Abstract
Extracorporeal life support (ECLS) has become increasingly popular as a salvage strategy for critically ill adults. Major advances in technology and the severe acute respiratory distress syndrome that characterized the 2009 influenza A(H1N1) pandemic have stimulated renewed interest in the use of venovenous extracorporeal membrane oxygenation (ECMO) and extracorporeal carbon dioxide removal to support the respiratory system. Theoretical advantages of ECLS for respiratory failure include the ability to rest the lungs by avoiding injurious mechanical ventilator settings and the potential to facilitate early mobilization, which may be advantageous for bridging to recovery or to lung transplantation. The use of venoarterial ECMO has been expanded and applied to critically ill adults with hemodynamic compromise from a variety of etiologies, beyond postcardiotomy failure. Although technology and general care of the ECLS patient have evolved, ECLS is not without potentially serious complications and remains unproven as a treatment modality. The therapy is now being tested in clinical trials, although numerous questions remain about the application of ECLS and its impact on outcomes in critically ill adults.
Keywords: extracorporeal circulation, extracorporeal membrane oxygenation, ARDS
The first heart-lung machine was used for human cardiac surgery in 1953. In 1972, Dr. Robert Bartlett reported on the successful use of extracorporeal membrane oxygenation (ECMO) outside of the operating room (1). This groundbreaking work led to the uptake of this technology in neonatal and pediatric populations. Since these early days of extracorporeal life support (ECLS), technologic innovations and ongoing use of ECMO in skilled centers have led to expanded use in adult populations. In more recent years, continued improvements in technology and in the management of patients receiving ECLS, as well as the heightened risk of severe acute respiratory distress syndrome (ARDS) during the 2009 to 2010 influenza A(H1N1) pandemic, have resulted in an increasing number of adult patients being supported with ECLS for cardiopulmonary failure after traditional treatment options have failed. Despite advances in care, indications and guidelines for the use of ECLS in the critically ill adult have yet to be firmly established.
Circuit Configurations, Components Common to All Forms of Extracorporeal Life Support, and Modes of Support
A basic circuit is composed of a blood pump, a membrane lung (or oxygenator), a heat exchanger, and cannulas and tubing. Depending on patient needs, partial to complete cardiopulmonary support (venoarterial [VA]-ECMO) or partial to complete pulmonary support (venovenous [VV]-ECMO) can be achieved. In a typical circuit, venous blood is drained out of a major vein, passed through a pump and a membrane lung for gas exchange, and oxygenated blood is then returned to a major artery (VA-ECMO) or vein (VV-ECMO) (Figure 1). An arteriovenous (AV) extracorporeal circuit that incorporates a pump or uses the patient’s own arterial pressure to drive blood across an oxygenator, or a VV-configured circuit with a low-flow pump, can partially support the respiratory system by effectively removing carbon dioxide (CO2) (extracorporeal CO2 removal [ECCO2R]).
Figure 1.
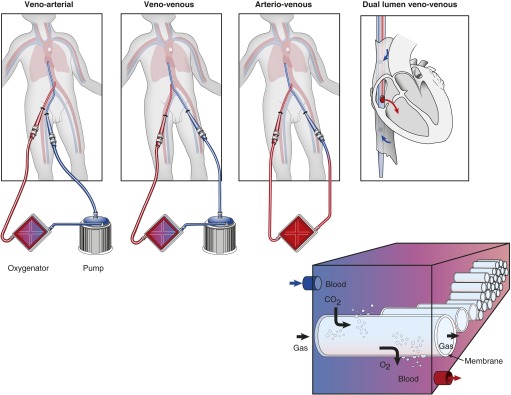
Common extracorporeal life support circuit configurations and schematic of gas exchange in the membrane lung.
Roller and centrifugal pumps are the two basic types of blood pumps used for ECLS, although in recent years adults are typically supported with centrifugal technology. A roller pump displaces blood through flexible tubing located inside a curved raceway to generate forward flow proportional to the pump speed and tubing size; this requires careful servo-regulation of pressures and a larger footprint and is generally inadequate for supporting adults over the longer term. Centrifugal pumps generate a pressure differential across the pump head via spinning pump components and centrifugal force, resulting in negative pressure in the drainage tubing and subsequent blood flow. The relationship between pump speed and blood flow is not directly related, requiring a flow meter. Modern pumps use magnetically driven or magnetically suspended impellers, which spin at the desired revolutions per minute to create blood flow while minimizing heat generation and blood–surface contact and therefore hemolysis (2, 3). Inlet pressure from the drainage limb and outlet pressure from the pump are monitored for excess negative or positive swings, respectively. Additional shunts (e.g., “bridges” between drainage and return limbs for weaning trials) and monitors (e.g., bubble detectors) can be added, but doing so may introduce additional access points and complicate the circuit.
The membrane lung (or oxygenator) is responsible for gas exchange (Figure 1). Oxygenation capacity is dependent on surface area of the membrane and contact with the blood phase. Oxygenator designs have evolved over time from flat sheets to hollow fiber (gas phase inside) membranes and from microporous to compressed microporous (“solid”) designs such that gas exchange occurs entirely by diffusion. Polymethylpentene hollow-fiber devices are best suited for longer-term ECLS and have been shown to have lower rates of hemolysis, better durability with lower pressure differential, and less plasma leakage (4). Fresh gas, or sweep gas, is introduced into the gas phase of the membrane (usually delivered as 100% oxygen, oxygen-ambient air, or oxygen-CO2 mixtures, controlled by a blender) and is adjusted to lower or maintain CO2 levels.
Cannulas and tubing size limit the flow rate achieved, which depends directly on the length and inversely on the radius of the conduits. For adults, typical cannulas range from 23 to 29F for venous drainage and 21 to 23F for blood return (and as small as 17–19F when in a VA configuration) with expected pressure flow characteristics available from the manufacturers. Vascular access can be obtained with extrathoracic percutaneous cannulation using the Seldinger technique, although central cannulation and/or a direct cutdown approach are also possible (5). The femoral vessels usually provide adequate access; a small distal perfusion cannula may be added to avoid or rescue limb ischemia. Alternative arterial access has been achieved in the subclavian and axillary arteries with adjunct synthetic grafting in adults (6, 7). A double-lumen cannula (20–31F) with drainage ports in the inferior and superior venae cavae and a return port positioned in the right atrium with flow directed across the tricuspid valve is available for VV-ECMO and offers single-site internal jugular access (Avalon Elite; Maquet Cardiovascular, Wayne, NJ) (Figure 1). In the United States, circuit components are currently approved for use by the Food and Drug Administration for short-term (6 h) cardiopulmonary bypass.
The goal of ECLS is to support gas exchange and systemic metabolic demands by providing oxygen delivery to the tissues. The degree of support provided for native heart or lung function is in large part dependent on blood flow (as well as patient hemoglobin, inlet hemoglobin saturation, and the properties of the membrane lung). Table 1 details the differences in cardiac and pulmonary effects of VA- versus VV-ECMO. When primary cardiac support is the goal, drainage of blood from the patient to the circuit results in decreased right and left heart filling pressures, reduction in pulmonary blood flow, cardiac unloading, and an improvement in end-organ perfusion. In VA-ECMO, because left ventricular afterload is increased by (usually) retrograde aortic flow, additional interventions may be required to prevent or relieve left ventricular overdistension in certain settings. Targeted flow rates in adults for VA- (and VV-) ECMO are usually 60 to 80 ml/kg/min. During cardiac support, mixed venous oxygen saturation is monitored from the venous drainage limb, and flow rates are adjusted to maintain adequate oxygen delivery.
Table 1.
Key Differences between Venoarterial and Venovenous Extracorporeal Membrane Oxygenation in the Steady State
| VA-ECMO | VV-ECMO | |
|---|---|---|
| Flow characteristics | Nonpulsatile | Nonpulsatile |
| No recirculation | Recirculation* | |
| Direct hemodynamic support | Partial to complete | None |
| LV effects | Decreased preload | None |
| Increased afterload | None | |
| Coronary oxygenation | Native ejection (patient) | Increased |
| RV effects | Decreased preload | None† |
| Decreased afterload | None† | |
| Pulmonary | Gas exchange | Gas exchange |
| Decreased pulmonary blood flow | Unchanged |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; LV = left ventricle; RV = right ventricle; VA = venoarterial; VV = venovenous.
Oxygenated perfusate blood mixed with venous blood, reinfused back into the extracorporeal circuit.
May have subtle effects on RV function via reversal of hypoxic pulmonary vasoconstriction, limiting positive intrathoracic pressure, and augmenting coronary oxygenation.
In the VV configuration, ventricular filling pressures and hemodynamics are unchanged in the steady state, but oxygen and carbon dioxide are exchanged via the membrane lung. Because both the drainage and return cannula are positioned in the venous system, mixing can occur. Recirculation, which is the combination of perfusate (oxygenated) blood and the patient’s venous blood reinfused into the circuit, can limit oxygen delivery. Here, the lungs sit in series (i.e., supraoxygenated perfusate blood is delivered back to the patient’s venous system or right atrium and then must traverse the pulmonary circulation) such that expected arterial oxygen saturations are lower (>85%), depending on the patient’s innate pulmonary function. In this setting, adequate oxygen delivery can be maintained provided cardiac output is sufficient, and especially because cardiac output may be augmented by limiting or removing positive pressure ventilation. CO2 removal is more efficient than oxygenation and thus requires substantially lower flow rates, smaller or pumpless systems, and smaller cannulas.
