Abstract
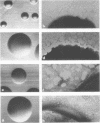
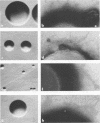
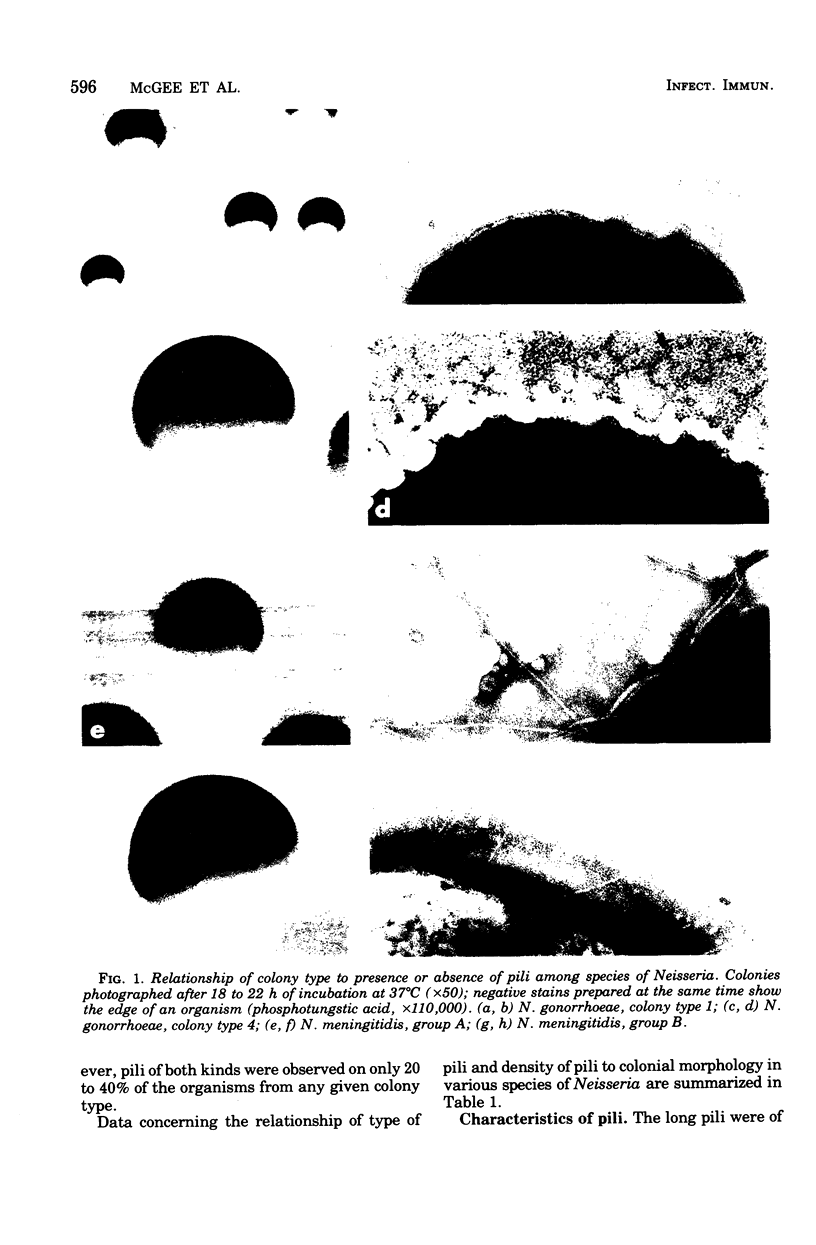
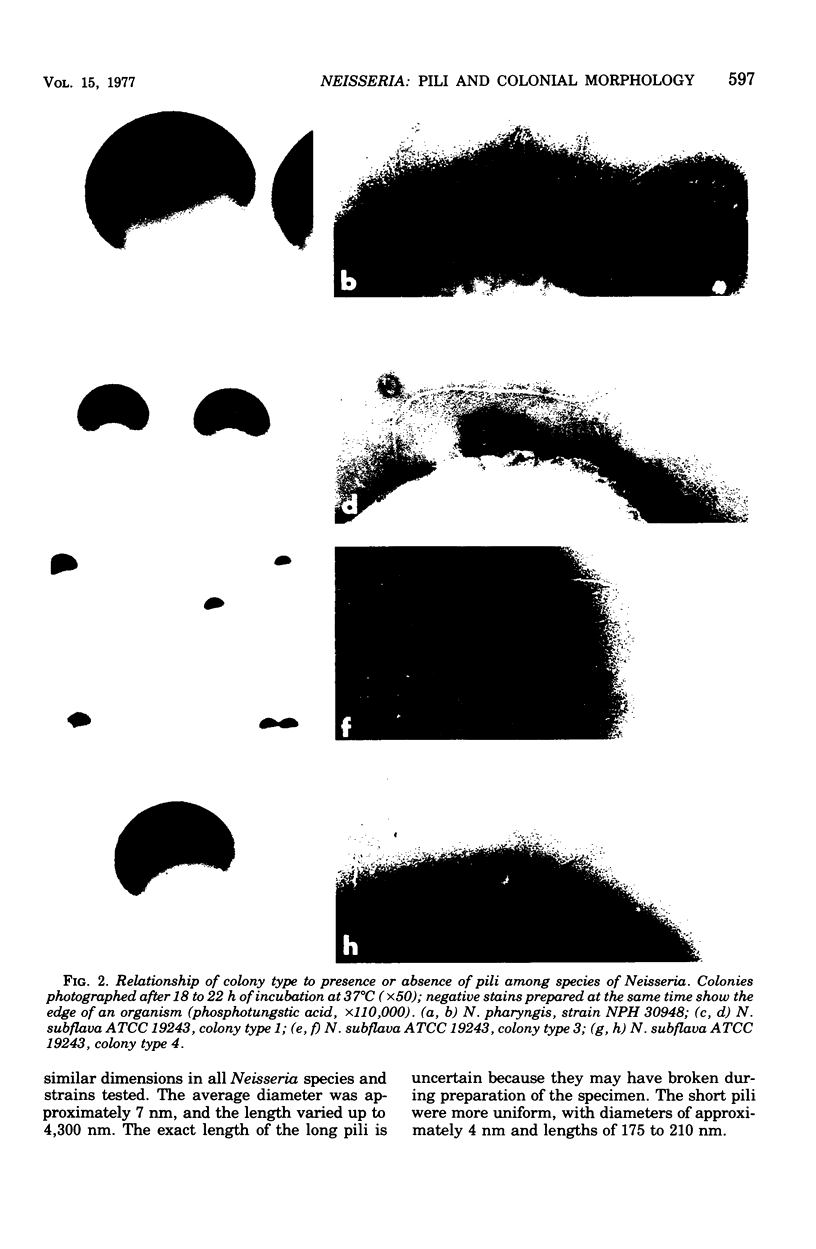
Growth in colonies with type 1 morphology and the presence of pili are characteristics that have been associated with virulence of gonococci for humans. To determine whether the presence of pili per se might be responsible for colony type 1 morphology, the relationship of pili to colony type was examined in various species of Neisseria. Short pili (175 to 210 nm in length) were seen only on nonpathogenic neisseria, whereas long pili (up to 4,300 nm) were seen on organisms of both nonpathogenic and pathogenic species. Although long pili, similar to those found on organisms from high-domed, type 1 colonies of gonococci, were observed on organisms from high-domed, type 1 colonies of nonpathogenic Neisseria species, they were also observed on low-convex, type 4 colonies of meningococci and nonpathogenic neisseria. Among meningococci there was no difference in the morphology of colonies consisting of organisms with many long pili and colonies consisting of organisms that completely lacked pili. Thus, there was no consistent relationship of pili to colonial morphology. Unless the pili of N. gonorrhoeae are unique among Neisseria species in their influence on colonial morphology, it is likely that factors other than pili determine colony type 1 morphology of gonococci. Whether these same factors, either alone or in conjunction with pili, are also responsible for gonococcal virulence warrants further investigation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975 Feb 1;141(2):297–305. doi: 10.1084/jem.141.