Abstract
Fasting glucose and hemoglobin A1c (HbA1c) are the standard measures for diagnosis and monitoring of diabetes. There has been recent interest in nontraditional markers of hyperglycemia, including fructosamine, glycated albumin and 1,5-anhydroglucitol (1,5-AG), as alternatives or adjuncts to standard measures. There is a growing literature linking these nontraditional markers with microvascular and macrovascular complications. Fructosamine and glycated albumin have also been shown to improve identification of persons with diabetes. However, long-term prospective studies with clinical outcomes are lacking. Some modern laboratory assays for fructosamine, glycated albumin and 1,5-AG have excellent performance. Expanded use of these tests has the potential to improve diabetes care as these measures may overcome limitations of HbA1c in certain patients, complement traditional measures by providing additional information on shorter-term glycemic control, and improve risk stratification for diabetes and its complications. Nonetheless, studies are needed to demonstrate if their routine use will benefit patients and improve outcomes.
Keywords: diabetes; fasting glucose; hemoglobin A1c; fructosamine; glycated albumin; 1,5-anhydroglucitol; hyperglycemia; biomarkers
Introduction
Hemoglobin A1c (HbA1c) has long been the standard measure used to monitor glycemic control in clinical practice and is routinely measured in all persons with diabetes. In addition to fasting glucose and 2-hour glucose, the American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), the World Health Organization (WHO), and other diabetes organizations now recommend the use of HbA1c for diagnosis of diabetes.1–6 First recommended in 2009, the addition of HbA1c to diagnostic criteria for diabetes has been controversial, largely attributable to limitations of the HbA1c test.7–9 There is growing interest in serum biomarkers of hyperglycemia, including fructosamine, glycated albumin, and 1,5-anhydroglucitol (1,5-AG), to be used as alternatives to or in conjunction with traditional measures10–13 These markers can overcome limitations of HbA1c in certain patients, could complement traditional measures in the clinic by providing additional information on shorter-term glycemic control, and may improve risk stratification for diabetes and its complications.
Markers of hyperglycemia
Traditional markers of hyperglycemia
Diabetes is a condition defined by elevations in glucose. Historically, glucose measured in the fasting state or glucose measured two hours after a carbohydrate challenge (oral glucose tolerance test) have been the standard measures used to diagnose diabetes and identify people at risk for diabetes (frequently termed “prediabetes”). HbA1c has been used widely since the 1980s and is the standard measure used for monitoring glycemic control in clinical practice.5 In red blood cells, HbA1c is hemoglobin that has glucose attached to the N-terminal valine of the beta chain and is reported as the proportion of total hemoglobin. Because the lifespan of red blood cells is approximately 120 days, HbA1c therefore reflects average glycemia over the past two to three months (since it is weighted towards the more recent months).13 Advantages of HbA1c include the lack of participant preparation (fasting is not necessary); high within-person reliability;14,15 and excellent standardization of the assay in most countries.16–18 Nonetheless, disadvantages of HbA1c include limited interpretability in the setting of altered red blood cell lifespan—levels are affected by changes in duration of red blood cell exposure to circulating blood glucose levels—and interference of some HbA1c assays by hemoglobin variants and several rare conditions (Table 1).7,19 These disadvantages have brought into focus possible roles for nontraditional glycemic markers in the clinic.
Table 1.
Characteristics of traditional and nontraditional markers of hyperglycemia
| Brief Description | Duration of glycemia reflected | Strengths | Limitations | |
|---|---|---|---|---|
| Traditional markers of hyperglycemia | ||||
| Fasting glucose | Direct measure of circulating blood glucose | Acute/immediate | Direct measure Widely accepted Inexpensive | Requires fasting; affected by acute illness and stress; pre-analytical issues (sample stability)*; moderate within-person variability |
| HbA1c | Proportion of hemoglobin that is glycated | 2–3 months | Reflects 2–3 month control Low within-person variability; no patient preparation needed; not affected by acute illness, stress or recent activity levels | Affected by alterations in red cell turnover; some methods for measurement can give inaccurate results in the presence of certain hemoglobin variants**; requires whole blood; cost |
| Nontraditional markers of hyperglycemia | ||||
| Fructosamine | Total serum protein glycation | 2–3 weeks | Does not require fasting; highly reliable automated methods are widely available; can be measured in serum or plasma; inexpensive | Affected by changes in serum protein metabolism (mostly albumin), thyroid dysfunction; limited evidence linking to outcomes |
| Glycated albumin | Proportion of albumin that is glycated | 2–3 weeks | Does not require fasting; can be measured in serum or plasma | Affected by changes in albumin metabolism, thyroid dysfunction; method performance may vary; availability in the US is limited; limited evidence linking to outcomes |
| 1,5-AG | Monosaccharide filtered by the kidney and normally reabsorbed; reabsorption inhibited and it is excreted at high levels of glycemia, so serum levels drop | 2–14 days | Does not require fasting; can be measured in serum or plasma; test is available from major laboratories in the US; expense | Affected by changes in renal threshold for glucose, dialysis or stage 4 or 5 kidney disease, pregnancy; limited evidence linking to outcomes |
See: Gambino R. Clin Chem. 2007 Dec;53(12):2040-1.