Systemic anticoagulation, usually with unfractionated heparin, is initiated typically at the time of cannulation to prevent circuit (and patient) thrombosis (Table 2). The ideal anticoagulation strategy and appropriate tests for monitoring (e.g., activating clotting time, anti-factor Xa or heparin assays, activated partial thromboplastin time, thromboelastography) in ECLS are controversial and should be based on laboratory capabilities and institutional standards.
Table 2.
Selected Patient-related Complications of Extracorporeal Life Support as Reported to the Extracorporeal Life Support Organization Registry, by Cardiac versus Respiratory Indication
| Cardiac Indication | Respiratory Indication | |
|---|---|---|
| Hemorrhagic complications, % | ||
| Gastrointestinal | 4 | 6 |
| Cannula site | 20 | 15 |
| Surgical site | 23 | 14 |
| Hemolysis | 7 | 6 |
| DIC | 4 | 3 |
| Central nervous system complications, % | ||
| Infarct* | 4 | 2 |
| Hemorrhage* | 2 | 4 |
| Pulmonary complications, % | ||
| Pneumothorax requiring treatment | 2 | 11 |
| Pulmonary hemorrhage | 3 | 7 |
| Infectious disease complications, % | ||
| Culture-proven infection | 14 | 18 |
| Musculoskeletal complications, % | ||
| Limb ischemia | <1 | <1 |
| Compartment syndrome | <1 | <1 |
| Fasciotomy | <1 | 0 |
| Amputation | <1 | 0 |
Definition of abbreviations: DIC = disseminated intravascular coagulation.
As detected by ultrasound or computed tomography.
Respiratory Support
Respiratory support can be considered while awaiting recovery from hypoxemic respiratory failure, hypercarbic respiratory failure, or massive air leak syndromes or as a way to bridge patients to lung transplantation. Among ECLS centers who voluntarily report to the Extracorporeal Life Support Organization (ELSO) registry, respiratory failure was the most common indication for adults as of January 2014, with more than 5,000 total runs as compared with approximately 4,000 runs for cardiac indications (http://elsonet.org).
As a respiratory support modality, ECLS is most appealing in its potential to reduce (or eliminate) the injurious effects of positive pressure ventilation. Ventilator-associated lung injury from overdistension of relatively spared lung units and cyclical recruitment–derecruitment exacerbates endothelial–epithelial barrier dysfunction, edema formation, and the release of inflammatory mediators in already injured lung (8). High concentrations of inspired oxygen can worsen cytotoxic damage (9). A low volume–low pressure ventilation strategy to reduce mechanical stretch improves outcomes in ARDS; however, some patients may develop refractory hypoxemia and/or have very poor lung compliance and severe respiratory acidosis with low Vt ventilation (LTVV) (10, 11).
Although the concept and triggers for using “salvage therapy” in ARDS remain poorly defined, ECLS may serve as an adjunct to (or in some cases in place of) traditional mechanical ventilation, achieving gas exchange while allowing for “lung rest” by minimizing the volume, pressure, and fraction of inspired oxygen (FiO2) delivered by the ventilator. Higher plateau pressures in patients treated with LTVV and with ECLS have been associated with death in severe ARDS, suggesting there may be no safe plateau pressure limit and that an aggressive strategy to protect the lungs may be beneficial (12, 13). In a small uncontrolled study comparing patients with ARDS treated with standard LTVV to those supported with ECCO2R to achieve very low Vts (4.2 ± 0.3 ml/kg) and end-inspiratory plateau pressures (25.0 ± 1.2 cm H2O), morphologic measures and inflammatory biomarkers of lung injury were improved in those treated with a very low volume–pressure strategy; however, this concept has not been tested in controlled trials (14). Goal ventilator settings for a patient on ECLS typically include a respiratory rate of 6 to 10 breaths per minute, peak inspiratory pressures of 20 to 25 cm H2O, positive end-expiratory pressure of 10 to 15 cm H2O, and FiO2 of 0.3 to 0.4, although optimal ventilator settings are unknown. Vts are usually well under 6 ml/kg of predicted body weight with this strategy.
Hypoxemic Respiratory Failure, ARDS, and the H1N1 Experience
Early randomized trials of ECMO for adults with severe acute respiratory failure and ECCO2R for severe ARDS showed no benefit of ECLS; however, ventilator strategies, circuit configurations, and technology have evolved substantially since these initial studies (15, 16). In the 1990s and 2000s, a number of observational studies from experienced ECLS centers reported survival rates of 52 to 75% in adults with severe respiratory failure supported with ECMO (Table 3) (17–22).
Table 3.
Recent Large Studies of Extracorporeal Life Support for Adults with Respiratory Failure
| Year | 1986–2006 | 1989–1995 | 1989–2003 | 2000–2012 | 2001–2006 | 2007–2010 | 2008–2012 | 2009 | 2009–2010 | 2009–2010 | 2009–2011 |
| First author | Brogan (121) | Lewandowski (18) | Hemmila (19) | Schmidt (128) | Peek (30) | Schmid (139) | Schmidt (136) | Davies (27) | Patroniti (23) | Noah (28) | Pham (12) |
| Source population | ELSO | Germany | Michigan | ELSO | UK* | Germany | France | ANZ | Italy | UK | France |
| H1N1 series | No | No | No | No | No | No (9 with H1N1) | No (36 with H1N1) | Yes | Yes | Yes | Yes |
| No. | 1,473 | 49 | 255 | 2,355 | 90 ECMO referred | 176 | 140 | 68 | 49 | 80 Referred, 69 ECMO | 123 |
| Age, yr | 34 (16–84) | 32 (14) | 38 (13) | 41 (28–54) | 40 (13) | 48 (16) | 44 (30–56) | 34 (27–43) | 39 (32–46) | 34 (28–46) | 42 (13) |
| Male sex, % | — | 57 | 49 | — | 57 | 61 | 50 | 57 | 38 | 50 | |
| BMI, kg/m2 | — | — | — | — | — | 30 (9) | 27 (24–32) | 29 (23–36) | 28 (24–35) | — | 31 (9) |
| SOFA | — | — | — | — | — | 12 (4) | 12 (10–15) | — | 7 (6–9) | 9 (7–10) | 10 (4) |
| APACHE II | — | 18 (5) | — | — | 20 (6) | — | — | — | — | — | — |
| VV-ECMO, % | 78 | 100 | 66 | 82 | — | 100 | 95 | — | — | 84 | 87 |
| PaO2/FiO2, mm Hg | — | 67 (28) | 55 (16) | 59 (48–75) | 76 (30) | 77 (47) | 53 (46–60) | 56 (48–63) | 63 (56–79) | 55 (46–63) | 63 (21) |
| Prior duration MV, d | — | 13 (9) | 4 (3) | 2 (0.8–6) | — | 6 (10) | 5 (1–11) | 2 (1–5) | 2 (1–5) | 4 (2–7) | 2 (1–5) |
| Vt, ml/kg PBW | — | 11 (4) | — | — | — | — | 6 (5–7) | 6 (5–7) | 6 (5–7) | 5 (4–7) | 7 (2) |
| Duration ECLS, d | 6 (3–12) | 23 (20) | 9 (8) | 7 (4–13) | 9 (6–16) | 12 (9) | 15 (8–30) | 10 (7–15) | 10 (7–17) | 9 (6–12) | 11 (8–22) |
| Mortality, % | 50 | 45 | 48 | 43 | 37 | 44 | 40 | 21 | 29 | 24 | 36 |
Definition of abbreviations: ANZ = Australia, New Zealand; APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; ECLS = extracorporeal life support; ELSO = Extracorporeal Life Support Organization; MV = mechanical ventilation; PBW = predicted body weight; SOFA = Sequential Organ Failure Assessment; VV-ECMO = venovenous extracorporeal membrane oxygenation.
Data are expressed as mean (SD) or median (interquartile range).
Conventional ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial.
The excess morbidity and mortality from viral pneumonia and ARDS observed in young persons during the H1N1 pandemic prompted an increase in the use of ECMO for respiratory failure (Table 3) (23–26). Investigators from Australia and New Zealand reported the first case series of 68 patients supported with ECMO for suspected or confirmed H1N1-associated ARDS, 79% of whom were still alive at the end of the study period (27). Those who received ECMO tended to be younger with less comorbid illness as compared with a mechanically ventilated group with confirmed H1N1 from the same centers and ECMO patients also had severe disease, with median (interquartile range) arterial PaO2/FiO2 ratios of 56 (48–63) and acute lung injury scores of 3.8 (3.5–4.0) (27). Noah and colleagues compared patients referred for ECMO support to four specialized centers in the UK to those with suspected or confirmed H1N1 from a prospective cohort (the Swine Flu Triage Study) and found that the risk of death was approximately 50% lower in those referred for ECMO, irrespective of three different statistical matching approaches (28). In a larger registry of patients with influenza A from France (the REVA/French Society of Intensive Care [SRLF] H1N1 registry), there was no difference in outcomes for those who received ECMO as compared with those who did not receive ECMO with severe ARDS when individuals were matched one-to-one, although 50% of the ECMO-treated patients went unmatched, and mortality was lower in this group as compared with matched patients receiving ECMO (12, 29). Survival in an H1N1 cohort from Utah (n = 47) not treated with rescue therapies was on par with reports in which ECMO was used (83%), highlighting the uncertainty that remains about the role of this therapy (and other rescue modalities) in patients with severe respiratory failure (8, 9, 12).
