Abstract
Impairment of stem cell function contributes to the progressive deterioration of tissue maintenance and repair with ageing. Evidence is mounting that age-dependent accumulation of DNA damage in both stem cells and cells that comprise the stem cell microenvironment are partly responsible for stem cell dysfunction with ageing. Here, we review the impact of the various types of DNA damage that accumulate with ageing on stem cell functionality, as well as the development of cancer. We discuss DNA-damage-induced cell intrinsic and extrinsic alterations that influence these processes, and review recent advances in understanding systemic adjustments to DNA damage and how they affect stem cells.
Even some of the most primitive forms of metazoan life rely on the regenerative capacities of stem cells. In higher animals, multiple tissues require a tissue-specific stem and progenitor cell pool for active replenishment during the lifespan of the organism. Stem cells have the unique capacity of long-term self-renewal, but this capacity also carries an intrinsic challenge: as stem cells are the most long-lived cells of the organism, the risk of acquiring genomic damage is increased. Several factors can contribute to the accumulation of DNA damage in stem cells of the adult organism, including telomere shortening, DNA replication stress and the failure of repair systems. Further, there is emerging evidence that aneuploidy contributes to the accumulation of genome instability in lineage-primed progenitor cells during ageing1,2.
Mechanisms of DNA damage induction have already been reviewed in many publications (see, for example, the recent review by Zeman and Cimprich3 on DNA replication stress). Our review focuses instead on the recent advances in the understanding of the outcome of genome instability in stem cells. There are two distinct consequences of DNA damage on the fate of cells. First, when DNA damage alters gene function through mutations or chromosomal rearrangements, the result can be aberrations in gene expression and activity, such as the dysregulation of genes that control stem cell differentiation and self-renewal, the inactivation of tumour suppressors or the activation of oncogenes4,5. Such changes can lead to cancerous growth, and tumorigenic alterations in stem cells can be particularly dangerous given the high inherent regenerative potential of these cells. To prevent such alterations, DNA damage checkpoints evolved as bona fide tumour suppressor mechanisms to limit the growth of damaged cells by inducing cell cycle arrest, cellular senescence or apoptosis6. As a side effect, the DNA damage response could compromise stem cell function and tissue renewal during ageing. DNA damage accumulation throughout life might underlie the declining regenerative potential of tissues and organs with ageing. Interestingly, the maintenance of stem cells does not rely solely on DNA damage responses that are cell autonomous. Recent evidence suggests that systemic adjustments to DNA damage could alter the regeneration of stem cell pools and influence clonal selection of subpopulations of stem cells with distinct functions7,8. As knowledge about the organismal consequences of DNA damage is only starting to emerge, we will provide an outlook on what to expect from integrated and organismal studies of responses to genome instability.
Consequences of DNA damage checkpoint activation in stem cells
Cellular DNA damage checkpoints determine the fate of cells that carry genomic damage (Fig. 1). DNA lesions trigger activation of signalling pathways, in particular of the protein kinase ATM (ataxia telangiectasia mutated) and the related kinase ATR (ataxia telangiectasia and Rad3-related), which mediates a cascade of post-translational modifications to chromatin and to proteins recruited to damaged DNA9. Stem cells that are deficient in either of these kinases are dysfunctional and are frequently exhausted prematurely, resulting in early ageing phenotypes10–14. The outputs of DNA damage checkpoint activation include cell cycle arrest, apoptosis and senescence — decisions that ATM and ATR coordinate with repair. Although ATM activation is central to the double-strand break response15, and ATR activation responds primarily to replication stress and exposure of single-stranded DNA16, in some cases the kinases cooperate, either in series or in parallel17–20. In addition to these classical checkpoint responses, there is emerging evidence that DNA-damage-induced differentiation eliminates damaged stem cells by inhibiting self-renewal and by pushing the damaged stem cells into the short-lived progenitor cell compartment8,11.
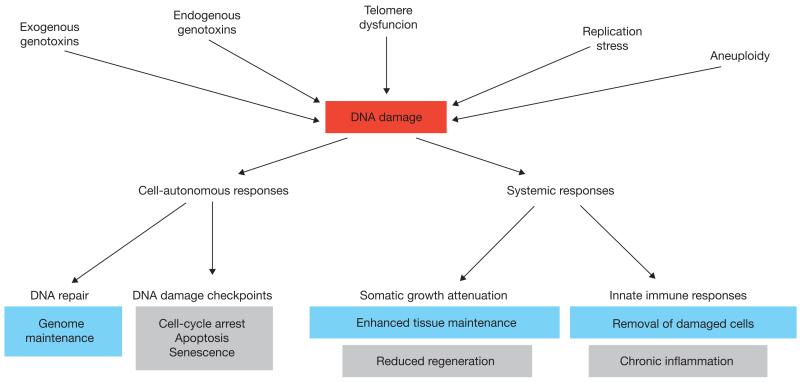
Figure 1.