See: www.ngsp.org for comprehensive list
Nontraditional markers of hyperglycemia
Fructosamine and glycated albumin are both ketoamines, which are formed as the result of a non-enzymatic process that binds glucose to serum proteins. In states of abnormally high glucose concentrations, as in persons with diabetes, serum proteins are exposed to greater concentrations of glucose and therefore experience increased glycation.20 Fructosamine assays measure total glycated serumprotein (mostly albumin, but also immunoglobulins and other circulating proteins), whereas glycated albumin is reported as the proportion of total albumin. The half-life of albumin and other serum proteins is shorter than that of red blood cells; thus measurements of fructosamine and glycated albumin reflect average glycemia over a shorter duration, approximately two to three weeks.20
1,5-AG is a 6-carbon monosaccharide obtained mainly from dietary sources, that reflects average glycemia over approximately the past 2–14 days.21–25 In states of normal glycemia, nearly 100% of 1,5-AG is reabsorbed by the renal tubule. However, at very high levels of glycemia (above the renal threshold, ~160–180 mg/dl), glucose competes with 1,5-AG for reabsorption by the renal tubule, and 1,5-AG is excreted in the urine, resulting in a drop in circulating 1,5-AG levels in the blood. Therefore, there is an inverse association between high levels of glucose and 1,5-AG21. Soybeans have particularly high levels of 1,5-AG, and certain foods such as rice, bread and beef contain modest levels; it is unclear to what extent dietary intake may affect circulating 1,5-AG levels and the interpretation of this test.21,22
Correlations of traditional markers of hyperglycemia with fructosamine, glycated albumin, and 1,5-anhydroglucitol
Fructosamine and glycated albumin are strongly associated with HbA1c and fasting glucose,26–30 and all four measures have been shown to be similarly correlated with mean glucose from continuous glucose monitoring over about 5 days in persons with diabetes.31 In settings where HbA1c testing is known to be problematic, fructosamine or glycated albumin may be a useful substitute. A difficulty, however, is that there are no established clinical cut-points and these assays are not standardized across instruments. Conversion equations can help estimate the ranges of fructosamine and glycated albumin test results that are similar to HbA1c targets. Various equations have been developed to convert fructosamine and glycated albumin to an “HbA1c equivalent”. For example, previous reports demonstrated that glycated albumin values in the range of 16% to 22%,27,32–34 and fructosamine levels around 312 μmol/L as reported by one study,27 are approximately equivalent to an HbA1c value of 7%. 1,5-AG is strongly inversely associated with HbA1c and fasting glucose in persons with diagnosed diabetes,27 but appropriate clinical targets are unclear. It should be noted that 1,5-AG is poorly correlated with fasting glucose and HbA1c in persons without diagnosed diabetes--the strongest correlations are observed at the highest glucose concentrations.27 (additionally cite Selvin in press) This suggests the utility of 1,5-AG may primarily be limited to persons with overtly elevated glucose.
Since these markers of hyperglycemia are measured on different scales, both clinicians and patients may benefit from being provided with equivalents. However, conversion equations for nontraditional glycemic markers have typically relied on single measurements (which may vary considerably over time, particularly in diabetic patients) and may differ depending on the underlying population from which they are derived, with uncertain generalizability. Furthermore, none of these markers are perfectly correlated, a function of differences in the physiology of each biomarker including the duration of glycemia reflected and other sources of biological and analytical variability. In fact, the discordance across traditional and nontraditional glycemic markers may suggest the complementary nature of these biomarkers. A benefit to the use of multiple measures is that they may each provide unique insight into different aspects of hyperglycemia and diabetes physiology.
Associations of nontraditional markers of hyperglycemia with complications
Cross-sectional studies
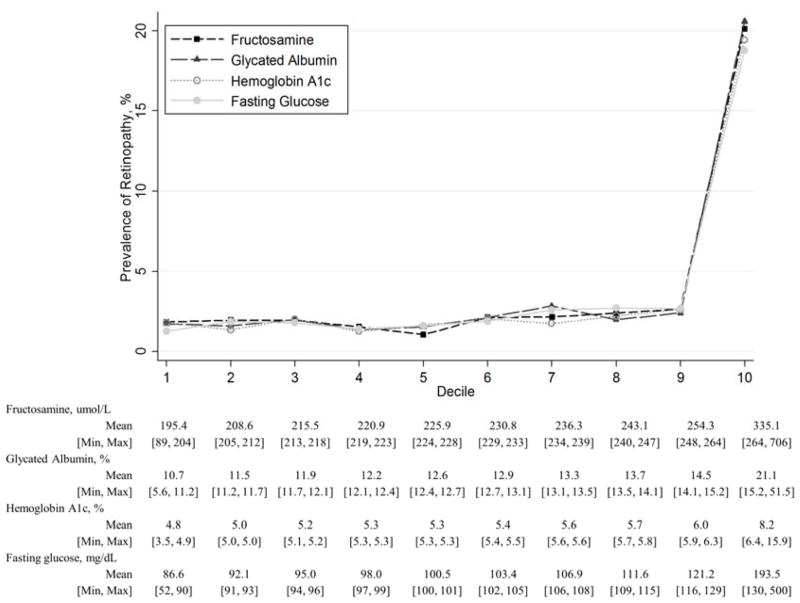
Cross-sectional studies have linked nontraditional markers of hyperglycemia with both microvascular and macrovascular complications. Fructosamine and glycated albumin have both been linked to prevalent retinopathy.35–37 In a recent analysis of 12,306 persons (958 with diabetes) in the Atherosclerosis Risk in Communities (ARIC) Study, we found an independent association of glycated albumin and fructosamine with retinopathy, with patterns of association very similar to those observed for HbA1c (Figure).29 In a Japanese cohort of more than 2,500 participants, the performance of glycated albumin and 1,5-AG to identify cases of retinopathy—as measured by the C-statistic—was shown to be comparable to fasting glucose and HbA1c.38 In a study of 1,575 Japanese adults without diagnosed diabetes, glycated albumin was associated with carotid artery intima-media thickness, a measure of subclinical atherosclerosis.39 Glycated albumin has been associated with prevalent kidney outcomes,40–42 and cardiovascular disease.42–49 Few studies have assessed the relationship of 1,5-AG to complications, although lower 1,5-AG concentrations have been linked to both prevalent coronary heart disease 50 and retinopathy51 in persons with diabetes. 1,5-AG has also been associated with measures of atherosclerosis and cardiovascular disease in a population without a history of diabetes.52
Figure.
Prevalence of retinopathy by deciles of fructosamine, glycated albumin, HbA1c, an fasting glucose, the Atherosclerosis Risk in Communities Study, N=9,445
Source: Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279–288. doi:10.1016/S2213-8587(13)70199-2.