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmros T., Hörstedt P., Winblad B. Scanning electron microscopic study of virulent and avirulent colonies of Neisseria gonorrhoeae. Infect Immun. 1975 Sep;12(3):630–637. doi: 10.1128/iai.12.3.630-637.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Holmquest A. N., Swanson J., Buchanan T. M., Wende R. D., Williams R. P. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974 May;9(5):897–902. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Gross J., Dourmashkin R. R., Taylor-Robinson D. Nonpilar surface appendages of colony type 1 and colony type 4 gonococci. Infect Immun. 1976 Jul;14(1):266–270. doi: 10.1128/iai.14.1.266-270.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976 Feb;13(2):608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A., Dourmashkin R. R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970 Apr;18(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyn A., Jephcott A. E., Ravn H. Neisseria gonorrhoeae. Colony variation II. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):435–436. doi: 10.1111/j.1699-0463.1971.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Blumershine R. V., Savage D. C. Association of Salmonella typhimurium with, and its invasion of, the ileal mucosa in mice. Infect Immun. 1975 Feb;11(2):365–370. doi: 10.1128/iai.11.2.365-370.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Whytock S., Green C. J., Carney F. E., Jr Effect of Neisseria gonorrhoeae on human and rabbit oviducts. Br J Vener Dis. 1974 Aug;50(4):279–288. doi: 10.1136/sti.50.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Wistreich G. A., Baker R. F. The presence of fimbriae (pili) in three species of Neisseria. J Gen Microbiol. 1971 Feb;65(2):167–173. doi: 10.1099/00221287-65-2-167. [DOI] [PubMed] [Google Scholar]