Cell-autonomous and systemic responses to DNA damage. Various sources of genotoxic stress induce DNA damage that can be removed by specialized DNA repair systems. Cell-autonomous DNA damage checkpoints halt the cell cycle to allow time for repair or, amid severe genome damage, trigger programmed cell death or cellular senescence. Although DNA damage checkpoint mechanisms protect against cancer, the associated removal of cells can contribute to ageing through declining regenerative stem cell pools (grey). Systemic DNA damage responses include attenuation of the somatic growth axis and triggering of innate immune responses, which might support longevity assurance (blue) by enhancing maintenance of tissue functionality and removal of damaged cells, but also contribute to ageing (grey) by damaging tissues and impairing regeneration.
The decision whether to arrest the cell cycle temporarily, to allow time to repair the damage, or to undergo apoptosis or differentiation to remove the damaged stem cell from the organism, depends not only on the type of damage encountered but also on the cell type and the developmental context. In addition, species differences may exist. Murine adult haematopoietic stem cells (HSCs) respond to low-level irradiation by initiating repair and remaining quiescent, although this reduces their long-term repopulating ability and may increase the risk of tumorigenesis resulting from gross chromosomal rearrangements21. A recent study reported that although irradiation of adult murine HSCs induced symmetric divisions to increase the stem cell capacity in the short term, long-term self-renewal of the HSCs was reduced after ionizing radiation22. Quiescent human umbilical cord blood cells, in contrast, tend to undergo p53-dependent apoptosis in response to similar doses of ionizing radiation23. Stem cell characteristics in response to DNA damage seem to also be important for cancer therapies. Cancer stem cells represent a subpopulation of cells in a tumour that are more resistant to DNA damaging agents than the bulk of the other tumour cells. In the context of chronic myelocytic leukaemia, the population of quiescent leukaemia-initiating cells is resistant to chemotherapy and must be forced into cycling to undergo apoptosis, for example by deletion of the c-Myc-destabilizing ubiquitin ligase component Fbxw7 (ref. 24).
Some stem cell populations, such as HSCs, mainly reside in a non-cycling state under homeostatic conditions. It is thought that the quiescent state protects stem cells from the harmful effects of elevated metabolic activity during the active phases of the cell cycle and from mutational hazards that can occur during DNA replication25. The importance of quiescence for HSC maintenance is seen in serial transplantation experiments, where HSCs completely exhaust after five to six rounds of transplantation. It was shown that proliferative stress leads to an accumulation of oxidative stress in transplanted HSCs, which restricts self-renewal capacity26. Other stem cells, such as LGR5+ (leucine-rich repeat-containing G-protein coupled receptor 5) stem cells of the intestinal epithelium, proliferate at a high rate. Quiescent and highly cycling stem cells seem to employ different pathways to repair DNA damage. Whereas actively cycling LGR5+ intestinal stem cells are able to use the highly accurate homologous recombination pathway27, this pathway cannot function in quiescent HSCs, as the homologous DNA sequence only becomes available during S phase of the cell cycle. Quiescent stem cells, such as HSCs and hair follicle bulge stem cells, instead rely on non-homologous end joining (NHEJ) to rapidly join the DNA ends21,28 — a process prone to error because of local end resection. Thus, although quiescence protects against replication-induced damage, it may indirectly lead to deletions or translocations arising from error-prone repair.
Telomere damage and aneuploidy in stem cell ageing
Excessively short, uncapped or dysfunctional telomeres are recognized as DNA damage by the checkpoint and repair machinery, and may result in loss or translocation of genetic material (Fig. 1). Stem cells in adult tissues exhibit some level of telomerase activity but still show significant shortening of telomeres during ageing29. Heterozygous mutations in telomere binding proteins — for example, TINF2 (TERF1-interacting nuclear factor 2) or POT1 (protection of telomeres 1) — lead to premature defects in tissue maintenance and increased rates of cancer in humans30,31. These defects predominantly affect the haematopoietic system, indicating that, in humans, HSCs are most sensitive to telomere capping defects caused by mutations in telomere-binding proteins. Recent work in mouse embryonic stem cells revealed that short telomeres lead to unstable differentiation32, a phenotype that could contribute to ageing-associated defects in tissue maintenance if similar perturbations occur in adult stem cells. Supporting this idea, telomere shortening in adult intestinal stem cells provokes marked increases in genome instability and defective differentiation in mice lacking the tumour suppressor p53 (ref. 33).