Prospective studies
Limited evidence from prospective studies suggests nontraditional markers may be useful for identification of persons at risk of developing microvascular and macrovascular complications. In addition to the associations with retinopathy in the above-mentioned ARIC Study, we found that both fructosamine and glycated albumin strongly predicted incident chronic kidney disease (CKD) over two decades of follow-up. The observed associations of fructosamine and glycated albumin with incident CKD were of similar magnitude to those observed for HbA1c.29 Analyses conducted in the DCCT/EDIC study of persons with type 1 diabetes also reported that glycated albumin was similarly associated with retinopathy and nephropathy as compared to HbA1c.28 Additionally, in an analysis of 84 persons with type 1 diabetes from the Wisconsin Diabetes Registry Study, fructosamine was associated with incident retinopathy.53 By contrast, in a Brazilian cohort of persons with diabetes, fasting glucose was associated with microvascular outcomes over about 5 years of follow-up, but fructosamine was not.54 In a recent prospective study in 2,095 Japanese persons (including approximately 100 with diabetes), 1,5-AG was associated with incident cardiovascular events during 11 years of follow-up.55
Clinical utility of nontraditional markers of hyperglycemia
For monitoring of short-term glycemic control
Nontraditional markers of hyperglycemia are not formally incorporated into clinical guidelines in the United States. However, various organizations in multiple countries, including the US, India, Australia and the United Kingdom, have suggested fructosamine as a useful alternative to HbA1c for monitoring glycemic control in persons with conditions that may interfere with the interpretation of the HbA1c test.11,12,56–61 Glycated albumin is used frequently in China, Japan and South Korea for monitoring intermediate glycemic control.62 Several assays have been developed to measure glycated albumin but the assays are not standardized, and therefore not necessarily equivalent. Some early studies raised serious concerns regarding the validity and reliability of fructosamine assays63, although second-generation assays had improved technical performance.64 Modern automated assays for fructosamine have shown high correlations with glucose and HbA1c, strong prognostic value, and very low CVs (approximately 3% in recent studies)29,31,65.
Whereas HbA1c reflects long-term, 2–3 month glycemic control, fructosamine and glycated albumin reflect hyperglycemia over the past 2 to 3 weeks. Thus, both have been proposed as useful markers of intermediate glycemic control. In clinical practice, HbA1c is typically measured at minimum every 6 months and more frequently (quarterly) in persons with recent therapy changes who are not meeting treatment goals.1,66
Fructosamine and glycated albumin may be quite useful to evaluate earlier response to changes in treatment. Glycated albumin has been shown to change faster than HbA1c in response to changes in medication or exercise.67,68 Compared to HbA1c, glycated albumin is more strongly correlated with continuous glucose measurements over 1 to 2 days, 69,70 and may more accurately reflect long-term glycemic variability and glucose excursions.71,72
1,5-AG is thought to reflect hyperglycemia over the past 2 weeks and is recommended by the manufacturer for use in persons with diabetes and HbA1c <8% to help identify patients with frequent hyperglycemic excursions.73,74 Indeed, 1,5-AG has been shown to be correlated with postprandial hyperglycemia in persons with diabetes and HbA1c <7%;75 and to be more strongly correlated with glucose variability as compared to HbA1c, fructosamine or glycated albumin over 2 to 3 days in persons with moderate glycemic control (HbA1c <8%).76,77
For diabetes screening or diagnosis
There is evidence that nontraditional markers of hyperglycemia may help to more accurately identify persons with diabetes. In several studies, fructosamine and glycated albumin had similar performance for the identification of persons with diabetes as compared to either fasting glucose or HbA1c.27,30,78–80 Furthermore, compared to using either test individually, sensitivity to identify cases of diabetes defined by 2-hour glucose was improved when glycated albumin was used in combination with either fasting glucose or HbA1c.80,81
A large proportion of persons identified as having pre-diabetes do not go onto develop diabetes, highlighting the need for strategies that will accurately identify persons who will progress to overt diabetes.82 It is possible that fructosamine or glycated albumin may be useful in early identification of high-risk persons. Recent studies have shown that both fructosamine and glycated albumin are associated with future risk of diabetes, independent of fasting glucose and HbA1c.29,83 1,5-AG has also been associated with future development of diabetes, but observed associations were lower in magnitude as compared to other markers of hyperglycemia and were not present in persons with fasting glucose or HbA1c in the non-diabetic range.83 Nonetheless, the evidence linking nontraditional biomarkers with future diabetes risk is sparse.
Utility of nontraditional markers in special populations
A focus in the literature has been the potential utility of fructosamine or glycated albumin for monitoring glycemic control in the setting of certain populations where HbA1c is thought to inaccurately reflect glycemia, including severe kidney disease.84 Recent studies have shown that, compared to HbA1c, glycated albumin is more strongly correlated with glucose in dialysis patients.85–92 Fructosamine and glycated albumin may also be useful for prediction of complications in persons with kidney failure. Indeed, fructosamine and glycated albumin have been both cross-sectionally and prospectively associated with microvascular, macrovascular and all-cause morbidity and mortality in dialysis patients, whereas many studies have reported no association of HbA1c with these outcomes.65,93–100 Nonetheless, despite their associations with clinical outcomes, fructosamine and glycated albumin may also be limited in this setting, since proteinuria and altered serum protein turnover may affect interpretation of these tests.101–103
1,5-AG has not been well studied in the setting of chronic kidney disease or dialysis. Because lowered plasma concentrations of 1,5-AG result from accelerated urine excretion due to competitive inhibition of glucose by the renal tubules, 1,5-AG may have a problematic interpretation in the setting of reduced kidney function. 1,5-AG was correlated with fasting glucose and HbA1c in persons with diabetes and mild to moderate CKD, but not in those with end stage renal disease (ESRD) (stages 4–5 CKD).104
There is also evidence to support the use of nontraditional markers of hyperglycemia in persons with other conditions that may decrease the lifespan of red blood cells. Fructosamine and glycated albumin have been shown to better reflect glucose levels in the setting of anemia, autologous blood donations and HIV, which may all result in artificially low HbA1c.105–108 There is also interest in whether fructosamine, glycated albumin, or 1,5-AG testing may play a role in the management of diabetes in patients with liver disease, but evidence for their performance in this setting is inconsistent.109–111 Furthermore, during pregnancy, glycated albumin may better reflect average glucose compared to HbA1c, which may be artificially elevated due to iron deficiency.112,113 Furthermore, measures of shorter-term glycemia may be especially important in gestational diabetes given the importance of frequent monitoring and strong associations between diabetes control in pregnancy and maternal and fetal outcomes.1,114
Conclusions
Nontraditional markers of hyperglycemia, fructosamine, glycated albumin, or 1,5-AG, may be useful for monitoring of glycemic control when short-term changes are of interest or as alternatives to HbA1c in settings in which HbA1c may be problematic. Fructosamine and glycated albumin may also aid in early identification of persons at future risk for diabetes. In clinical or epidemiologic studies where fasting glucose or HbA1c measurements are not available but where serum or plasma specimens were collected, fructosamine or glycated albumin may be particularly useful to identify persons with undiagnosed hyperglycemia. Furthermore, in resource-intensive randomized clinical trials of short duration (<6 months), fructosamine and glycated albumin may be useful to evaluate responses to glucose-lowering interventions. Additionally, the complementary nature of these different tests of hyperglycemia warrants exploration into the potential utility of fructosamine, glycated albumin, and/or 1,5-AG in the development of risk prediction models for diabetes and its complications.
