.3) | |
| Humidifier or vaporizer | ||||
| Cold, portable, or room | 6 (55) | 10 (42) | Undefined | |
| Hot, portable, or room | 2 (18) | 6 (25) | 0.4 (0.03–4.4) | |
| Supplemental oxygen with in-line humidifier | 3 (75) | 8 (42) | 2.6 (0.2–29.1) | |
| Nebulizer-related exposure | ||||
| Received inhaled or nebulized therapy (not MDI) | 40 (83) | 71 (84) | 0.7 (0.2–2.2) | |
| Nebulizer type | ||||
| Compressed air | 35 (97) | 65 (94) | 2.6 (0.2–29.1) | |
| Ultrasonic | 2 (6) | 10 (14) | 0.2 (0.02–1.5) | |
| Mixing inhaled therapy with fluids | ||||
| Sterile saline | 17 (35) | 38 (45) | 0.6 (0.3–1.3) | |
| Sterile water | 3 (17) | 8 (20) | 1.2 (0.2–8.6) | |
| Tap water, unboiled | 1 (6) | 0 (0) | Undefined | |
| Replacing nebulizer (compressed air) or medication cup (ultrasonic) | 1.6 (0.5–5.0) | |||
| Never replaced | 7 (18) | 14 (21) | ||
| Replaced <1 time/d | 28 (74) | 50 (75) | ||
| Replaced ≥1 time/d | 3 (8) | 3 (4) | ||
| Cleaning nebulizer | ||||
| Disposable parts | 1.2 (0.7–1.9) | |||
| Never cleaned | 8 (18) | 18 (22) | ||
| Cleaned disposable parts <1 time/d | 9 (20) | 21 (26) | ||
| Cleaned disposable parts ≥1 time/d | 28 (62) | 43 (52) | ||
| Nondisposable parts | 0.6 (0.3–1.1) | |||
| Never cleaned | 27 (63) | 40 (53) | ||
| Cleaned nondisposable parts <1 time/d | 14 (33) | 24 (32) | ||
| Cleaned nondisposable parts ≥1 time/d | 2 (5) | 11 (15) | ||
| Fluid used most often to clean nebulizer | ||||
| Sterile saline | 0 (0) | 1 (1) | Undefined | |
| Sterile water | 0 (0) | 1 (1) | Undefined | |
| Boiled bottled water | 1 (2) | 2 (2) | 2.4 (0.2–39.7) | |
| Unboiled bottled water | 1 (2) | 0 (0) | Undefined | |
| Boiled tap water | 4 (9) | 13 (15) | 0.5 (0.1–1.9) | |
| Unboiled tap water | 27 (61) | 50 (60) | 0.9 (0.4–2.4) | |
| Detergent or soap | 10 (23) | 19 (22) | 1.1 (0.5–2.7) | |
| Disinfecting solution | 40 (91) | 78 (94) | 0.9 (0.2–4.2) | |
| Vinegar | 13 (30) | 22 (26) | 1.1 (0.4–2.6) | |
| Allowed nebulizer to dry between uses | 35 (90) | 63 (90) | 1.1 (0.3–4.8) | |
| Other procedures | ||||
| Nasal washes | 11 (23) | 14 (17) | 1.5 (0.5–4.1) | |
| Bronchoscopy | 0 (0) | 0 (0) | NA |
Definition of abbreviations: CI = confidence interval; MDI = metered-dose inhaler; NA = not applicable; NTM = nontuberculous mycobacterial; OR = odds ratio.
For nonordinal variables, the reference group for any given category was all those who reported “no” for a particular category. For ordinal variables, the odds ratio was estimated from a fitted logistic regression model, with the odds corresponding to each level of increase. Proportions and odds ratios are reported only for those who had valid values (i.e., nonmissing) for the reported exposures.
With respect to soil exposure, a minority of cases and control subjects reported having any of the assessed outdoor soil exposures (Table 1). The most common activity was watering plants, reported by 29% of cases and 23% of control subjects, followed by planting or digging in the soil, reported by 23% of both cases and control subjects. Similarly, nebulizer exposure was quite similar between cases and control subjects, with 83% of cases and 84% of control subjects receiving inhaled or nebulizer therapy. Cleaning habits for these nebulizers were again quite comparable between cases and control subjects (Table 1).