In addition to telomere-related damage at chromosome ends, increases in losses and gains of chromosomes and chromosomal regions also contribute to the accumulation of genome instability with age1,34 (Fig. 1). Although the underlying mechanisms remain largely unexplored, one contributing factor may be age-related telomere shortening33 as well as decreases in the expression of BUBR1 (also known as BUB1B; BUB1 mitotic checkpoint serine/threonine kinase B), a core component of a mitotic checkpoint that ensures proper attachment of duplicated chromosomes to the mitotic spindle before anaphase onset35. Mutant mice in which the decline of BubR1 is accelerated develop premature ageing phenotypes owing to early senescence of certain progenitor cell populations and loss of regenerative potential2. Furthermore, transgenic mice with sustained high levels of BubR1 throughout life are less susceptible to age-related aneuploidization and show a marked extension in healthy lifespan1. A recent study showing that induction of aneuploidy in neural stem cells leads to microcephaly underscores the importance of chromosomal integrity for tissue development and maintenance36. However, the analysis of the role of euploidy-controlling genes in ageing clearly needs to be extended to other aneuploidy models that have so far mostly been used for short-term cancer studies in early life37.
Alterations of the stem cell environment with ageing
How age-related accumulation of DNA damage affects the functionality of stem cells in tissue maintenance has primarily been studied from a stem-cell-intrinsic perspective. However, evidence is mounting that changes in the stem cell microenvironment (or stem cell niche) and in the systemic circulatory environment also contribute to the ageing-associated decline in stem cell function (Fig. 2). Importantly, studies using telomere-dysfunctional mice show that genotoxic stress provokes cell-extrinsic alterations that impair HSC function, and ageing-associated defects in HSC differentiation, characterized by profound suppression of lymphopoiesis38.
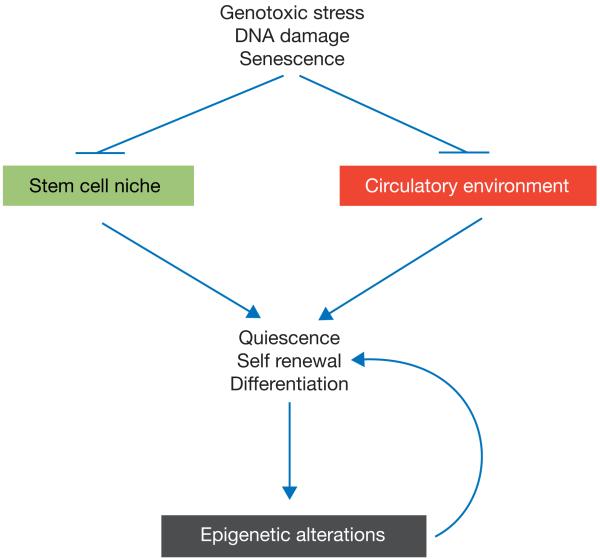
Figure 2.
Impact of DNA damage on the stem cell environment. The accumulation of DNA damage and senescent cells during ageing leads to alterations in the stem cell niche and the systemic circulatory environment. Both processes can interfere with signalling pathways (such as Notch, Wnt and Sprouty1) that are required for the maintenance of stem cell quiescence, self-renewal and differentiation. Disturbances in these basic stem cell parameters lead to alterations in the epigenetic landscape of the DNA of ageing stem cells, further aggravating alterations in stem cell quiescence, self-renewal and differentiation.
Given their apparent role in the ageing-related stem cell decline, it is of interest to investigate such stem-cell-extrinsic mechanisms of ageing in greater detail (Fig. 2). One such mechanism of muscle stem cell deterioration involves the Delta-like 1 (DLL1)-ligand–Notch-receptor signalling pathway. Studies on mouse models provide compelling evidence that skeletal muscle ageing is characterized by defects in DLL1-mediated activation of quiescent satellite cells following injury, resulting in impaired tissue repair39–41. These defects were rescued by exposure to a young blood circulatory environment (see below). In addition, Wnt signalling pathways play a key role in regulating stem cell fate and self-renewal in different organ compartments and cancer (reviewed in ref. 42). Studies on skeletal muscle ageing revealed that ageing influences muscle stem cell function by affecting Wnt signalling activity. Specifically, studies in mice provided experimental evidence for hyperactivation of canonical Wnt signalling in ageing skeletal muscle, which perturbs tissue repair and homeostasis through altering stem cell fate, resulting in increased fibrosis43,44. The study also showed that ageing-associated alterations in the blood serum contribute to increases in canonical Wnt signalling activity44. In contrast to the negative effects of the canonical Wnt signalling pathway, non-canonical Wnt signalling mediated by Wnt7A was reported to enhance muscle stem cell self-renewal and muscle fibre regeneration45,46. Interestingly, Wnt7A activation ameliorated muscular dystrophy47, but whether it can reverse ageing-associated impairments in muscle regeneration remains to be elucidated. Niche-mediated mechanisms were also found to impair muscle regenerative potential during ageing (Fig. 2). FGF2 expression is chronically elevated in old muscles, especially in the stem cell niche48. Increased FGF2 signalling in the aged muscle impairs Sprouty1-mediated maintenance of stem cell quiescence49, thus diminishing the pool of functional satellite cells with age.