Additional studies of fructosamine, glycated albumin and 1,5-AG could help address uncertainty in this area. First, prospective associations of these three nontraditional glycemic markers with clinical complications are largely uncharacterized. Large epidemiologic population-based cohort studies are needed to fully characterize long-term risk associations and to better establish the prognostic value of these biomarkers. Such studies would inform relevant clinical cut-points, performance of these markers for risk stratification, and comparative predictive ability. Second, clinical studies with repeat assessments of glucose and HbA1c and those involving continuous glucose monitoring studies are needed to rigorously characterize associations with average glucose in persons with type 1 and type 2 diabetes. Such studies may help establish construct validity and utility of nontraditional markers for monitoring glycemic control. Finally, randomized clinical trials can determine whether use of these tests can improve care and outcomes for persons with diabetes. It is possible that one or more of these biomarkers may be an efficient and appropriate alternative to HbA1c in some patients and strategies that combine multiple tests for glycemia may be beneficial in certain settings.
Acknowledgments
C.M. Parrinello is supported by NIH/NHLBI Cardiovascular Epidemiology training grant T32HL007024. E. Selvin is supported by NIH/NIDDK grant R01DK089174. The authors thank Dr. David B. Sacks (Department of Laboratory Medicine, National Institutes of Health) for his thoughtful review of a draft of this manuscript.
References
**Of major importance
*Of importance
- 1.Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 (Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.International A, Committee E, Diabe- A, Federation ID, Committee IE, International T. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboratio. Eur Heart J. 2013;34(39):3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 4.Consultation IDF. [Accessed March 29, 2014];WHO IRIS: Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. 2006 Available at: http://apps.who.int/iris/handle/10665/43588.
- 5.World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. 2011. pp. 1–25. [PubMed] [Google Scholar]
- 6.International Diabetes Federation Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. 2012:1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [Google Scholar]
- 7.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518–23. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inzucchi SE. Clinical practice. Diagnosis of diabetes. N Engl J Med. 2012;367(6):542–50. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 9*.Sacks DB. Hemoglobin A1c in diabetes: panacea or pointless? Diabetes. 2013;62(1):41–3. doi: 10.2337/db12-1485. This commentary discusses the current clinical utility of HbA1c, as well as its limitations. The author mentions fructosamine and glycated albuminas potential alternatives to HbA1c, however also notes the need for additional studies since there is a lack of data linking these markers to clinical outcomes from clinical trials and prospective studies, as well as no established clinical cut-points for use in persons with diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.True MW. Circulating biomarkers of glycemia in diabetes management and implications for personalized medicine. J Diabetes Sci Technol. 2009;3(4):743–7. doi: 10.1177/193229680900300421. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2769973&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [Accessed June 20, 2014];Johns Hopkins POC-IT Guides: Alternative markers of glycemia: fructosamine, glycated albumin, 1,5-AG. Available at: http://www.hopkinsguides.com/hopkins/ub/view/Johns_Hopkins_Diabetes_Guide/547055/all/Alternative_markers_of_glycemia:_fructosamine__glycated_albumin__1_5_AG.
- 12.Joslin Diabetes Center. [Accessed June 20, 2014];Home Blood Glucose (Sugar) Monitoring, Hemoglobin A1C Testing, and Fructosamine Tests. Available at: http://www.joslin.org/info/home_blood_glucose_sugar_monitoring_hemoglobin_a1c_testing_and_fructosamine_tests.html.