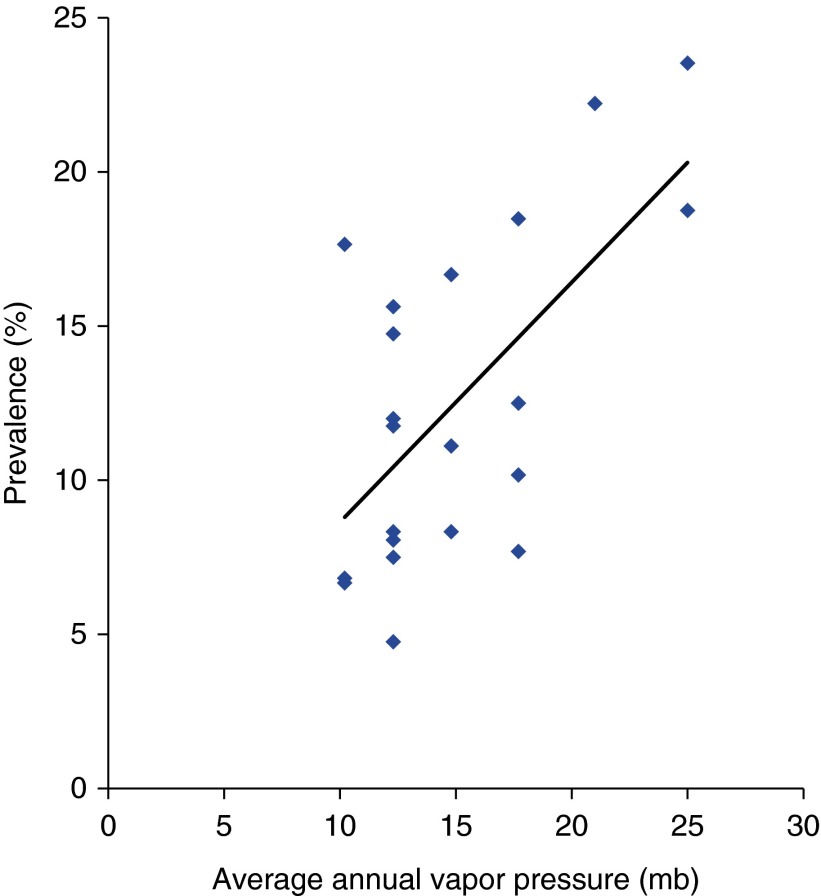
When center-level prevalence was correlated with measured climatic factors, average annual vapor pressure, which reflects atmospheric water vapor content at a specific temperature, was significantly predictive of center prevalence, with an R2 of 0.4 (Figure 1) (P < 0.002). Precipitation, temperature, and relative humidity were not predictive of center prevalence.
Figure 1.
The correlation of CF center prevalence with average annual atmospheric water vapor content (R2 = 0.4).
Discussion
We found very few individual behaviors associated with incident NTM infections. In contrast, we found a strong association of atmospheric water content within a geographic area with the incidence of NTM infections among patients in that area. With respect to individual behaviors, although a comprehensive list of behaviors was assessed for both cases and control subjects, the only factors found to be significantly associated with incident NTM infection were swimming in an indoor pool, living in a single-family residence, and reporting observations of rusty or unclean tap water from household plumbing. Although a minority of cases and control subjects reported swimming, this behavior has a strong biologic plausibility for increased aerosol exposure, given that mycobacteria are known to form biofilms and that the large surface area and closed environment of an indoor swimming pool are likely to facilitate exposure to high quantities of mycobacteria through aerosolization from biofilms.
Mycobacteria have been isolated from swimming pools (9) and from whirlpool therapy baths (10) and are known to be resistant to chlorine (11). Thus, susceptible persons have an increased risk of infection in such an environment. Residence in a single-family home is likely to be a surrogate measure for higher socioeconomic status, which has been shown to be associated with prevalent disease (1). The rusty or unclear appearance of water could be related to unmeasured plumbing factors, but, beyond that, no clear explanation is apparent. In addition, given the number of comparisons made, this factor could be a spurious finding. Because this was an exploratory study, we did not adjust for multiple comparisons.