The Conventional ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial randomized patients with severe ARDS to conventional mechanical ventilation versus referral to a single ECMO center (Glenfield Hospital, Leicaster, UK) (30). CESAR enrolled 180 adults (from 766 screened), 18 to 65 years of age, with severe but reversible respiratory failure, and acute lung injury (or Murray) scores greater than 3.0 or a pH less than 7.20 despite optimal mechanical ventilation. Patients receiving high-level mechanical ventilation for more than 7 days and/or with a contraindication to anticoagulation were excluded (31). There was a significantly higher rate of survival without disability at 6 months in those allocated to the intervention (consideration of ECMO) versus the control (conventional management) arm (63 vs. 47%, respectively; relative risk, 0.69; 95% confidence interval, 0.05–0.97; P = 0.03). However, several important caveats about CESAR should be considered. First, only 75% of those referred for ECMO actually received it, as treatment with a standardized protocol including LTVV, diuresis, and prone positioning allowed some to avoid cannulation. Second, practice in the control arm was not standardized. Patients referred for ECMO were more likely to receive LTVV ventilation for longer periods of time as compared with those conventionally managed in the referring hospitals, which could have accounted for the differences in outcome. Third, three of the patients in the control group were lost to follow-up at 6 months and could have changed the results depending on their disability status. Fourth, interhospital transport was not without risk, as five patients died during the transport process (although the primary analysis was intention to treat). Although there were significant differences in lengths of stay and cost between the two groups, ECMO referral was demonstrated to be cost effective by the investigators (30, 32).
Although CESAR demonstrated that care at an ECMO-based center may improve outcomes in adults with severe ARDS, the limitations discussed above make it impossible to conclude that ECMO itself provides benefit. Thus, there remains clinical equipoise to conduct a large clinical trial to define the role of ECMO in adults with severe ARDS. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) is an ongoing randomized, multicenter, open-label trial that will compare 60-day all-cause mortality in patients with severe ARDS treated with rapid initiation (within 3–6 h after optimal medical management) of ECMO plus very low volume–pressure settings with those receiving standardized LTVV (ClinicalTrials.gov, NCT01470703).
Hypercarbic Respiratory Failure and Extracorporeal Carbon Dioxide Removal
CO2 clearance is more efficient than oxygenation and depends largely on sweep flow and membrane characteristics, rather than blood flow (33). Pumpless systems, which use a patient’s arteriovenous pressure gradient to create a shunt from artery to vein (e.g., interventional lung assist [iLA]; NovaLung, Heilbronn, Germany), and circuits similar to those used for renal ultrafiltration that incorporate low-flow pumps and small or dual-lumen venous cannulas (with avoidance of arterial cannulation) have been studied for this purpose (14, 34–37). A retrospective series of 90 patients with ARDS demonstrated rapid normalization of CO2 and modest improvements in oxygenation using iLA, although vasopressors were uniformly required to establish sufficient shunt flow (1–2 L/min) and the rate of lower limb ischemia was 10% (35). A follow-up prospective randomized trial (the Xtravent-study), which compared iLA and very LTVV (approximately 3 ml/kg) to traditional LTVV in adults with severe ARDS (n = 79) found no difference in the primary endpoint of ventilator-free days at 28 and 60 days (38).
Although the use of ECCO2R has not been robustly studied in acute exacerbations of airways disease, it stands to reason that because the primary abnormality is ventilatory failure, the potential for lower flow rates and/or pumpless configurations may offer a more favorable side effect profile as compared with VV-ECMO. ECCO2R may also allow for the minimization of sedation and promotion of early mobilization in certain patients. Several small studies have been published on the use of ECCO2R to avoid or replace mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease (39–41). In two studies using lung-assist systems, low flow rates achieved improvements in respiratory acidosis, avoidance of invasive ventilation in many patients, and, in one study, shorter hospital lengths of stay as compared with age-, diagnosis-, and acuity-matched control subjects receiving mechanical ventilation (37, 39, 41). In one of these reports, the ECCO2R circuit consisted of a 15.5F coaxial cannula (placed in the internal jugular or femoral position) with a centrifugal pump to enhance CO2 clearance to achieve targeted flow rates of 0.3–0.5 L/min (Hemolung Respiratory Assist System; ALung Technologies, Inc, Pittsburgh, PA) (37, 39). In a pilot study of five patients with acute exacerbations of chronic obstructive pulmonary disease, ECCO2R and dual-lumen small cannulae (20–23F) were successfully used for early extubation and ambulation (40).
Bridge to Lung Transplantation and Primary Graft Dysfunction
As mechanically ventilated patients have a worse survival after lung transplant, there has been a renewed interest in the use of ECLS as a bridging strategy to transplantation, although this approach remains controversial (42). Small series have reported the use of ECLS alone or in combination with standard mechanical ventilation, with mixed results (43–48). In an analysis of lung transplant recipients in the United Network for Organ Sharing Registry from 1987 to 2008, the use of preoperative ECMO (n = 51) resulted in higher rates of retransplantation (as compared with recipients not requiring preoperative life support) and was an independent predictor of death after transplantation (47). One-year survival in patients bridged to lung transplantation from ECMO has been reported to be between 62 and 75% with duration of mechanical support before transplant inversely related to outcomes (49, 50). A strategy of “awake ECMO” for patients awaiting lung transplantation has been reported more recently and offers the theoretical advantage of early mobilization, rehabilitation, and avoidance of endotracheal intubation and its associated complications (49, 51–56). Fuehner and colleagues reported a retrospective single-center experience comparing awake ECMO recipients to a historical control group who had received invasive ventilation as a bridge to transplant and found a higher likelihood of survival at 6 months in the awake ECMO group (80 vs. 50%, P = 0.02) and shorter post-transplant ventilator courses despite similar durations of bridging therapies and rates of waiting list deaths (53).
The most appropriate ECLS configuration for a given patient being bridged to lung transplant depends on the underlying disease process. Patients with significant right heart failure and/or hemodynamic compromise may require VA-ECMO support, whereas those with obstructive or restrictive lung disease can be supported with VV-ECMO or ECCO2R. Given the uncertainty of the available donor pool and the possibility of prolonged waitlist time on bridging therapy, the ideal extracorporeal circuit would have the potential for long-term use. Several cases have been reported describing the use of pulmonary artery to left atrial shunts including a parallel oxygenator (iLA; NovaLung) to support patients with pulmonary arterial hypertension and right ventricular failure for up to 62 days while awaiting transplant (57–59).
In the post-transplant period, VV- and VA-ECMO have been used to support patients who develop primary graft dysfunction (PGD). Although there have been no controlled studies, single-center observations suggest the use of ECMO in severe PGD with inadequate gas exchange despite maximal medical management has greater than 60% survival in the immediate post-transplant period, especially when instituted early (within 24 h), although significant ECMO-related complications have been reported and long-term survival as well as allograft function are generally poor in recipients with severe PGD (60–64). In a review of 151 cases reported to ELSO, 42% of patients receiving ECMO for post-transplant PGD survived to hospital discharge, although this estimate is susceptible to selection and reporting bias (65). Elevated oxygenation index (mean airway pressure × FiO2/PaO2 ≥ 30) after transplant predicted the development of severe PGD likely to require ECMO in a single study, with excellent survival when ECMO was initiated early in response to this marker (80 vs. 15% for those with late or no intervention, P = 0.02) (63). It has been suggested that early ECMO be considered for severe PGD in those who are at greatest risk for developing graft failure (e.g., recipient diagnosis of pulmonary hypertension) (66, 67). There has been limited experience with the use of ECLS to enhance organ procurement in both brain- and cardiac-death donors (68–70).
Cardiac Support
A number of options exist for mechanical circulatory support in cardiac failure. In acutely decompensated patients, a temporary bridging strategy may best serve patients as the most appropriate destination therapy is being determined. There are no controlled trials comparing other short-term devices (i.e., intraaortic balloon counterpulsation or temporary ventricular assist devices [VAD]) to VA-ECMO for refractory cardiogenic shock, but ECLS may be used as a salvage strategy in this setting (71–73). Failure to wean from intraoperative cardiopulmonary bypass (postcardiotomy), acute myocardial infarction, ischemic cardiomyopathy, acute myocarditis, and postpartum cardiomyopathy are common indications for VA-ECMO. Successful transitioning from ECMO directly to cardiac transplant has been described, as has bridging to cardiac retransplantation after allograft failure, although patients supported with VADs had better survival than those bridged with ECMO while awaiting retransplant in the United Network for Organ Sharing Registry (74–77). Short-term mechanical circulatory support may be the most feasible approach for patients presenting remotely, and mobile ECLS services have been developed for this purpose (78). Reported survival rates for VA-ECMO vary widely depending on the indication but average around 40% in reports from experienced centers (71, 75, 79–82).