Parabiosis experiments — a surgical technique in which two animals are joined to establish a common circulatory system40 — provided a proof of concept that alterations in the cell-extrinsic system contribute to impairments in stem cell function during ageing. In experiments where young and old mice were joined, cell-extrinsic factors from the young mice were found to restore neural and muscle stem cell function in the old mice40,50. As well as underscoring the critical importance of the circulatory environment in age-related stem cell malfunction, these experiments provided a proof of concept that it is possible to rejuvenate aged stem cells and the capacity of tissue regeneration. Identification of the key cell-extrinsic factors involved could provide molecular entry points for the development of therapies aimed at improving human health and lifespan51.
Epigenetic modifications in response to DNA damage lead to the induction of p16Ink4a, a key marker of cellular senescence (Fig. 2). An age-related increase in p16Ink4a expression has been observed in various tissue compartments of ageing mice52. Deletion of p16Ink4a increases the regenerative capacity of haematopoietic and neuronal stem cells as well as that of pancreatic islets during ageing53–55. Thus, senescence of stem cells and differentiated cells may represent an important cell-intrinsic mechanism driving age-related loss of tissue regenerative potential. Furthermore, studies designed to dissect the mechanism of precocious skeletal muscle degeneration in BubR1 progeroid mice uncovered that senescent cells may also limit tissue regeneration by altering the stem-cell niche through the factors they secrete, including pro-inflammatory cytokines and proteases that disrupt tissue architecture, commonly referred to as the senescence-associated secretory phenotype (SASP)56 (see below: ‘Systemic adjustments to genomic damage’). The key observation supporting this concept was that clearance of senescent cells from these mice attenuated skeletal muscle degeneration57.
The role of the immune system in senescent cell clearance and tissue ageing is complex and remains to be defined. Senescent cells in tumours and pre-malignant lesions are efficiently cleared by the immune system58,59, but they accumulate in multiple tissues during ageing60. Whether an ageing-associated decline in immune function contributes to this process remains to be investigated. It is possible that ageing-associated impairment in the lymphoid differentiation potential of HSCs as well as the upregulation of senescent checkpoints in peripheral lymphocytes leads to reductions in the clearance of senescent cells from ageing tissues61,62.
Systemic adjustments to genomic damage
Beyond the tissue microenvironment, the systemic adjustments of the ageing organism are increasingly being implicated in determining the regenerative capacities of the stem cell compartments (Fig. 1). Whereas young organisms grow and thrive, the endocrine growth environment declines with ageing. However, as ageing cells accumulate DNA damage, the risk for mutation-driven tumorigenesis steadily increases. It is thought that the endocrine shift antagonizes the increased cancer risk by depriving cancer cells of growth factors. The somatic growth (or somatotropic) axis is controlled by growth hormone (GH) and insulin-like growth factor 1 (IGF-1) signalling63. GH is secreted from the pituitary and, through GH receptor (GHR) activation, stimulates IGF-1 production in target tissues. IGF-1 in turn regulates cellular growth and survival signalling through IGF-1 receptor (IGF-1R) signalling. In young animals, the somatic growth axis is required for body growth, but GH–IGF-1 levels decline with ageing63. Moderate reduction of IGF-1 activity is associated with elevated general stress resistance, prolonged tissue maintenance and extension of lifespan in organisms ranging from nematode worms to mice, whereas high IGF-1 activity can support cancerous growth in mice64–66. Intriguingly, mice that age prematurely as a result of mutations in nucleotide excision repair (NER) genes that are defective in the human progeroid (premature ageing-like) Cockayne syndrome and XPF-ERCC1 (XFE) progeria show low expression levels of somatotropic genes in various tissues and reduced IGF-1 levels in their circulation67,68. It is likely that the attenuation of the somatotropic axes accounts for the reduced body growth and apparent cancer protection of those types of progeroid mice69. Mechanistically, persistence of DNA lesions that lead to blockage of transcription (in Cockayne syndrome and XFE) results in cellular attenuation of the somatotropic mediators GHR and IGF-1R (ref. 70). Somatotropic attenuation in response to unrepaired DNA damage might thus counteract the detrimental consequences of genome instability by enhancing tissue maintenance and systemically reducing proliferation of potentially damaged