Persons with CF, as well as other susceptible persons, likely have multiple exposures to nontuberculous mycobacteria, which limits the ability to attribute all infections to a single cause. In addition, identifying the risk associated with specific behaviors is limited by the inability to measure these behaviors precisely, both because persons may not recall their behaviors specifically and because the variation is minimal. Nonetheless, it is possible that patients can frequently be exposed to, and sometimes infected from, their household water system. Nontuberculous mycobacteria are common in municipal water supplies, with 35% of samples from 21 states found to test positive for nontuberculous mycobacteria (12). In one study, strains from the water system that were identical to those in the patient were found in 7 (41%) of 17 patients sampled. The only specific risk factor identified in that study was the temperature of the hot water heater, with higher temperatures conferring a reduced risk of nontuberculous mycobacteria isolation from the plumbing (7). Aerosolized water droplets are likely an important portal of entry into the airways by nontuberculous mycobacteria. However, because exposures to aerosolized water droplets are common and PNTM disease is rare, such exposure is likely a necessary but insufficient cause. More precise ascertainment of risk would need to include information related to intensity of exposure. For example, ascertainment of risk related to showering would need to include information such as shower duration, temperature of water in water heater, size of room, type of ventilation, and type of showerhead and sampling from the showerhead. Other studies of CF severity have identified “modifier” genes associated with more severe disease (13, 14); thus, it is not unreasonable to speculate that other genes exist that predispose individuals to NTM infection and disease.
We did not find soil exposure to be a risk factor in our study, although exposure to soil has been associated with risk of reactivity to Mycobacterium avium in a population-based study (15) conducted in Florida, a noted high-prevalence area (1), with a dose–response effect reported. That study found that persons who had participated in 6 or more years of a soil-related occupation, such as lawn and landscaping services or farming, were about three times more likely to be infected with M. avium, as measured by M. avium sensitin skin test, than study subjects who had never had a soil-related occupation, whereas water exposures such as showering or swimming were not associated with M. avium reactivity. The types of risk factors that are important in a given population depend in part on the prevalence of those factors in the population. In our study, soil-related behaviors were not very common, and the intensity of exposure was not measured.
In another recent population-based case–control study in a non-CF population, the overall findings were similar to ours: Most common individual exposures to aerosolized water or soil were not associated with an increased risk of disease. The only individual-level behavioral risk factors identified were using a spray bottle to spray plants (OR, 2.7). In contrast, frequent swimming pool use and washing dishes by hand were associated with a lower risk of disease. The authors in that study did not distinguish between indoor and outdoor swimming pool use, and they hypothesized that this behavior was protective because it was associated with better overall health (3).
The strong association with climatic variables is consistent with the known geographic distribution of NTM exposure and PNTM disease, first described in relation to sensitivity to purified protein derivative from Mycobacterium intracellulare (PPD-B) by Edwards and colleagues in their studies conducted among Navy recruits (16). These latter studies showed a concentration of PPD-B reactivity in the hotter, more humid areas of the Southeast among Navy recruits entering the service between 1958–1965 who had previously been lifetime residents of a single county (16). More recently, Adjemian and colleagues identified a higher prevalence of PNTM disease in the southeastern and western regions of the United States (1). In addition, in a separate analysis, PNTM disease clusters were identified in many of these same areas, including parts of California, Hawaii, Florida, and Louisiana (17). In this same study of spatial clustering of PNTM disease, using available data sources, the authors identified evapotranspiration and the proportion of the county that comprised surface water as risk factors for clustering of prevalent disease (17). These factors relate in turn to the ability of the atmosphere to remove water from surfaces.
In our present study, we did not measure those factors directly, but instead were able to measure average annual water vapor pressure, a more direct measure of the moisture content in the air. This factor was the one factor most strongly correlated with NTM infection prevalence among CF patients across the 21 CF centers. The consistency of these findings in different populations (CF patients and adults >65 years of age nationally) is striking and provides an environmental explanation for the previously observed higher sensitivity to M. intracellulare in hot, humid regions concentrated in the southeastern area of the United States. Surface waters are an important source of household water supplies in most urban areas, and different types of surface waters (and higher moisture content of the air) favor mycobacterial growth. In one of the few environmental studies in which different groundwater sources were sampled, recovery of M. avium and M. intracellulare was highest in the acidic, brown-water swamps of the southeastern coastal plain and was correlated with warmer temperature and low pH (18). Atmospheric water vapor absorbed from this environment is also very likely to contain high concentrations of mycobacteria. The only other study in which researchers evaluated the role of climate in disease risk among patients with CF found that the presence of Pseudomonas aeruginosa was associated with warmer annual ambient temperatures (19), suggesting that climatic factors may play a role in disease risk for other pathogens as well in this population.