ECLS may be considered in patients requiring emergent cardiac catheterization. Small series have described the successful use of ECLS for patients who suffer a cardiac arrest during percutaneous intervention or transcatheter aortic valve implantation, while providing therapeutic hypothermia (83, 84). A retrospective series from Japan of 86 patients described the use of VA-ECMO to assist in revascularization for patients in refractory cardiac arrest (85). Intraarrest percutaneous intervention with ECMO support was achieved in 71% of the cohort (with concurrent therapeutic hypothermia in 37% of cases); 29 and 24% of patients survived to 30 days and had favorable neurologic outcomes, respectively (85). As compared with the more traditional bridging devices used in the catheterization laboratory, ECLS provides additional gas-exchange support for those patients with oxygenation issues and/or underlying lung disease and supports right ventricular function in those patients with biventricular failure.
Extracorporeal Cardiopulmonary Resuscitation
Although there have been no controlled studies, two observational, single-center studies have suggested extracorporeal cardiopulmonary resuscitation (ECPR) confers a survival benefit in adults suffering in-hospital cardiac arrest (86, 87). Investigators from Taiwan prospectively compared the use of ECPR in adults with witnessed in-hospital cardiac arrest to those treated with traditional cardiopulmonary resuscitation (CPR) (87). Patients were eligible for ECPR if a cardiac etiology was felt to be the cause of the arrest and after failure of return of spontaneous circulation for at least 10 minutes (n = 59); all others received standard CPR (n = 113). After propensity matching, the risk of death at discharge, 30 days, and 1 year was approximately halved among those who had received ECPR, and there were significant differences in survival to discharge even among those who had been resuscitated for prolonged periods of time (>60 min). Treatment assignment (ECPR vs. traditional CPR) was not randomly allocated, and there were baseline differences between the groups not accounted for by matching, thus raising concerns about residual bias and confounding in this study (88). Single-center series have reported ∼30% survival rates to hospital discharge combining ECPR with traditional resuscitation efforts in experienced centers where ECLS can be rapidly deployed (87, 89–92).
Potential Additional Uses in Critical Illness
ECLS has been used to rescue critically ill adults for etiologies outside of primary cardiopulmonary failure, but evidence supporting the expansion of ECLS use in the critical care setting is lacking. In a retrospective series of 52 patients with septic shock supported with VA-ECMO, 75% of whom had at least three organ failures, only 15% survived to hospital discharge, and thus sepsis remains a controversial indication in adults (93). Because myocardial depression can occur in some patients with sepsis and given advances in ECLS technology, this may be an important area for future study. In a recent retrospective series of 14 patients supported with VA-ECMO for refractory septic shock and documented acute severe left ventricular dysfunction, 71% were discharged home and were alive after 13 months (94). In trauma patients, the risks of systemic anticoagulation and the consumptive coagulopathy that often develops need to be strongly considered when evaluating such a patient for ECLS. In the 1990s, modified extracorporeal circuits were used to reverse hypothermia via countercurrent rewarming and to assist in massive resuscitation, and several successful reports or small series of ECMO for support of trauma patients followed (95–100). Miniaturized ECLS circuits have allowed for interhospital transfer to trauma centers and successful evacuation of severely injured soldiers, even when war zone cannulation is required (101, 102). A number of case reports have been published describing the use of VV-ECMO or ECCO2R in patients with ARDS due to severe burns and/or smoke inhalation injury (103–108). Existing literature on the use of ECLS for burn patients was summarized recently by Asmussen and colleagues; to date, no large prospective series exist for this indication (109). Finally, because the leading cause of death in acute drug intoxication is cardiovascular collapse, ECLS has been successfully used for overdose after traditional supportive measures fail (110–113). Daubin and colleagues recently published a retrospective report of 17 cases of severe poisoning requiring ECLS for refractory shock from a variety of drugs over a 10-year period (114). Survival to hospital discharge was 76% without significant neurologic sequelae. There was a high rate (60%) of cannula-related complications, although most of these occurred before a change in technique to provide a distal shunt for limb perfusion, as has been seen in prior series (114, 115).
Relative Contraindications and Complications
There are no absolute contraindications to ECLS, and each patient should be evaluated on a case-by-case basis and in the context of the individual center’s experience. ECLS can be associated with a number of complications, however, including bleeding, thrombosis, infection, and distal limb ischemia. Patient-related complications by VA versus VV configuration as voluntarily reported to ELSO are presented in Table 2, acknowledging that these data may be subject to reporting bias. With improvements in technology and changes in clinical practice (e.g., miniaturized, coated circuits; conservative anticoagulation and transfusion strategies), complication rates may decrease over time. Patients with a history of recent central nervous system hemorrhage and other contraindications to anticoagulation are generally considered high-risk candidates for extracorporeal support. Although anticoagulation can be withheld or reduced for brief periods of time, the use of ECLS in patients with bleeding diathesis (e.g., diffuse alveolar hemorrhage or intracranial bleeding) is supported by case reports only (116–120). Rates of central nervous system infarction or hemorrhage and surgical bleeding were roughly 5 and 30%, respectively, in a large series of adult patients with respiratory failure supported with ECLS from the ELSO registry, 78% of whom were supported with a VV configuration (121). The morbidity associated with VA-ECMO for cardiac failure is higher (as one would anticipate with cannulation of a major artery and in patients receiving ECPR or ECLS postcardiotomy) with frequent reporting of major bleeding (41%), infection (31%), and lesser but considerable rates of lower extremity ischemia (17%), neurologic complications (13%), stroke (6%), and lower extremity amputation (5%) (79). In a series of 81 patients who received ECMO for refractory cardiogenic shock, the majority (57%) experienced at least one major complication related to ECLS (82).
Life-limiting comorbidities or additional organ failures that are not considered reversible limit the potential benefit of ECLS. Advanced vascular disease or ease of vascular access should also be considered when assessing a patient’s candidacy. Increasing age has been consistently associated with worse outcomes across many observational studies, although individual patients of advanced age may be considered candidates depending on indication for support and comorbid illness(es) (121–123). Renal failure has been repeatedly shown to predict a poor prognosis in patients on VA-ECMO (78, 82, 122–124). Acute myocarditis as an indication for VA support appears to be associated with better survival, as does male sex (19, 82, 125, 126). When considering patients for respiratory support, longer duration of mechanical ventilation before ECMO clearly predicts a poor prognosis (particularly when the patient has been mechanically ventilated for more than 7 d), and the CESAR trial excluded those patients who had been on high pressure (>30 cm H2O) or high FiO2 (>0.80) for longer than 7 days before study entry (17–19, 30, 127). The ELSO registry was recently used to develop and validate a survival equation for adults with respiratory failure requiring ECMO, the Respiratory ECMO Survival Prediction Score (RESP-score), incorporating many of the above-mentioned pre-ECMO variables, with good discrimination in a small validation cohort (c-statistic = 0.92; 95% confidence interval, 0.89–0.97) (128).
In addition to patient-related complications, equipment-related failures can occur. Observations from the last 2 decades suggest relatively low rates of circuit rupture (2%) and clotting (20%) (121). Polymethylpentene hollow-fiber oxygenators and heparin-coated circuits have improved gas exchange capabilities, reduced shear stress and hemolysis, and improved durability when compared with earlier generation silicone oxygenators (129). Oxygenator failure occurred in 12 and 7% of adult respiratory and cardiac cases, respectively, with widely variable run times (www.elso.org). Bicaval dual-lumen catheters (Avalon Elite; Maquet) for VV-ECMO offers single-site cannulation and may enhance patient mobilization and reduce recirculation; however, care must be taken to place these cannulas under direct visualization using either fluoroscopic or echocardiographic guidance, as major vessel injury and right ventricular rupture have been reported (130, 131).
Resource Use and Long-Term Outcomes
The provision of ECLS services is a resource-intense endeavor that ideally includes a multidisciplinary care team, transport services, ongoing educational/simulation programs, equipment updates, and follow-up care. In the CESAR trial, health-care costs per ECMO-referred patient were more than double those for patients managed conventionally (difference in costs, $65,519, with longer intensive care unit and hospital stays in the ECMO group, even among nonsurvivors), but ECMO treatment translated to a small gain in quality-adjusted life years at 6 months and a favorable lifetime predicted cost-utility of roughly $31,000 per life-year (30, 132).
Although current ELSO guidelines outline important requirements for ECMO centers, including the need for an experienced physician program director, a full-time ECMO coordinator, and ongoing training and protocolization for specialists, there is no formal accreditation process for centers and specialists at this time. To this end, this review is accompanied by an international consensus statement on program development for centers offering ECMO for adults with respiratory failure (133). To provide appropriate care, an ECMO center should complete at least six runs annually; adequate center volume may be closer to 15 or 20 annual ECLS runs, as these thresholds have recently been tied to better survival in neonatal and pediatric populations (www.elso.org) (134, 135).