- 13.Goldstein DE, Little RR, Lorenz RA, et al. Tests of Glycemia in Diabetes. Diabetes Care. 2004;27(7):1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–51. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 15.Meigs JB, Nathan DM, Cupples LA, Wilson PWF, Singer DE. Tracking of glycated hemoglobin in the original cohort of the framingham heart study. J Clin Epidemiol. 1996;49(4):411–417. doi: 10.1016/0895-4356(95)00513-7. [DOI] [PubMed] [Google Scholar]
- 16.Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418:63–71. doi: 10.1016/j.cca.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little RR, Rohlfing CL, Sacks DB. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205–14. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 18.Rohlfing CL, Parvin Ca, Sacks DB, Little RR. Comparing analytic performance criteria: Evaluation of HbA1c certification criteria as an example. Clin Chim Acta. 2014;433:259–63. doi: 10.1016/j.cca.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacks DB, John WG. Interpretation of hemoglobin a1c values. JAMA. 2014;311(22):2271–2. doi: 10.1001/jama.2014.6342. [DOI] [PubMed] [Google Scholar]
- 20.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. [Accessed April 5, 2014];Clin Chem. 1987 33(12):2153–63. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3319287. [PubMed] [Google Scholar]
- 21.Buse JB, Freeman JLR, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5(3):355–63. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 22.Dungan KM. (GlycoMark™) as a marker of short-term glycemic control and glycemic excursions. 2008:9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Kim WJ, Park C-Y. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine. 2013;43(1):33–40. doi: 10.1007/s12020-012-9760-6. [DOI] [PubMed] [Google Scholar]
- 24.Yamanouchi T, Akanuma Y. Serum 1,5-anhydroglucitol (1,5 AG): new clinical marker for glycemic control. [Accessed July 25, 2014];Diabetes Res Clin Pract. 1994 24 (Suppl):S261–8. doi: 10.1016/0168-8227(94)90259-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7859616. [DOI] [PubMed] [Google Scholar]
- 25.Yamanouchi T. Clinical usefulness of glycaemic control. Lancet. 1994;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 26.Ai M, Otokozawa S, Schaefer EJ, et al. Glycated albumin and direct low density lipoprotein cholesterol levels in type 2 diabetes mellitus. Clin Chim Acta. 2009;406(1–2):71–4. doi: 10.1016/j.cca.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58(12):1648–1655. doi: 10.1373/clinchem.2012.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Nathan DM, McGee P, Steffes MW, Lachin JM. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63(1):282–90. doi: 10.2337/db13-0782. This was an important study conducted in a subsample of participants from the Diabetes Control and Complications Trial (DCCT), to assess the association of short-term and intermediate glycemia on microvascular and macrovascular complications in persons with type 1 diabetes. Over a mean of 6.5 years of follow-up, this paper reported similar associations of HbA1c and glycated albumin with microvascular complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279–288. doi: 10.1016/S2213-8587(13)70199-2. This has been the largest population-based study, with the longest follow-up, to assess prospective associations of fructosamine and glycated albumin with incident diabetes and microvascular outcomes in persons with and without diabetes. This study included more than 12,000 participants (nearly 1,000 with diabetes), who were followed for about 20 years. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Li H, Wang Z, et al. Glycated albumin is a potential diagnostic tool for diabetes mellitus. Clin Med (Northfield Il) 2012;12(6):568–571. doi: 10.7861/clinmedicine.12-6-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck R, Steffes M, Xing D, et al. The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol, and continuous glucose monitoring. Pediatr Diabetes. 2011;12(8):690–5. doi: 10.1111/j.1399-5448.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung CH, Hwang Y-C, Kim KJ, et al. Development of an HbA1c-Based Conversion Equation for Estimating Glycated Albumin in a Korean Population with a Wide Range of Glucose Intolerance. PLoS One. 2014;9(4):e95729. doi: 10.1371/journal.pone.0095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue K, Tsujimoto T, Yamamoto-Honda R, et al. A newer conversion equation for the correlation between HbA1c and glycated albumin. [Accessed May 4, 2014];Endocr J. 2014 doi: 10.1507/endocrj.ej13-0450. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24681757. [DOI] [PubMed]
- 34.Tahara Y. Analysis of the method for conversion between levels of HbA1c and glycated albumin by linear regression analysis using a measurement error model. Diabetes Res Clin Pract. 2009;84(3):224–9. doi: 10.1016/j.diabres.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34(4):960–967. doi: 10.2337/dc10-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He B-B, Wei L, Gu Y-J, et al. Factors associated with diabetic retinopathy in chinese patients with type 2 diabetes mellitus. Int J Endocrinol. 2012;2012:157940. doi: 10.1155/2012/157940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita S, Kasayama S, Deguchi R, et al. Glycated Albumin, Rather than Hba1c, Reflects Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus. J Diabetes Metab. 2013;4(6):4–7. doi: 10.4172/2155-6156.1000278. [DOI] [Google Scholar]
- 38.Mukai N, Yasuda M, Ninomiya T, et al. Thresholds of various glycemic measures for diagnosing diabetes based on prevalence of retinopathy in community-dwelling Japanese subjects: the Hisayama Study. Cardiovasc Diabetol. 2014;13(1):45. doi: 10.1186/1475-2840-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furusyo N, Koga T, Ai M, et al. Plasma glycated albumin level and atherosclerosis: results from the Kyushu and Okinawa Population Study (KOPS) Int J Cardiol. 2013;167(5):2066–72. doi: 10.1016/j.ijcard.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 40.Kondaveeti SB, DK, Mishra S, Kumar RA, Shaker IA. Evaluation of glycated albumin and microalbuminuria as early risk markers of nephropathy in type 2 diabetes mellitus. J Clin Diagn Res. 2013;7(7):1280–3. doi: 10.7860/JCDR/2013/5145.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Y, Pu LJ, Lu L, Zhang Q, Zhang RY, Shen WF. Serum advanced glycation end-products and receptors as prognostic biomarkers in diabetics undergoing coronary artery stent implantation. Can J Cardiol. 28(6):737–43. doi: 10.1016/j.cjca.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Lu L, Pu LJ, Xu XW, et al. Association of serum levels of glycated albumin, C-reactive protein and tumor necrosis factor-alpha with the severity of coronary artery disease and renal impairment in patients with type 2 diabetes mellitus. Clin Biochem. 2007;40(11):810–6. doi: 10.1016/j.clinbiochem.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Jin C, Lu L, Zhang RY, et al. Association of serum glycated albumin, C-reactive protein and ICAM-1 levels with diffuse coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta. 2009;408(1–2):45–9. doi: 10.1016/j.cca.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Pu LJ, Zhang Q, et al. Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2009;206(2):540–5. doi: 10.1016/j.atherosclerosis.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 45.Lu L, Jin Pu L, Chen QJ, et al. Increased glycated albumin and decreased esRAGE concentrations are associated with in-stent restenosis in Chinese diabetic patients. Clin Chim Acta. 2008;396(1–2):33–7. doi: 10.1016/j.cca.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Song SO, Kim KJ, Lee B-W, Kang ES, Cha BS, Lee HC. Serum glycated albumin predicts the progression of carotid arterial atherosclerosis. Atherosclerosis. 2012;225(2):450–5. doi: 10.1016/j.atherosclerosis.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Moon JH, Chae MK, Kim KJ, et al. Decreased endothelial progenitor cells and increased serum glycated albumin are independently correlated with plaque-forming carotid artery atherosclerosis in type 2 diabetes patients without documented ischemic disease. [Accessed May 4, 2014];Circ J. 2012 76(9):2273–9. doi: 10.1253/circj.cj-11-1499. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22664650. [DOI] [PubMed] [Google Scholar]