A strength of the present study is that incident infections were measured, which enabled us to relate exposures in the 4-month period prior to mycobacteria sampling to conversion by the time of sampling. Because the incubation period for PNTM disease is not clear, it has been difficult to precisely relate specific exposures to subsequent infection and disease. Thus, because cases were likely to represent incident NTM infections, this clustering represents more recent infection in the area of residence at the time of the study rather than infection in some remote past. However, one limitation of this approach should be acknowledged. Because only a single negative culture prior to the first positive was required for an enrolled subject to be counted as a case, due to sampling variability, some of those persons classified as incident cases may have had a culture at some time in the past. For this reason, some of the incident infections detected could represent prevalent infections acquired previously. Furthermore, because most (70%) patients with CF with NTM infections had only a single positive out of three cultures (5), the numbers were too small to study outcomes limited to those who had more than one positive. In addition, the small sample size overall limited the statistical power to find significant associations, particularly those less frequent exposures such as Jacuzzi use. Finally, an additional strength of this study is that it was conducted prior to the widespread use of macrolides among patients with CF (20), such that replication of this type of study in the future would be quite difficult.
The few risk factors identified that are associated with individual behaviors, as well as the strong risk associated with the ambient vapor pressure, likely relate to both measurement issues and appropriate ways of measuring risk. Vapor pressure is a climatic factor that can be measured very precisely and likely has a more direct relationship with mycobacterial growth. In contrast, individual behaviors are more difficult to measure because persons have to recall their behavior over a given time period and also because a given behavior may have a varied risk level, depending on the environment in which this risk occurs. In addition, because host susceptibility plays such an important role in disease development, the relevance of an individual behavior interacts with host susceptibility as well. Other than adjusting the temperature of hot water heaters (7) and reducing exposure to indoor swimming pools, specific protective behaviors have not yet been identified.
Updated guidelines for infection prevention and control from the Cystic Fibrosis Foundation include a recommendation that persons with CF avoid whirlpools and hot tub spas (21), which is based on findings regarding Pseudomonas in these settings. However, with respect to swimming pools, the updated Cystic Fibrosis Foundation guidelines permit swimming in pools with adequate disinfection. In a recent study of aerosols and water samples from therapy pools and hot tubs, researchers found high levels of NTM infections in these settings, but they also reported that concentrations can be reduced through halogen (chlorine or bromide) disinfection (22). Future research should be focused on identifying other modifiable high-risk behaviors or conditions that could reduce the risk of exposure or reinfection among persons already known to be at high risk for NTM infection.
Acknowledgments
Acknowledgment
The authors thank Dr. Andrew Comrie for his guidance on understanding climatic factors and Dr. Pamela A. Shaw for her guidance on data analysis. They also like to thank Ruben Montes de Oca for his support with data management.
Footnotes
Supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health; and the Cystic Fibrosis Foundation (CFF A914).
Author Contributions: K.N.O.: study concept and design; acquisition of data. D.R.P., J.A., K.N.O., A.G.F.: analysis and interpretation of data. D.R.P., J.A., K.N.O., A.G.F.: drafting of the manuscript. D.R.P., J.A., K.N.O., M.R.K.: critical revision of the manuscript for important intellectual content. J.A., D.R.P.: statistical analysis. K.N.O., D.R.P.: study supervision.