Little is known about the long-term sequelae of ECLS in adult survivors. In the absence of randomized trials with comparable control groups, it is difficult to distinguish morbidity related to critical illness and organ failures from complications related to ECLS itself. French investigators have reported a 6-month survival rate approaching 60% for patients with severe ARDS treated with ECMO, although survivors had impaired health-related quality-of-life scores in physical and emotional well-being as compared with control subjects (136). Similarly, in a cohort of French patients who received VA-ECMO for refractory cardiogenic shock, survivors had long-term deficits in physical and social function as compared with control subjects, although they had better quality-of-life indices than patients with chronic medical conditions and those recovering from ARDS (82). Longitudinal data from the CESAR trial demonstrated no difference in validated surveys of health status, respiratory symptoms, or spirometric measures by treatment allocation at 6 months (30). In a small series of adults followed for 0.5 to 12 years after ECMO, 52% had abnormal neurocognitive function, with a high incidence of neuroradiologic abnormalities, especially among those who had received VA-ECMO (75%) (137). Among 87 adults who had received ECLS for predominantly cardiac indications, 50% suffered neurologic injury and 9 of 10 nonsurvivors with brain autopsies available demonstrated areas of hemorrhage or hypoxic ischemic injury (138).
Conclusions
ECLS is being increasingly used to support critically ill adults who have failed conventional management. The pioneering work of well-established centers, technologic advances in circuit components, and the experience gained in the 2009 to 2010 influenza A(H1N1) pandemic have allowed for the successful rescue of many patients, but significant questions remain regarding the appropriate patient population, the optimal circuitry and cannulation technique, ventilation strategies, patient mobility, outcomes, and cost-effectiveness. Controlled trials are needed to better define indications and best practices for this therapy.
Acknowledgments
Acknowledgment
The authors thank medical illustrator Dr. Francois Luks and Drs. Mitchell Levy and Steven Kawut for their thoughtful review of the manuscript.
Footnotes
C.E.V. is supported by the American Heart Association grant 11FTF7400032 and the National Institute of General Medical Sciences grant P20GM103652.
CME will be available for this article at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201404-0736CI on July 21, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bartlett RH, Gazzaniga AB, Fong SW, Jefferies MR, Roohk HV, Haiduc N. Extracorporeal membrane oxygenator support for cardiopulmonary failure. Experience in 28 cases. J Thorac Cardiovasc Surg. 1977;73:375–386. [PubMed] [Google Scholar]
- 2.Lawson DS, Ing R, Cheifetz IM, Walczak R, Craig D, Schulman S, Kern F, Shearer IR, Lodge A, Jaggers J. Hemolytic characteristics of three commercially available centrifugal blood pumps. Pediatr Crit Care Med. 2005;6:573–577. doi: 10.1097/01.pcc.0000163282.63992.13. [DOI] [PubMed] [Google Scholar]
- 3.Tamari Y, Lee-Sensiba K, Leonard EF, Parnell V, Tortolani AJ. The effects of pressure and flow on hemolysis caused by Bio-Medicus centrifugal pumps and roller pumps. Guidelines for choosing a blood pump. J Thorac Cardiovasc Surg. 1993;106:997–1007. [PubMed] [Google Scholar]
- 4.Horton S, Thuys C, Bennett M, Augustin S, Rosenberg M, Brizard C. Experience with the Jostra rotaflow and QuadroxD oxygenator for ECMO. Perfusion. 2004;19:17–23. doi: 10.1191/0267659104pf702oa. [DOI] [PubMed] [Google Scholar]
- 5.Pranikoff T, Hirschl RB, Remenapp R, Swaniker F, Bartlett RH. Venovenous extracorporeal life support via percutaneous cannulation in 94 patients. Chest. 1999;115:818–822. doi: 10.1378/chest.115.3.818. [DOI] [PubMed] [Google Scholar]
- 6.Hysi I, Fabre O, Renaut C, Guesnier L. Extracorporeal membrane oxygenation with direct axillary artery perfusion. J Card Surg. 2014;29:268–269. doi: 10.1111/jocs.12229. [DOI] [PubMed] [Google Scholar]
- 7.Javidfar J, Brodie D, Costa J, Miller J, Jurrado J, LaVelle M, Newmark A, Takayama H, Sonett JR, Bacchetta M. Subclavian artery cannulation for venoarterial extracorporeal membrane oxygenation. ASAIO J. 2012;58:494–498. doi: 10.1097/MAT.0b013e318268ea15. [DOI] [PubMed] [Google Scholar]
- 8.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 9.Martin DS, Grocott MP. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit Care Med. 2013;41:423–432. doi: 10.1097/CCM.0b013e31826a44f6. [DOI] [PubMed] [Google Scholar]
- 10.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 11.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, Mourvillier B, Ara-Somohano C, Bastien O, Zogheib E, et al. REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 13.Hager DN, Krishnan JA, Hayden DL, Brower RG ARDS Clinical Trials Network. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 15.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC, II, Thomas AN, Proctor HJ, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 16.Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF, Jr, Weaver LK, Dean NC, Thomas F, East TD, Pace NL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 17.Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112:759–764. doi: 10.1378/chest.112.3.759. [DOI] [PubMed] [Google Scholar]
- 18.Lewandowski K, Rossaint R, Pappert D, Gerlach H, Slama KJ, Weidemann H, Frey DJ, Hoffmann O, Keske U, Falke KJ. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med. 1997;23:819–835. doi: 10.1007/s001340050418. [DOI] [PubMed] [Google Scholar]
- 19.Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults Ann Surg 2004240595–605.[Discussion, pp. 605–607] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullrich R, Lorber C, Röder G, Urak G, Faryniak B, Sladen RN, Germann P. Controlled airway pressure therapy, nitric oxide inhalation, prone position, and extracorporeal membrane oxygenation (ECMO) as components of an integrated approach to ARDS. Anesthesiology. 1999;91:1577–1586. doi: 10.1097/00000542-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Rich PB, Awad SS, Kolla S, Annich G, Schreiner RJ, Hirschl RB, Bartlett RH. An approach to the treatment of severe adult respiratory failure. J Crit Care. 1998;13:26–36. doi: 10.1016/s0883-9441(98)90026-0. [DOI] [PubMed] [Google Scholar]
- 22.Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH.Extracorporeal life support for 100 adult patients with severe respiratory failure Ann Surg 1997226544–564.[Discussion, pp. 565–566] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong GL, Brammer L, Finelli L. Timely assessment of the severity of the 2009 H1N1 influenza pandemic. Clin Infect Dis. 2011;52:S83–S89. doi: 10.1093/cid/ciq013. [DOI] [PubMed] [Google Scholar]
- 25.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 26.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, et al. California Pandemic (H1N1) Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 27.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 28.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 29.Cooper DJ, Hodgson CL. Extracorporeal membrane oxygenation rescue for H1N1 acute respiratory distress syndrome: equipoise regained. Am J Respir Crit Care Med. 2013;187:224–226. doi: 10.1164/rccm.201211-2052ED. [DOI] [PubMed] [Google Scholar]
- 30.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. [Google Scholar]
- 31.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 32.Zwischenberger JB, Lynch JE. Will CESAR answer the adult ECMO debate? Lancet. 2009;374:1307–1308. doi: 10.1016/S0140-6736(09)61630-5. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Bréchot N, Merceron S, Luyt CE, Trouillet JL, Chastre J, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013;39:838–846. doi: 10.1007/s00134-012-2785-8. [DOI] [PubMed] [Google Scholar]
- 34.Liebold A, Reng CM, Philipp A, Pfeifer M, Birnbaum DE. Pumpless extracorporeal lung assist - experience with the first 20 cases. Eur J Cardiothorac Surg. 2000;17:608–613. doi: 10.1016/s1010-7940(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 35.Bein T, Weber F, Philipp A, Prasser C, Pfeifer M, Schmid FX, Butz B, Birnbaum D, Taeger K, Schlitt HJ. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med. 2006;34:1372–1377. doi: 10.1097/01.CCM.0000215111.85483.BD. [DOI] [PubMed] [Google Scholar]
- 36.Ricci D, Boffini M, Del Sorbo L, El Qarra S, Comoglio C, Ribezzo M, Bonato R, Ranieri VM, Rinaldi M. The use of CO2 removal devices in patients awaiting lung transplantation: an initial experience. Transplant Proc. 2010;42:1255–1258. doi: 10.1016/j.transproceed.2010.03.117. [DOI] [PubMed] [Google Scholar]