cells. However, reconstitution of IGF-1 in mouse models for Hutchinson-Gilford Progeria syndrome (HGPS) rescued the severe postnatal growth retardation and thus prolonged the short lifespan of the mice71. These findings suggest that although there are detrimental consequences during early life when the animals need to grow and thrive, attenuation of the GH–IGF1-mediated growth axis in response to DNA damage might promote tissue maintenance and suppress cancer development amid accumulating genome instability with ageing (Fig. 1). The extension of tissue functionality by reduced IGF-1 signalling might predominantly benefit differentiated cell types such as the post-mitotic somatic tissues of nematode worms64 and neurons in mammals72. In contrast, studies in mice with increased genome instability owing to a mutation in the Sirtuin gene Sirt6 suggest that systemically reduced somatotropic signalling negatively impacts regenerative capacity. Full-body Sirt6 knockout animals show similar features as the above-mentioned NER-deficient progeroid mice, including reduced somatotropic activity and growth, cachexia, kyphosis and leukopenia. Strikingly, Sirt6 HSCs were proficient in reconstituting lethally irradiated wild-type recipients, indicating that the bone marrow defects in Sirt6 knockout animals were non-cell-autonomous73. This is reminiscent of the defects in HSC differentiation in telomere-dysfunctional mice that were rescued by HSC transplantation into wild-type mice (see above)38. Although it remains unclear if suppression of IGF-1 also contributes to the latter phenotype, these observations suggest that systemic adjustments that arise from persistent DNA damage reduce the growth-supporting environment and result in declining stem-cell-mediated regeneration. Re-establishing a growth-permissive environment might therefore provide the therapeutic means of reconstituting regenerative capacities with ageing. However, it will be important to counterweigh such approaches to avoid the cancer-promoting consequences of growth-factor signalling. It will be of pivotal interest to better understand how adjustments of the endocrine growth environment during ageing affect the balance between maintenance of differentiated cell types and the regenerative capacities of their stem cell niches.
Cellular DNA damage signalling, including activation of the tumour suppressor p53, triggers both innate and adaptive immune responses58,59 (Fig. 1). Cells that have entered senescence as a consequence of DNA damage acquire a SASP, including secretion of pro-inflammatory cytokines and chemokines that might contribute to the sterile inflammation observed in a variety of tissues74 and the circulatory environment75 in response to ageing. The functional consequences of these inflammatory changes have yet to be explored in animal models of ageing. However, there is evidence that the increased secretion of pro-inflammatory signals from cells that carry DNA damage could have multiple effects on tissue maintenance and cancer formation by: impairing the functionality of stem cells in ageing tissues (see above and refs 7,38,76); promoting the immunologic clearance of senescent tumour cells and premalignant cells58,59; influencing tissue remodelling in response to injury77; and stimulating the growth of cancerous cells78.
The systemic consequences of innate immune responses to genome instability in regenerative tissues have recently been demonstrated in the Caenorhabditis elegans system. In adult C. elegans, the only stem cell niche is in the germ line, which contains mitotically active germ cells that differentiate through meiosis into gametes, whereas somatic tissues are post-mitotic. DNA damage specifically in the germ line was shown to trigger an ancestral innate immune response that evokes stress resistance throughout somatic tissues by activating the ubiquitin proteasome system (UPS)79. It was suggested that the elevated somatic endurance extends the reproductive lifespan to allow germ cells to restore genome stability before resuming offspring generation80. It will be interesting to explore whether the innate immune responses to DNA damage in mammalian stem cells can impact the endurance of differentiated tissues.
Clonal drifts in the stem cell pool and selection of aberrant clones
Ageing of the stem cell compartment is not always associated with a decrease in stem cell number (Fig. 3). In fact, the number of immunophenotypically defined stem cells increases during ageing in the haematopoietic system both in mice and humans81. However, the functionality of stem cells on a per-cell basis decreases during ageing82–84. Furthermore, in the haematopoietic system the clonal composition of stem cells can change during ageing85. Current data indicate that ageing-associated clonal drifts in the HSC compartment are induced by both cell-intrinsic and cell-extrinsic processes7,83.
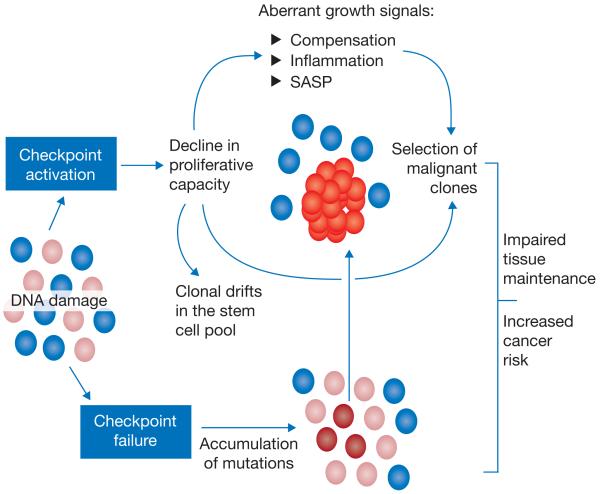
Figure 3.