- 48.Shen Y, Pu LJ, Lu L, Zhang Q, Zhang RY, Shen WF. Glycated albumin is superior to hemoglobin A1c for evaluating the presence and severity of coronary artery disease in type 2 diabetic patients. Cardiology. 2012;123(2):84–90. doi: 10.1159/000342055. [DOI] [PubMed] [Google Scholar]
- 49.Shen Y, Lu L, Ding FH, et al. Association of increased serum glycated albumin levels with low coronary collateralization in type 2 diabetic patients with stable angina and chronic total occlusion. Cardiovasc Diabetol. 2013;12(1):165. doi: 10.1186/1475-2840-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujiwara T, Yoshida M, Yamada H, et al. Lower 1,5-anhydroglucitol is associated with denovo coronary artery disease in patients at high cardiovascular risk. Heart Vessels. 2014 doi: 10.1007/s00380-014-0502-y. [DOI] [PubMed] [Google Scholar]
- 51.Kim WJ, Park CY, Park SE, et al. Serum 1,5-anhydroglucitol is associated with diabetic retinopathy in Type 2 diabetes. Diabet Med. 2012;29(9):1184–90. doi: 10.1111/j.1464-5491.2012.03613.x. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe K, Suzuki T, Ouchi M, et al. Relationship between postprandial glucose level and carotid artery stiffness in patients without diabetes or cardiovascular disease. BMC Cardiovasc Disord. 2013;13(1):11. doi: 10.1186/1471-2261-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen RM, LeCaire TJ, Lindsell CJ, Smith EP, D’Alessio DJ. Relationship of Prospective GHb to Glycated Serum Proteins in Incident. Diabetes Care. 2008;31(1):151–153. doi: 10.2337/dc07-1465. Additional. [DOI] [PubMed] [Google Scholar]
- 54.Cardoso CRL, Salles GF. Predictors of development and progression of microvascular complications in a cohort of Brazilian type 2 diabetic patients. J Diabetes Complications. 2008;22(3):164–70. doi: 10.1016/j.jdiacomp.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 55*.Watanabe M, Kokubo Y, Higashiyama A, Ono Y, Miyamoto Y, Okamura T. Serum 1,5-anhydro-D-glucitol levels predict first-ever cardiovascular disease: an 11-year population-based cohort study in Japan, the Suita study. Atherosclerosis. 2011;216(2):477–83. doi: 10.1016/j.atherosclerosis.2011.02.033. This was a large population-based prospective study with long-term follow-up. It included approximately 2,000 persons (only about 30 people with diabetes) and assessed the association of 1,5-AG with incident cardiovascular events over about 11 years. [DOI] [PubMed] [Google Scholar]
- 56.Colagiuri S, Dickinson S, Girgis S, Colagiuri R. National Evidence Based Guideline for Blood Glucose Control in Type 2 Diabetes. Diabetes Aust NHMRC. 2009 Jul;2009 [Google Scholar]
- 57.Kesavadev J, Sadikot S, Wangnoo S, et al. Consensus guidelines for glycemic monitoring in type 1/type 2 & GDM. Diabetes Metab Syndr Clin Res Rev. 2014 doi: 10.1016/j.dsx.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 58.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61–99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (update) London: 2008. [PubMed] [Google Scholar]
- 60. [Accessed June 20, 2014];Diabetes UK Guide to Diabetes: Testing. Available at: http://www.diabetes.org.uk/Guide-to-diabetes/Monitoring/Testing/
- 61.Association for Clinical Biochemistry. [Accessed June 20, 2014];Fructosamine (plasma, serum) Available at: http://www.acb.org.uk/docs/default-source/amalc/fructosamine-3.pdf.
- 62.Araki T, Ishikawa Y, Okazaki H, et al. Introduction of glycated albumin measurement for all blood donors and the prevalence of a high glycated albumin level in Japan. J Diabetes Investig. 2012;3(6):492–7. doi: 10.1111/j.2040-1124.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Upton J, Lever M, Sadler WA, George PM, Boswell DR. The quality of performance of the fructosamine test. [Accessed April 19, 2014];N Z Med J. 1988 101(854):606–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3173855. [PubMed] [Google Scholar]
- 64.Cefalu WT, Bell-Farrow AD, Petty M, Izlar C, Smith JA. Clinical validation of a second-generation fructosamine assay. [Accessed July 29, 2014];Clin Chem. 1991 37(7):1252–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1855298. [PubMed] [Google Scholar]
- 65*.Shafi T, Sozio SM, Plantinga LC, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2013;36(6):1522–33. doi: 10.2337/dc12-1896. This was a large prospective cohort study of persons on hemodialysis over a median follow-up of 3.5 years, and describes associations of fructosamine and glycated albumin with clinical outcomes, which had not been previously described in this population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Executive summary: standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1 January):S5–13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 67.Shima K, Komatsu M, Noma Y, Miya K. Glycated Albumin (GA) is More Advantageous than Hemoglobin A1c for Evaluating the Efficacy of Sitagliptin in Achieving Glycemic Control in Patients with Type 2 Diabetes. [Accessed May 4, 2014];Intern Med. 2014 53(8):829–35. doi: 10.2169/internalmedicine.53.1364. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24739602. [DOI] [PubMed] [Google Scholar]
- 68.Moura BP, Amorim PR, Silva BP, Franceschini SC, Reis JS, Marins JC. Effect of a short-term exercise program on glycemic control measured by fructosamine test in type 2 diabetes patients. Diabetol Metab Syndr. 2014;6(1):16. doi: 10.1186/1758-5996-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshiuchi K, Matsuhisa M, Katakami N, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. [Accessed May 20, 2014];Endocr J. 2008 55(3):503–7. doi: 10.1507/endocrj.k07e-089. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18445997. [DOI] [PubMed] [Google Scholar]
- 70.Suwa T, Ohta A, Matsui T, et al. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM) [Accessed May 19, 2014];Endocr J. 2010 57(2):135–40. doi: 10.1507/endocrj.k09e-234. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19926921. [DOI] [PubMed] [Google Scholar]
- 71.Koga M, Murai J, Morita S, Saito H, Kasayama S. Comparison of annual variability in HbA1c and glycated albumin in patients with type 1 vs. type 2 diabetes mellitus. J Diabetes Complications. 27(3):211–3. doi: 10.1016/j.jdiacomp.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA. 2010;303(22):2291–2. doi: 10.1001/jama.2010.785. [DOI] [PubMed] [Google Scholar]
- 73. [Accessed June 20, 2014];Clinical Guide: 1,5-Anhydroglucitol (1,5-AG) Blood Test. Available at: http://www.glycomark.com/docs/default-source/downloads/glycomarkclinicalguide.pdf?sfvrsn=8.