- 37.Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39:1382–1387. doi: 10.1097/CCM.0b013e31820eda45. [DOI] [PubMed] [Google Scholar]
- 38.Bein T, Weber-Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, Muellenbach R, Dembinski R, Graf BM, Wewalka M, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burki NK, Mani RK, Herth FJ, Schmidt W, Teschler H, Bonin F, Becker H, Randerath WJ, Stieglitz S, Hagmeyer L, et al. A novel extracorporeal CO2 removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest. 2013;143:678–686. doi: 10.1378/chest.12-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abrams DC, Brenner K, Burkart KM, Agerstrand CL, Thomashow BM, Bacchetta M, Brodie D. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10:307–314. doi: 10.1513/AnnalsATS.201301-021OC. [DOI] [PubMed] [Google Scholar]
- 41.Kluge S, Braune SA, Engel M, Nierhaus A, Frings D, Ebelt H, Uhrig A, Metschke M, Wegscheider K, Suttorp N, et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med. 2012;38:1632–1639. doi: 10.1007/s00134-012-2649-2. [DOI] [PubMed] [Google Scholar]
- 42.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, et al. International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: Thirtieth adult lung and heart-lung transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Lang G, Taghavi S, Aigner C, Rényi-Vámos F, Jaksch P, Augustin V, Nagayama K, Ghanim B, Klepetko W. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation. 2012;93:729–736. doi: 10.1097/TP.0b013e318246f8e1. [DOI] [PubMed] [Google Scholar]
- 44.Hämmäinen P, Schersten H, Lemström K, Riise GC, Kukkonen S, Swärd K, Sipponen J, Silverborn M, Dellgren G. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant. 2011;30:103–107. doi: 10.1016/j.healun.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 45.de Perrot M, Granton JT, McRae K, Cypel M, Pierre A, Waddell TK, Yasufuku K, Hutcheon M, Chaparro C, Singer L, et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30:997–1002. doi: 10.1016/j.healun.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Toyoda Y, Bhama JK, Shigemura N, Zaldonis D, Pilewski J, Crespo M, Bermudez C.Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation J Thorac Cardiovasc Surg 20131451065–1070.[Discussion, pp. 1070–1061] [DOI] [PubMed] [Google Scholar]
- 47.Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH.Should lung transplantation be performed for patients on mechanical respiratory support? The US experience J Thorac Cardiovasc Surg 2010139765–773.e761 [DOI] [PubMed] [Google Scholar]
- 48.Haneya A, Philipp A, Mueller T, Lubnow M, Pfeifer M, Zink W, Hilker M, Schmid C, Hirt S. Extracorporeal circulatory systems as a bridge to lung transplantation at remote transplant centers. Ann Thorac Surg. 2011;91:250–255. doi: 10.1016/j.athoracsur.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Crotti S, Iotti GA, Lissoni A, Belliato M, Zanierato M, Chierichetti M, Di Meo G, Meloni F, Pappalettera M, Nosotti M, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest. 2013;144:1018–1025. doi: 10.1378/chest.12-1141. [DOI] [PubMed] [Google Scholar]
- 50.Dellgren G, Riise GC, Swärd K, Gilljam M, Rexius H, Liden H, Silverborn M. Extracorporeal membrane oxygenation as a bridge to lung transplantation: a long-term study. Eur J Cardiothorac Surg. 2014;21:21. doi: 10.1093/ejcts/ezu112. [DOI] [PubMed] [Google Scholar]
- 51.Del Sorbo L, Ranieri VM, Keshavjee S. Extracorporeal membrane oxygenation as “bridge” to lung transplantation: what remains in order to make it standard of care? Am J Respir Crit Care Med. 2012;185:699–701. doi: 10.1164/rccm.201202-0193ED. [DOI] [PubMed] [Google Scholar]
- 52.Nosotti M, Rosso L, Tosi D, Palleschi A, Mendogni P, Nataloni IF, Crotti S, Tarsia P. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:55–59. doi: 10.1093/icvts/ivs433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, Olsson KM, Greer M, Sommer W, Welte T, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–768. doi: 10.1164/rccm.201109-1599OC. [DOI] [PubMed] [Google Scholar]
- 54.Olsson KM, Simon A, Strueber M, Hadem J, Wiesner O, Gottlieb J, Fuehner T, Fischer S, Warnecke G, Kühn C, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant. 2010;10:2173–2178. doi: 10.1111/j.1600-6143.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 55.Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, Zemmel D, Galuskin K, Morrone TM, Boerem P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014;18:R38. doi: 10.1186/cc13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia JP, Kon ZN, Evans C, Wu Z, Iacono AT, McCormick B, Griffith BP. Ambulatory veno-venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg. 2011;142:755–761. doi: 10.1016/j.jtcvs.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 57.Schmid C, Philipp A, Hilker M, Arlt M, Trabold B, Pfeiffer M, Schmid FX. Bridge to lung transplantation through a pulmonary artery to left atrial oxygenator circuit. Ann Thorac Surg. 2008;85:1202–1205. doi: 10.1016/j.athoracsur.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Camboni D, Philipp A, Arlt M, Pfeiffer M, Hilker M, Schmid C. First experience with a paracorporeal artificial lung in humans. ASAIO J. 2009;55:304–306. doi: 10.1097/MAT.0b013e31819740a0. [DOI] [PubMed] [Google Scholar]
- 59.Strueber M, Hoeper MM, Fischer S, Cypel M, Warnecke G, Gottlieb J, Pierre A, Welte T, Haverich A, Simon AR, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant. 2009;9:853–857. doi: 10.1111/j.1600-6143.2009.02549.x. [DOI] [PubMed] [Google Scholar]
- 60.Glassman LR, Keenan RJ, Fabrizio MC, Sonett JR, Bierman MI, Pham SM, Griffith BP.Extracorporeal membrane oxygenation as an adjunct treatment for primary graft failure in adult lung transplant recipients J Thorac Cardiovasc Surg 1995110723–726.[Discussion, pp. 726–727] [DOI] [PubMed] [Google Scholar]
- 61.Bittner HB, Lehmann S, Rastan A, Garbade J, Binner C, Mohr FW, Barten MJ.Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation and graft recovery Ann Thorac Surg 201294942–949.[Author reply, pp. 949–950] [DOI] [PubMed] [Google Scholar]
- 62.Hartwig MG, Walczak R, Lin SS, Davis RD. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg. 2012;93:366–371. doi: 10.1016/j.athoracsur.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 63.Fiser SM, Kron IL, McLendon Long S, Kaza AK, Kern JA, Tribble CG. Early intervention after severe oxygenation index elevation improves survival following lung transplantation. J Heart Lung Transplant. 2001;20:631–636. doi: 10.1016/s1053-2498(01)00249-2. [DOI] [PubMed] [Google Scholar]
- 64.Bermudez CA, Adusumilli PS, McCurry KR, Zaldonis D, Crespo MM, Pilewski JM, Toyoda Y. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg. 2009;87:854–860. doi: 10.1016/j.athoracsur.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 65.Fischer S, Bohn D, Rycus P, Pierre AF, de Perrot M, Waddell TK, Keshavjee S. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: analysis of the Extracorporeal Life Support Organization (ELSO) registry. J Heart Lung Transplant. 2007;26:472–477. doi: 10.1016/j.healun.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 66.Fang A, Studer S, Kawut SM, Ahya VN, Lee J, Wille K, Lama V, Ware L, Orens J, Weinacker A, et al. Lung Transplant Outcomes Group. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest. 2011;139:782–787. doi: 10.1378/chest.09-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT working group on primary lung graft dysfunction part VI: Treatment. J Heart Lung Transplant. 2005;24:1489–1500. doi: 10.1016/j.healun.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Fondevila C, Hessheimer AJ, Ruiz A, Calatayud D, Ferrer J, Charco R, Fuster J, Navasa M, Rimola A, Taurá P, et al. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant. 2007;7:1849–1855. doi: 10.1111/j.1600-6143.2007.01846.x. [DOI] [PubMed] [Google Scholar]
- 69.Migliaccio ML, Zagli G, Cianchi G, Lazzeri C, Bonizzoli M, Cecchi A, Anichini V, Gensini GF, Peris A. Extracorporeal membrane oxygenation in brain-death organ and tissues donors: a single-centre experience. Br J Anaesth. 2013;111:673–674. doi: 10.1093/bja/aet323. [DOI] [PubMed] [Google Scholar]
- 70.Bodzin AS, Hirose H, West S, Hasz R, Maley WR, Cavarocchi NC. Outcome of organs procured from donors on extracorporeal membrane oxygenation support: an analysis of kidney and liver allograft data. Clin Transplant. 2014;28:816–820. doi: 10.1111/ctr.12384. [DOI] [PubMed] [Google Scholar]
- 71.Pagani FD, Lynch W, Swaniker F, Dyke DB, Bartlett R, Koelling T, Moscucci M, Deeb GM, Bolling S, Monaghan H, et al. Extracorporeal life support to left ventricular assist device bridge to heart transplant: a strategy to optimize survival and resource utilization. Circulation. 1999;100:II206–II210. doi: 10.1161/01.cir.100.suppl_2.ii-206. [DOI] [PubMed] [Google Scholar]