Consequences of DNA damage on clonal selection in tissue stem cells during ageing. Stem cells in an organ consist of different subpopulations. Ageing-associated accumulation of DNA damage activates checkpoints that remove damaged stem cells by inducing apoptosis, cell cycle arrest or differentiation. This can lead to clonal drifts or imbalances in the pool of remaining stem cells. Checkpoint activation (top) in a growing number of stem cells in ageing tissues impairs the proliferative capacity of these stem cells, which will in turn increase the selective pressure for the outgrowth of mutant cell clones. This process is further accelerated by aberrant growth signals originating from compensatory feedback loops to maintain tissue homeostasis, the accumulation of senescent cells exhibiting a secretory phenotype (SASP), or inflammation as a consequence of immune reactions targeting damaged cells. It is also possible that some stem cells will escape the induction of checkpoints in response to DNA damage (bottom). Checkpoint-deficient stem cells have an increased risk of acquiring mutations that will lead to a selective growth advantage in the context of damage accumulation, checkpoint induction and growth inhibition in the pool of ageing stem cells (top).
In the haematopoietic system, ageing is characterized by a decrease in lymphopoiesis and an increase in myelopoiesis, and clonal drifts in the composition of HSCs seem to contribute to these alterations84,85. The pool of HSCs consists of different subpopulations, including lymphoid-biased HSCs and myeloid-biased HSCs. During ageing, the population of lymphoid-biased HSCs decreases while the population of myeloid-biased cells is maintained, although the latter population exhibits reduced functionality on a per-cell basis84. The drifts in the clonal composition of HSCs are thought to contribute to the decline of immune function and to the increased risk of myeloid leukaemia with age61. There is emerging evidence that drifts in the clonal composition of stem cells occur also in other organ systems, such as skeletal muscle86. It is tempting to speculate that these drifts in clonality at the stem cell level lead to changes in the composition and function of various tissues. However, studies on intestinal stem cells (ISCs) have shown that clonal drifts in stem cell compartments can also be neutral87,88. In addition, these studies revealed evidence that oncogenic mutations can lead to clonal selection of ISCs (ref. 89). The clonal advantage of mutant ISCs can be condition-dependent; for example, the selection of p53-deficient ISCs over wild-type ISCs was found to be dependent on the context of chronic inflammation90. Studies on human colon crypts revealed an increase in chromosomal imbalances with increasing age, suggesting that clonal selection may favour mutant stem cells during ageing34.
Molecular mechanisms that lead to the evolution of clonal drifts in ageing stem cell compartments remain to be defined. Interestingly, it was shown that the accumulation of DNA damage leads to the evolution of clonal drifts in HSCs by inducing a BATF (basic leucine zipper transcription factor, ATF-like)-dependent differentiation checkpoint that results in preferential depletion of lymphoid-biased HSCs (ref. 8). It is possible that DNA damage, which occurs in HSCs during physiological ageing in humans91, could also contribute to ageing-associated drifts in clonality in human HSCs. In agreement with this idea, patients with myelodysplastic syndrome (MDS) — an ageing-associated bone marrow failure syndrome characterized by increased myelopoiesis and leukaemia risk — display telomere shortening and BATF induction in CD34+ HSCs (ref. 8).
Clonal evolutions in stem cells may not only contribute to tissue ageing but also to the development of cancer (Fig. 3). Work on HSCs revealed that leukaemia-initiating cells (LICs) show an increase in clonal selection and an elevated malignant potential when the proliferative competition of non-transformed stem and progenitor cells declines92,93. These mechanisms could be highly relevant for the age-dependent increase in malignancies originating from stem and progenitor cells. A series of recent studies identified an age-dependent accumulation of mutations in human HSCs (refs 94,95). Interestingly, these mutations occur frequently in human leukaemia94,96. These data suggest that mutations at the stem cell level can occur before the development of disease symptoms or full-blown leukaemia. Many of these ageing-associated, pre-leukaemic mutations in HSCs affect genes involved in the control of the epigenome, supporting the concept that ageing selects for alterations at the epigenetic level that lead to clonal expansion of aberrant stem cells (Fig. 2), thus setting the stage for the evolution of malignancies. Mechanisms that enhance clonal selection of aberrant stem cells in ageing tissues remain to be delineated. In addition to cell-intrinsic processes and loss of proliferative competition, it is possible that alterations in the systemic blood circulatory environment contribute to this process. In support of this assumption, the accumulation of DNA damage in ageing telomere-dysfunctional mice leads to premature evolution of clonal drifts of HSCs. Interestingly, these changes were associated with a loss of HSC quiescence76, and loss of quiescence was shown to impair the function of HSCs by inducing alterations in the DNA methylation landscape97 (Fig. 2).