Originally Published in Press as DOI: 10.1513/AnnalsATS.201404-184OC on July 28, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirac MA, Horan KL, Doody DR, Meschke JS, Park DR, Jackson LA, Weiss NS, Winthrop KL, Cangelosi GA. Environment or host? A case-control study of risk factors for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:684–691. doi: 10.1164/rccm.201205-0825OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Steagall WK, Glasgow CG, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivier KN, Weber DJ, Wallace RJ, Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, et al. Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 6.Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA. 2009;106:16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falkinham JO., III Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis. 2011;17:419–424. doi: 10.3201/eid1703.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevots DR, Adjemian J, Fernandez AG, Knowles M, Olivier KN. Environmental exposures to nontuberculous mycobacteria among persons with cystic fibrosis: the risk of individual exposures and the association of climatic factors in disease prevalence [abstract] Am J Respir Crit Care Med. 2010;181.1:A2604. [Google Scholar]
- 9.Dailloux M, Hartemann P, Beurey J. Study on the relationship between isolation of mycobacteria and classical microbiological and chemical indicators of water quality in swimming pools. Zentralbl Bakteriol Mikrobiol Hyg [B] 1980;171:473–486. [PubMed] [Google Scholar]
- 10.Havelaar AH, Berwald LG, Groothuis DG, Baas JG. Mycobacteria in semi-public swimming-pools and whirlpools. Zentralbl Bakteriol Mikrobiol Hyg [B] 1985;180:505–514. [PubMed] [Google Scholar]
- 11.Taylor RH, Falkinham JO, III, Norton CD, LeChevallier MW. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl Environ Microbiol. 2000;66:1702–1705. doi: 10.1128/aem.66.4.1702-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covert TC, Rodgers MR, Reyes AL, Stelma GN., Jr Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol. 1999;65:2492–2496. doi: 10.1128/aem.65.6.2492-2496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, et al. Gene Modifier Study Group. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353:1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 14.Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci. 2010;1214:57–69. doi: 10.1111/j.1749-6632.2010.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed C, von Reyn CF, Chamblee S, Ellerbrock TV, Johnson JW, Marsh BJ, Johnson LS, Trenschel RJ, Horsburgh CR., Jr Environmental risk factors for infection with Mycobacterium avium complex. Am J Epidemiol. 2006;164:32–40. doi: 10.1093/aje/kwj159. [DOI] [PubMed] [Google Scholar]
- 16.Edwards LB, Acquaviva FA, Livesay VT, Cross FW, Palmer CE. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis. 1969;99(4 Suppl):12–14. [PubMed] [Google Scholar]
- 17.Adjemian J, Olivier KN, Seitz AE, Falkinham JO, III, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186:553–558. doi: 10.1164/rccm.201205-0913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschner RA, Jr, Parker BC, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria: Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. Am Rev Respir Dis. 1992;145:271–275. doi: 10.1164/ajrccm/145.2_Pt_1.271. [DOI] [PubMed] [Google Scholar]
- 19.Collaco JM, McGready J, Green DM, Naughton KM, Watson CP, Shields T, Bell SC, Wainwright CE, Cutting GR ACFBAL Study Group. Effect of temperature on cystic fibrosis lung disease and infections: a replicated cohort study. PLoS ONE. 2011;6:e27784. doi: 10.1371/journal.pone.0027784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cystic Fibrosis Trust Antibiotic treatment for cystic fibrosis: report of the UK Cystic Fibrosis Trust Antibiotic Group, 3rd edition.Bromley, UK: UK Cystic Fibrosis Trust; May 2009. Available from: https://www.cysticfibrosis.org.uk/media/82010/CD_Antibiotic_treatment_for_CF_May_09.pdf [Google Scholar]
- 21.Saiman L, Siegel JD, LiPuma JJ, Brown RJ, Bryson EA, Chambers MJ, Downer VS, Fliege J, Hazle LA, et al. Infection Prevention and Control Guideline for Cystic Fibrosis: 2013 update. Infect Control Hosp Epidemiol. 2014;35(Suppl 1):S1–S67. doi: 10.1086/676882. [DOI] [PubMed] [Google Scholar]
- 22.Glazer CS, Martyny JW, Lee B, Sanchez TL, Sells TM, Newman LS, Murphy J, Heifets L, Rose CS. Nontuberculous mycobacteria in aerosol droplets and bulk water samples from therapy pools and hot tubs. J Occup Environ Hyg. 2007;4:831–840. doi: 10.1080/15459620701634403. [DOI] [PubMed] [Google Scholar]