- 72.Pagani FD, Aaronson KD, Swaniker F, Bartlett RH.The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device Ann Thorac Surg 200171S77–S81.[Discussion, pp. S82–S85] [DOI] [PubMed] [Google Scholar]
- 73.Smedira NG, Blackstone EH.Postcardiotomy mechanical support: risk factors and outcomes Ann Thorac Surg 200171S60–S66.[Discussion, pp. S82–S85] [DOI] [PubMed] [Google Scholar]
- 74.Khan MS, Mery CM, Zafar F, Adachi I, Heinle JS, Cabrera AG, Fraser CD, Jr, Morales DL. Is mechanically bridging patients with a failing cardiac graft to retransplantation an effective therapy? Analysis of the United Network of Organ Sharing database. J Heart Lung Transplant. 2012;31:1192–1198. doi: 10.1016/j.healun.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Takayama H, Truby L, Koekort M, Uriel N, Colombo P, Mancini DM, Jorde UP, Naka Y. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32:106–111. doi: 10.1016/j.healun.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Lehmann S, Uhlemann M, Etz CD, Garbade J, Schroeter T, Borger M, Misfeld M, Bittner HB, Mohr FW. Extracorporeal membrane oxygenation: experience in acute graft failure after heart transplantation. Clin Transplant. 2014;28:789–796. doi: 10.1111/ctr.12380. [DOI] [PubMed] [Google Scholar]
- 77.D’Alessandro C, Golmard JL, Barreda E, Laali M, Makris R, Luyt CE, Leprince P, Pavie A. Predictive risk factors for primary graft failure requiring temporary extra-corporeal membrane oxygenation support after cardiac transplantation in adults. Eur J Cardiothorac Surg. 2011;40:962–969. doi: 10.1016/j.ejcts.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 78.Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Léger P, Pavie A, Combes A, Leprince P. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program) Eur Heart J. 2013;34:112–120. doi: 10.1093/eurheartj/ehs081. [DOI] [PubMed] [Google Scholar]
- 79.Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 80.Bermudez CA, Rocha RV, Toyoda Y, Zaldonis D, Sappington PL, Mulukutla S, Marroquin OC, Toma C, Bhama JK, Kormos RL. Extracorporeal membrane oxygenation for advanced refractory shock in acute and chronic cardiomyopathy. Ann Thorac Surg. 2011;92:2125–2131. doi: 10.1016/j.athoracsur.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 81.Lamarche Y, Cheung A, Ignaszewski A, Higgins J, Kaan A, Griesdale DE, Moss R. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J Thorac Cardiovasc Surg. 2011;142:60–65. doi: 10.1016/j.jtcvs.2010.07.075. [DOI] [PubMed] [Google Scholar]
- 82.Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Léger P, Pavie A, Chastre J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36:1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 83.Nagao K, Hayashi N, Kanmatsuse K, Arima K, Ohtsuki J, Kikushima K, Watanabe I. Cardiopulmonary cerebral resuscitation using emergency cardiopulmonary bypass, coronary reperfusion therapy and mild hypothermia in patients with cardiac arrest outside the hospital. J Am Coll Cardiol. 2000;36:776–783. doi: 10.1016/s0735-1097(00)00779-8. [DOI] [PubMed] [Google Scholar]
- 84.Arlt M, Philipp A, Voelkel S, Schopka S, Husser O, Hengstenberg C, Schmid C, Hilker M. Early experiences with miniaturized extracorporeal life-support in the catheterization laboratory. Eur J Cardiothorac Surg. 2012;42:858–863. doi: 10.1093/ejcts/ezs176. [DOI] [PubMed] [Google Scholar]
- 85.Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, Higashi A, Itakura K, Sera A, Inoue I, et al. Should we emergently revascularize occluded coronaries for cardiac arrest?: rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605–1613. doi: 10.1161/CIRCULATIONAHA.111.067538. [DOI] [PubMed] [Google Scholar]
- 86.Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, Song YB, Hahn JY, Choi SH, Gwon HC, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011;39:1–7. doi: 10.1097/CCM.0b013e3181feb339. [DOI] [PubMed] [Google Scholar]
- 87.Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 88.Höglund P, Nilsson LA, Rehnqvist N.CPR with assisted extracorporeal life support Lancet 20083721878–1879.[Author reply, pp. 1879–1880] [DOI] [PubMed] [Google Scholar]
- 89.Wu MY, Lee MY, Lin CC, Chang YS, Tsai FC, Lin PJ. Resuscitation of non-postcardiotomy cardiogenic shock or cardiac arrest with extracorporeal life support: the role of bridging to intervention. Resuscitation. 2012;83:976–981. doi: 10.1016/j.resuscitation.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Chung SY, Sheu JJ, Lin YJ, Sun CK, Chang LT, Chen YL, Tsai TH, Chen CJ, Yang CH, Hang CL, et al. Outcome of patients with profound cardiogenic shock after cardiopulmonary resuscitation and prompt extracorporeal membrane oxygenation support. A single-center observational study. Circ J. 2012;76:1385–1392. doi: 10.1253/circj.cj-11-1015. [DOI] [PubMed] [Google Scholar]
- 91.Haneya A, Philipp A, Diez C, Schopka S, Bein T, Zimmermann M, Lubnow M, Luchner A, Agha A, Hilker M, et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83:1331–1337. doi: 10.1016/j.resuscitation.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 92.Chen YS, Chao A, Yu HY, Ko WJ, Wu IH, Chen RJ, Huang SC, Lin FY, Wang SS. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41:197–203. doi: 10.1016/s0735-1097(02)02716-x. [DOI] [PubMed] [Google Scholar]
- 93.Huang CT, Tsai YJ, Tsai PR, Ko WJ. Extracorporeal membrane oxygenation resuscitation in adult patients with refractory septic shock. J Thorac Cardiovasc Surg. 2013;146:1041–1046. doi: 10.1016/j.jtcvs.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 94.Bréchot N, Luyt CE, Schmidt M, Leprince P, Trouillet JL, Léger P, Pavie A, Chastre J, Combes A. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013;41:1616–1626. doi: 10.1097/CCM.0b013e31828a2370. [DOI] [PubMed] [Google Scholar]
- 95.Biderman P, Einav S, Fainblut M, Stein M, Singer P, Medalion B. Extracorporeal life support in patients with multiple injuries and severe respiratory failure: a single-center experience? J Trauma Acute Care Surg. 2013;75:907–912. doi: 10.1097/TA.0b013e3182a8334f. [DOI] [PubMed] [Google Scholar]
- 96.Arlt M, Philipp A, Voelkel S, Rupprecht L, Mueller T, Hilker M, Graf BM, Schmid C. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation. 2010;81:804–809. doi: 10.1016/j.resuscitation.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 97.Bein T, Osborn E, Hofmann HS, Zimmermann M, Philipp A, Schlitt HJ, Graf BM. Successful treatment of a severely injured soldier from Afghanistan with pumpless extracorporeal lung assist and neurally adjusted ventilatory support. Int J Emerg Med. 2010;3:177–179. doi: 10.1007/s12245-010-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gentilello LM, Cobean RA, Offner PJ, Soderberg RW, Jurkovich GJ.Continuous arteriovenous rewarming: Rapid reversal of hypothermia in critically ill patients J Trauma. 199232316–325.[Discussion, pp. 325–317] [DOI] [PubMed] [Google Scholar]
- 99.Madershahian N, Wittwer T, Strauch J, Franke UF, Wippermann J, Kaluza M, Wahlers T. Application of ECMO in multitrauma patients with ARDS as rescue therapy. J Card Surg. 2007;22:180–184. doi: 10.1111/j.1540-8191.2007.00381.x. [DOI] [PubMed] [Google Scholar]
- 100.Perchinsky MJ, Long WB, Hill JG, Parsons JA, Bennett JB. Extracorporeal cardiopulmonary life support with heparin-bonded circuitry in the resuscitation of massively injured trauma patients. Am J Surg. 1995;169:488–491. doi: 10.1016/S0002-9610(99)80201-3. [DOI] [PubMed] [Google Scholar]
- 101.Bein T, Zonies D, Philipp A, Zimmermann M, Osborn EC, Allan PF, Nerlich M, Graf BM, Fang R. Transportable extracorporeal lung support for rescue of severe respiratory failure in combat casualties. J Trauma Acute Care Surg. 2012;73:1450–1456. doi: 10.1097/TA.0b013e3182782480. [DOI] [PubMed] [Google Scholar]
- 102.Zimmermann M, Philipp A, Schmid FX, Dorlac W, Arlt M, Bein T. From Baghdad to Germany: use of a new pumpless extracorporeal lung assist system in two severely injured US soldiers. ASAIO J. 2007;53:e4–e6. doi: 10.1097/MAT.0b013e3180574b37. [DOI] [PubMed] [Google Scholar]
- 103.McCunn M, Reynolds HN, Cottingham CA, Scalea TM, Habashi NM. Extracorporeal support in an adult with severe carbon monoxide poisoning and shock following smoke inhalation: a case report. Perfusion. 2000;15:169–173. doi: 10.1177/026765910001500213. [DOI] [PubMed] [Google Scholar]
- 104.Chou NK, Chen YS, Ko WJ, Huang SC, Chao A, Jan GJ, Lin FY, Wang SS, Chu SH. Application of extracorporeal membrane oxygenation in adult burn patients. Artif Organs. 2001;25:622–626. doi: 10.1046/j.1525-1594.2001.025008622.x. [DOI] [PubMed] [Google Scholar]