Outlook
A variety of mechanisms contribute to the accumulation of genomic damage in ageing stem cells. The relative contribution of these sources of genome instability to human stem cell ageing remains to be delineated. The downstream signals responding to genomic damage determine the consequences for stem cell functionality, but remain incompletely understood. These signals function primarily to protect ageing tissues from cancer formation. However, the same pathways can promote tissue dysfunction and selection of malignant clones during ageing, involving both cell-intrinsic and cell-extrinsic alterations that are activated in response to genome damage. It will be of tremendous interest whether interference with the molecular DNA damage response systems could improve tissue function during ageing and slow the age-dependent increase in cancer. In principle, such approaches would aim to impair age-dependent accumulation of genomic damage and/or alleviate toxic ageing-promoting responses to such genomic insults. Experimental evidence indicates that both approaches work in mouse models of ageing1,98,99. Future research will determine if these approaches can be translated to increase human health-span during ageing.
ACKNOWLEDGEMENTS
We thank the Else Kröner-Fresenius-Foundation for funding the 5th Else Kröner-Fresenius-Symposium in Molecular Medicine on the ‘Role of Stem Cells in Aging, Diseases, and Cancer’. This meeting fostered the discussion on DNA damage in stem cells and was the basis of this Review. We apologize to our colleagues for omitting numerous references due to space limitations.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Axel Behrens, Mammalian Genetics Laboratory, Cancer Research UK London Research Institute, 44 Lincoln’s Inn Fields, London, WC2A 3LY, UK; School of Medicine, King’s College London, Guy’s Campus, London, SE1 1UL, UK.
Jan M. van Deursen, Department of Pediatric and Adolescent Medicine and the Department of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine, Rochester, Minnesota 55905, USA
K. Lenhard Rudolph, Leibniz Institute of Age Research, Fritz Lipmann Institute e.V., Jena, 07745, Germany; Research Group on Molecular Aging, Faculty of Medicine, Friedrich-Schiller-University, Jena, Germany.
Björn Schumacher, Institute for Genome Stability in Ageing and Disease, Medical Faculty, University of Cologne, 50931 Cologne; Cologne Excellence Cluster for Cellular Stress Responses in Aging-Associated Diseases (CECAD), Institute for Genetics, and Systems Biology of Cologne, University of Cologne, Zülpicher Str. 47a, 50674 Cologne, Germany.
References
- 1.Baker DJ, et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat. Cell Biol. 2013;15:96–102. doi: 10.1038/ncb2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker DJ, Weaver RL, van Deursen JM. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Reports. 2013;3:1164–1174. doi: 10.1016/j.celrep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nat. Cell Biol. 2014;16:2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb LA, Harris CC. Advances in chemical carcinogenesis: a historical review and prospective. Cancer Res. 2008;68:6863–6872. doi: 10.1158/0008-5472.CAN-08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Ju Z, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat. Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 11.Inomata K, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Takubo K, et al. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Ruzankina Y, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maryanovich M, et al. The ATM–BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat. Cell. Biol. 2012;14:535–541. doi: 10.1038/ncb2468. [DOI] [PubMed] [Google Scholar]
- 15.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 16.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiff T, et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25:5775–5782. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammazzalorso F, Pirzio LM, Bignami M, Franchitto A, Pichierri P. ATR and ATM differently regulate WRN to prevent DSBs at stalled replication forks and promote replication fork recovery. EMBO J. 2010;29:3156–3169. doi: 10.1038/emboj.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jazayeri A, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 20.Cuadrado M, et al. ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J. Exp. Med. 2006;203:297–303. doi: 10.1084/jem.20051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohrin M, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Insinga A, et al. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proc. Natl Acad. Sci. USA. 2013;110:3931–3936. doi: 10.1073/pnas.1213394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milyavsky M, et al. A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Cell Stem Cell. 2010;7:186–197. doi: 10.1016/j.stem.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Takeishi S, et al. Ablation of Fbxw7 eliminates leukemia-initiating cells by preventing quiescence. Cancer Cell. 2013;23:347–361. doi: 10.1016/j.ccr.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 26.Yahata T, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 27.Hua G, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sotiropoulou PA, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 2010;12:572–582. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- 29.Vaziri H, et al. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc. Natl Acad. Sci. USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsay AJ, et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 2013;45:526–530. doi: 10.1038/ng.2584. [DOI] [PubMed] [Google Scholar]
- 31.Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pucci F, Gardano L, Harrington L. Short telomeres in ESCs lead to unstable differentiation. Cell Stem Cell. 2013;12:479–486. doi: 10.1016/j.stem.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begus-Nahrmann Y, et al. p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat. Genet. 2009;41:1138–1143. doi: 10.1038/ng.426. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh JC, Van Den Berg D, Kang H, Hsieh CL, Lieber MR. Large chromosome deletions, duplications, and gene conversion events accumulate with age in normal human colon crypts. Aging Cell. 2013;12:269–279. doi: 10.1111/acel.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker DJ, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 36.Marthiens V, et al. Centrosome amplification causes microcephaly. Nat. Cell Biol. 2013;15:731–740. doi: 10.1038/ncb2746. [DOI] [PubMed] [Google Scholar]