- 74.Rubinow KB, Hirsch IB. Reexamining Metrics for Glucose Control. JAMA. 2014;305(11):1132–1133. doi: 10.1001/jama.2011.314. [DOI] [PubMed] [Google Scholar]
- 75.Won JC, Park C-Y, Park H-S, et al. 1,5-Anhydroglucitol reflects postprandial hyperglycemia and a decreased insulinogenic index, even in subjects with prediabetes and well-controlled type 2 diabetes. Diabetes Res Clin Pract. 2009;84(1):51–7. doi: 10.1016/j.diabres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Suh S, Joung JY, Jin SM, et al. Strong correlation between glycaemic variability and total glucose exposure in type 2 diabetes is limited to subjects with satisfactory glycaemic control. Diabetes Metab. 2014 doi: 10.1016/j.diabet.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Sun J, Dou J-T, Wang X-L, et al. Correlation between 1,5-anhydroglucitol and glycemic excursions in type 2 diabetic patients. [Accessed May 4, 2014];Chin Med J (Engl) 2011 124(22):3641–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22340217. [PubMed] [Google Scholar]
- 78.Furusyo N, Koga T, Ai M, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS) Diabetologia. 2011;54(12):3028–36. doi: 10.1007/s00125-011-2310-6. [DOI] [PubMed] [Google Scholar]
- 79.Zhang T, He H, Yang H-L, et al. Study of glycated albumin cut-off point in diabetes mellitus and impaired glucose regulation. [Accessed May 4, 2014];Sichuan Da Xue Xue Bao Yi Xue Ban. 2014 45(2):274–7. 298. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24749356. [PubMed] [Google Scholar]
- 80.Li Q, Pan J, Ma X, et al. Combined utility of hemoglobin A1c and glycated albumin in diabetic screening. [Accessed May 4, 2014];Zhonghua Yi Xue Za Zhi. 2011 91(26):1813–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22093780. [PubMed] [Google Scholar]
- 81.Ma X-J, Pan J-M, Bao Y-Q, et al. Combined assessment of glycated albumin and fasting plasma glucose improves the detection of diabetes in Chinese subjects. Clin Exp Pharmacol Physiol. 2010;37(10):974–9. doi: 10.1111/j.1440-1681.2010.05417.x. [DOI] [PubMed] [Google Scholar]
- 82.Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pr. 2008;14(7):933–946. doi: 10.4158/EP.14.7.933. [DOI] [PubMed] [Google Scholar]
- 83.Juraschek SP, Steffes MW, Miller ER, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35(11):2265–70. doi: 10.2337/dc12-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shipman KE, Jawad M, Sullivan KM, Ford C, Gama R. HbA1c is a reliable test for type 2 diabetes in primary care irrespective of chronic kidney disease. [Accessed July 25, 2014];BMJ. 2014 348:g3780. doi: 10.1136/bmj.g3780. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24942952. [DOI] [PubMed] [Google Scholar]
- 85.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73(9):1062–8. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 86.Little RR, Rohlfing CL, Tennill AL, et al. Measurement of Hba(1C) in patients with chronic renal failure. Clin Chim Acta. 2013;418:73–6. doi: 10.1016/j.cca.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sany D, Elshahawy Y, Anwar W. Glycated albumin versus glycated hemoglobin as glycemic indicator in hemodialysis patients with diabetes mellitus: variables that influence. [Accessed March 29, 2014];Saudi J Kidney Dis Transpl. 2013 24(2):260–73. Available at: http://www.sjkdt.org/temp/SaudiJKidneyDisTranspl242260-6464221_175722.pdf. [PubMed] [Google Scholar]
- 88.Chen F-K, Sun X-F, Zhang D, et al. Glycated albumin may be a choice, but not an alternative marker of glycated hemoglobin for glycemic control assessment in diabetic patients undergoing maintenance hemodialysis. [Accessed March 29, 2014];Chin Med J (Engl) 2013 126(17):3295–300. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24033952. [PubMed] [Google Scholar]
- 89.Meyer L, Chantrel F, Imhoff O, et al. Glycated albumin and continuous glucose monitoring to replace glycated haemoglobin in patients with diabetes treated with haemodialysis. Diabet Med. 2013;30(11):1388–9. doi: 10.1111/dme.12294. [DOI] [PubMed] [Google Scholar]
- 90.Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int. 30(1):72–9. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 91.Nagayama H, Inaba M, Okabe R, et al. Glycated albumin as an improved indicator of glycemic control in hemodialysis patients with type 2 diabetes based on fasting plasma glucose and oral glucose tolerance test. Biomed Pharmacother. 2009;63(3):236–40. doi: 10.1016/j.biopha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Park H-I, Kim YS, Lee J, Kim Y, Shin SJ. Performance characteristics of glycated albumin and its clinical usefulness in diabetic patients on hemodialysis. Korean J Lab Med. 2009;29(5):406–14. doi: 10.3343/kjlm.2009.29.5.406. [DOI] [PubMed] [Google Scholar]
- 93.Yamada S, Inaba M, Shidara K, et al. Association of glycated albumin, but not glycated hemoglobin, with peripheral vascular calcification in hemodialysis patients with type 2 diabetes. Life Sci. 2008;83(13–14):516–9. doi: 10.1016/j.lfs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Kumeda Y, Inaba M, Shoji S, et al. Significant correlation of glycated albumin, but not glycated haemoglobin, with arterial stiffening in haemodialysis patients with type 2 diabetes. Clin Endocrinol (Oxf) 2008;69(4):556–61. doi: 10.1111/j.1365-2265.2008.03202.x. [DOI] [PubMed] [Google Scholar]