- 105.Kornberger E, Mair P, Oswald E, Hörmann C, Ohler K, Balogh D. Inhalation injury treated with extracorporeal CO2 elimination. Burns. 1997;23:354–359. doi: 10.1016/s0305-4179(96)00111-8. [DOI] [PubMed] [Google Scholar]
- 106.Patton ML, Simone MR, Kraut JD, Anderson HL, III, Haith LR., Jr Successful utilization of ECMO to treat an adult burn patient with ARDS. Burns. 1998;24:566–568. doi: 10.1016/s0305-4179(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 107.Chian CF, Wu CP, Chen CW, Su WL, Yeh CB, Perng WC. Acute respiratory distress syndrome after zinc chloride inhalation: survival after extracorporeal life support and corticosteroid treatment. Am J Crit Care. 2010;19:86–90. doi: 10.4037/ajcc2009908. [DOI] [PubMed] [Google Scholar]
- 108.Thompson JT, Molnar JA, Hines MH, Chang MC, Pranikoff T. Successful management of adult smoke inhalation with extracorporeal membrane oxygenation. J Burn Care Rehabil. 2005;26:62–66. doi: 10.1097/01.bcr.0000150303.15345.79. [DOI] [PubMed] [Google Scholar]
- 109.Asmussen S, Maybauer DM, Fraser JF, Jennings K, George S, Keiralla A, Maybauer MO. Extracorporeal membrane oxygenation in burn and smoke inhalation injury. Burns. 2013;39:429–435. doi: 10.1016/j.burns.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 110.Weinberg RL, Bouchard NC, Abrams DC, Bacchetta M, Dzierba AL, Burkart KM, Brodie D. Venoarterial extracorporeal membrane oxygenation for the management of massive amlodipine overdose. Perfusion. 2014;29:53–56. doi: 10.1177/0267659113498807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McVey FK, Corke CF. Extracorporeal circulation in the management of massive propranolol overdose. Anaesthesia. 1991;46:744–746. doi: 10.1111/j.1365-2044.1991.tb09770.x. [DOI] [PubMed] [Google Scholar]
- 112.Mégarbane B, Leprince P, Deye N, Guerrier G, Résière D, Bloch V, Baud FJ. Extracorporeal life support in a case of acute carbamazepine poisoning with life-threatening refractory myocardial failure. Intensive Care Med. 2006;32:1409–1413. doi: 10.1007/s00134-006-0257-8. [DOI] [PubMed] [Google Scholar]
- 113.Auzinger GM, Scheinkestel CD. Successful extracorporeal life support in a case of severe flecainide intoxication. Crit Care Med. 2001;29:887–890. doi: 10.1097/00003246-200104000-00041. [DOI] [PubMed] [Google Scholar]
- 114.Daubin C, Lehoux P, Ivascau C, Tasle M, Bousta M, Lepage O, Quentin C, Massetti M, Charbonneau P. Extracorporeal life support in severe drug intoxication: a retrospective cohort study of seventeen cases. Crit Care. 2009;13:R138. doi: 10.1186/cc8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Massetti M, Bruno P, Babatasi G, Neri E, Khayat A. Cardiopulmonary bypass and severe drug intoxication. J Thorac Cardiovasc Surg. 2000;120:424–425. doi: 10.1067/mtc.2000.107825. [DOI] [PubMed] [Google Scholar]
- 116.Lee JH, Kim SW. Successful management of warfarin-exacerbated diffuse alveolar hemorrhage using an extracorporeal membrane oxygenation. Multidiscip Respir Med. 2013;8:16. doi: 10.1186/2049-6958-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel JJ, Lipchik RJ.Systemic lupus-induced diffuse alveolar hemorrhage treated with extracorporeal membrane oxygenation: a case report and review of the literature J Intensive Care Med 201429104–109 [DOI] [PubMed] [Google Scholar]
- 118.Mongero LB, Brodie D, Cunningham J, Ventetuolo C, Kim H, Sylvan E, Bacchetta MD.Extracorporeal membrane oxygenation for diffuse alveolar hemorrhage and severe hypoxemic respiratory failure from silicone embolism Perfusion 201025249–252.[Discussion, pp. 253–254] [DOI] [PubMed] [Google Scholar]
- 119.Muellenbach RM, Kredel M, Kunze E, Kranke P, Kuestermann J, Brack A, Gorski A, Wunder C, Roewer N, Wurmb T. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury. J Trauma Acute Care Surg. 2012;72:1444–1447. doi: 10.1097/TA.0b013e31824d68e3. [DOI] [PubMed] [Google Scholar]
- 120.Hwang GJ, Sheen SH, Kim HS, Lee HS, Lee TH, Gim GH, Hwang SM, Lee JJ. Extracorporeal membrane oxygenation for acute life-threatening neurogenic pulmonary edema following rupture of an intracranial aneurysm. J Korean Med Sci. 2013;28:962–964. doi: 10.3346/jkms.2013.28.6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35:2105–2114. doi: 10.1007/s00134-009-1661-7. [DOI] [PubMed] [Google Scholar]
- 122.Mendiratta P, Wei JY, Gomez A, Podrazik P, Riggs AT, Rycus P, Gossett J, Prodhan P. Cardiopulmonary resuscitation requiring extracorporeal membrane oxygenation in the elderly: a review of the Extracorporeal Life Support Organization registry. ASAIO J. 2013;59:211–215. doi: 10.1097/MAT.0b013e31828fd6e5. [DOI] [PubMed] [Google Scholar]
- 123.Flécher E, Anselmi A, Corbineau H, Langanay T, Verhoye JP, Félix C, Leurent G, Tulzo YL, Malledant Y, Leguerrier A.Current aspects of extracorporeal membrane oxygenation in a tertiary referral centre: determinants of survival at follow-up Eur J Cardiothorac Surg(In press) [DOI] [PubMed] [Google Scholar]
- 124.Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87:778–785. doi: 10.1016/j.athoracsur.2008.12.079. [DOI] [PubMed] [Google Scholar]
- 125.Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, Sasako Y, Nakatani T, Nonogi H, Miyazaki S. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26:2185–2192. doi: 10.1093/eurheartj/ehi411. [DOI] [PubMed] [Google Scholar]
- 126.Saito S, Nakatani T, Kobayashi J, Tagusari O, Bando K, Niwaya K, Nakajima H, Miyazaki S, Yagihara T, Kitamura S. Is extracorporeal life support contraindicated in elderly patients? Ann Thorac Surg. 2007;83:140–145. doi: 10.1016/j.athoracsur.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 127.Mols G, Loop T, Geiger K, Farthmann E, Benzing A. Extracorporeal membrane oxygenation: a ten-year experience. Am J Surg. 2000;180:144–154. doi: 10.1016/s0002-9610(00)00432-3. [DOI] [PubMed] [Google Scholar]
- 128.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, Scheinkestel C, Cooper DJ, Brodie D, Pellegrino V, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 129.Khoshbin E, Roberts N, Harvey C, Machin D, Killer H, Peek GJ, Sosnowski AW, Firmin RK. Poly-methyl pentene oxygenators have improved gas exchange capability and reduced transfusion requirements in adult extracorporeal membrane oxygenation. ASAIO J. 2005;51:281–287. doi: 10.1097/01.mat.0000159741.33681.f1. [DOI] [PubMed] [Google Scholar]
- 130.Hirose H, Yamane K, Marhefka G, Cavarocchi N. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg. 2012;7:36. doi: 10.1186/1749-8090-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Javidfar J, Wang D, Zwischenberger JB, Costa J, Mongero L, Sonett J, Bacchetta M. Insertion of bicaval dual lumen extracorporeal membrane oxygenation catheter with image guidance. ASAIO J. 2011;57:203–205. doi: 10.1097/MAT.0b013e3182155fee. [DOI] [PubMed] [Google Scholar]
- 132.Thalanany MM, Mugford M, Hibbert C, Cooper NJ, Truesdale A, Robinson S, Tiruvoipati R, Elbourne DR, Peek GJ, Clemens F, et al. CESAR Trial Group. Methods of data collection and analysis for the economic evaluation alongside a national, multi-centre trial in the UK: conventional ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) BMC Health Serv Res. 2008;8:94. doi: 10.1186/1472-6963-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, De Backer D, Fan E, Ferguson N, Fortenberry J, et al. Position paper for the Organization of Extracorporeal Membrane Oxygenation Programs for Acute Respiratory Failure in Adult Patients. Am J Respir Crit Care Med. 2014;190:488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 134.Freeman CL, Bennett TD, Casper TC, Larsen GY, Hubbard A, Wilkes J, Bratton SL. Pediatric and neonatal extracorporeal membrane oxygenation: does center volume impact mortality? Crit Care Med. 2014;42:512–519. doi: 10.1097/01.ccm.0000435674.83682.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karamlou T, Vafaeezadeh M, Parrish AM, Cohen GA, Welke KF, Permut L, McMullan DM. Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg. 2013;145:470–475. doi: 10.1016/j.jtcvs.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 136.Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, Trouillet JL, Bréchot N, Nieszkowska A, Dupont H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Risnes I, Wagner K, Nome T, Sundet K, Jensen J, Hynås IA, Ueland T, Pedersen T, Svennevig JL. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2006;81:1401–1406. doi: 10.1016/j.athoracsur.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 138.Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68:1543–1549. doi: 10.1001/archneurol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schmid C, Philipp A, Hilker M, Rupprecht L, Arlt M, Keyser A, Lubnow M, Müller T. Venovenous extracorporeal membrane oxygenation for acute lung failure in adults. J Heart Lung Transplant. 2012;31:9–15. doi: 10.1016/j.healun.2011.07.013. [DOI] [PubMed] [Google Scholar]