- 37.Schvartzman JM, Sotillo R, Benezra R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat. Rev. Cancer. 2010;10:102–115. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Z, et al. Alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in telomere-dysfunctional mice. Blood. 2010;115:1481–1489. doi: 10.1182/blood-2009-08-237230. [DOI] [PubMed] [Google Scholar]
- 39.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 40.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 41.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr. Opin. Cell Biol. 2013;25:254–264. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 45.Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat. Cell Biol. 2012;14:186–191. doi: 10.1038/ncb2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Von Maltzahn J, Renaud JM, Parise G, Rudnicki MA. Wnt7a treatment ameliorates muscular dystrophy. Proc. Natl Acad. Sci. USA. 2012;109:20614–20619. doi: 10.1073/pnas.1215765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shea KL, et al. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10:362–369. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janzen V, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 54.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 55.Molofsky AV, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 60.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Geiger H, Rudolph KL. Immunoaging induced by hematopoietic stem cell aging. Curr. Opin. Immunol. 2011;23:532–536. doi: 10.1016/j.coi.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- 64.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 65.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 66.DiGiovanni J, et al. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 2000;60:1561–1570. [PubMed] [Google Scholar]
- 67.Niedernhofer LJ, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 68.van der Pluijm I, et al. Impaired genome maintenance suppresses the growth hormone-–insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 2006;5:e2. doi: 10.1371/journal.pbio.0050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wijnhoven SW, Hoogervorst EM, de Waard H, van der Horst GT, van Steeg H. Tissue specific mutagenic and carcinogenic responses in NER defective mouse models. Mutat. Res. 2007;614:77–94. doi: 10.1016/j.mrfmmm.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 70.Garinis GA, et al. Persistent transcription-blocking DNA lesions trigger somatic growth attenuation associated with longevity. Nat. Cell Biol. 2009;11:604–615. doi: 10.1038/ncb1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marino G, et al. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl Acad. Sci. USA. 2010;107:16268–16273. doi: 10.1073/pnas.1002696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen E, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mostoslavsky R, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 74.Reinhardt HC, Schumacher B. The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 2012;28:128–136. doi: 10.1016/j.tig.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonafe M, et al. A gender-dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur. J. Immunol. 2001;31:2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::aid-immu2357>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 76.Song Z, Zhang J, Ju Z, Rudolph KL. Telomere dysfunctional environment induces loss of quiescence and inherent impairments of hematopoietic stem cell function. Aging Cell. 2012;11:449–455. doi: 10.1111/j.1474-9726.2012.00802.x. [DOI] [PubMed] [Google Scholar]
- 77.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ermolaeva MA, et al. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature. 2013;501:416–420. doi: 10.1038/nature12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ermolaeva MA, Schumacher B. Systemic DNA damage responses: organismal adaptations to genome instability. Trends Genet. 2014 doi: 10.1016/j.tig.2013.12.001. http://dx.doi.org/10.1016/j.tig.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pang WW, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rossi DJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 2011;208:2691–2703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 87.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 88.Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 89.Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 2014;15:62–69. doi: 10.1002/embr.201337799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vermeulen L, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342:995–998. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]
- 91.Rube CE, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Henry CJ, Marusyk A, Zaberezhnyy V, Adane B, DeGregori J. Declining lymphoid progenitor fitness promotes aging-associated leukemogenesis. Proc. Natl Acad. Sci. USA. 2010;107:21713–21718. doi: 10.1073/pnas.1005486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bilousova G, Marusyk A, Porter CC, Cardiff RD, DeGregori J. Impaired DNA replication within progenitor cell pools promotes leukemogenesis. PLoS Biol. 2005;3:e401. doi: 10.1371/journal.pbio.0030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jan M, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Science Transl. Med. 2012;4:149ra118. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Busque L, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat. Genet. 2012;44:1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welch JS, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beerman I, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12:413–425. doi: 10.1016/j.stem.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 98.Choudhury AR, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 99.Tomas-Loba A, et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]