- 95.Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int Suppl. 2010;(117):S41–5. doi: 10.1038/ki.2010.193. [DOI] [PubMed] [Google Scholar]
- 96.Murea M, Moran T, Russell GB, et al. Glycated Albumin, Not Hemoglobin A1c, Predicts Cardiovascular Hospitalization and Length of Stay in Diabetic Patients on Dialysis. Am J Nephrol. 2012;36(5):488–496. doi: 10.1159/000343920. [DOI] [PubMed] [Google Scholar]
- 97.Ding FH, Lu L, Zhang RY, et al. Impact of elevated serum glycated albumin levels on contrast-induced acute kidney injury in diabetic patients with moderate to severe renal insufficiency undergoing coronary angiography. Int J Cardiol. 2013;167(2):369–73. doi: 10.1016/j.ijcard.2011.12.101. [DOI] [PubMed] [Google Scholar]
- 98.Fukuoka K, Nakao K, Morimoto H, et al. Glycated albumin levels predict long-term survival in diabetic patients undergoing haemodialysis. Nephrology (Carlton) 2008;13(4):278–83. doi: 10.1111/j.1440-1797.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 99**.Freedman BI, Andries L, Shihabi ZK, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol. 2011;6(7):1635–43. doi: 10.2215/CJN.11491210. This was one of the first prospective studies to assess associations of glycated albumin with clinical outcomes in a population of persons with diabetes and ESRD. It included 444 participants with diabetes and included follow-up just over 2 years. [DOI] [PubMed] [Google Scholar]
- 100.Isshiki K, Nishio T, Isono M, et al. Glycated Albumin Predicts the Risk of Mortality in Type 2 Diabetic Patients on Hemodialysis: Evaluation of a Target Level for Improving Survival. Ther Apher Dial. 2013 doi: 10.1111/1744-9987.12123. [DOI] [PubMed] [Google Scholar]
- 101.Ma W-Y, Wu C-C, Pei D, et al. Glycated albumin is independently associated with estimated glomerular filtration rate in nondiabetic patients with chronic kidney disease. Clin Chim Acta. 2011;412(7–8):583–6. doi: 10.1016/j.cca.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 102.Okada T, Nakao T, Matsumoto H, et al. Influence of proteinuria on glycated albumin values in diabetic patients with chronic kidney disease. [Accessed May 24, 2014];Intern Med. 2011 50(1):23–9. doi: 10.2169/internalmedicine.50.4129. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21212569. [DOI] [PubMed] [Google Scholar]
- 103.Chen H-S, Wu T-E, Lin H-D, et al. Hemoglobin A(1c) and fructosamine for assessing glycemic control in diabetic patients with CKD stages 3 and 4. Am J Kidney Dis. 2010;55(5):867–74. doi: 10.1053/j.ajkd.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 104.Kim WJ, Park C-Y, Lee K-B, et al. Serum 1,5-anhydroglucitol concentrations are a reliable index of glycemic control in type 2 diabetes with mild or moderate renal dysfunction. Diabetes Care. 2012;35(2):281–6. doi: 10.2337/dc11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim S, Min W-K, Chun S, Lee W, Park H-I. Glycated albumin may be a possible alternative to hemoglobin A1c in diabetic patients with anemia. Clin Chem Lab Med. 2011;49(10):1743–7. doi: 10.1515/CCLM.2011.646. [DOI] [PubMed] [Google Scholar]
- 106.Koga M, Hashimoto K, Murai J, et al. Usefulness of glycated albumin as an indicator of glycemic control status in patients with hemolytic anemia. Clin Chim Acta. 2011;412(3–4):253–7. doi: 10.1016/j.cca.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Sugimoto T, Hashimoto M, Hayakawa I, et al. Alterations in HbA1c resulting from the donation of autologous blood for elective surgery in patients with diabetes mellitus. Blood transfus. 2014;12 (Suppl 1):s209–13. doi: 10.2450/2013.0271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32(9):1591–3. doi: 10.2337/dc09-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koga M, Kasayama S, Kanehara H, Bando Y. CLD (chronic liver diseases)-HbA1C as a suitable indicator for estimation of mean plasma glucose in patients with chronic liver diseases. Diabetes Res Clin Pract. 2008;81(2):258–62. doi: 10.1016/j.diabres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Bando Y, Kanehara H, Toya D, Tanaka N, Kasayama S, Koga M. Association of serum glycated albumin to haemoglobin A1C ratio with hepatic function tests in patients with chronic liver disease. Ann Clin Biochem. 2009;46(Pt 5):368–72. doi: 10.1258/acb.2009.008231. [DOI] [PubMed] [Google Scholar]
- 111.Koga M, Murai J, Saito H, et al. 1,5-Anhydroglucitol levels are low irrespective of plasma glucose levels in patients with chronic liver disease. Ann Clin Biochem. 2011;48(Pt 2):121–5. doi: 10.1258/acb.2010.010053. [DOI] [PubMed] [Google Scholar]
- 112.Hashimoto K, Noguchi S, Morimoto Y, et al. A1C but not serum glycated albumin is elevated in late pregnancy owing to iron deficiency. Diabetes Care. 2008;31(10):1945–8. doi: 10.2337/dc08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hashimoto K, Osugi T, Noguchi S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care. 2010;33(3):509–11. doi: 10.2337/dc09-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]