Abstract
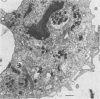
The interaction between Coxiella burnetii and peritoneal macrophages obtained from immune guinea pigs was studied by transmission electron microscopy. Phagocytosis and subsequent fate of ingested phase I and II rickettsiae were compared. Phase I rickettsiae were more resistant to phagocytosis than were phase II organisms. Macrophages from phase I- and II-immunized animals were equally capable of phagocytizing rickettsiae. Phase I and II rickettsiae previously treated with normal serum multiplied and destroyed macrophages from guinea pigs that had been immunized with phase II rickettsiae. Phase II organisms were initially suppressed in macrophages from phase I-immunized animals, but eventually multiplied in these cells. In contrast, only phase I organisms were destroyed by macrophages from phase I-immunized animals. Treatment of rickettsiae with immune serum enhanced ingestion by macrophages and potentiated the destruction of organisms by both types of macrophages. The macrophage migration inhibition assay was performed on peritoneal exudate cells from immune animals. Migration of peritoneal macrophages from phase I-immunized guinea pigs was inhibited, whereas macrophages from phase II-immunized animals migrated when cells were cultured in the presence of killed, intact phase I or II C. burnetii.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila F. R., Schultz R. M., Tompkins W. A. Specific macrophage immunity to vaccinia virus: macrophage-virus interaction. Infect Immun. 1972 Jul;6(1):9–16. doi: 10.1128/iai.6.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREZINA R., KAZAR J. STUDY OF THE ANTIGENIC STRUCTURE OF COXIELLA BURNETI. IV. PHAGOCYTOSIS AND OPSONIZATION IN RELATION TO THE PHASES OF C. BURNETI. Acta Virol. 1965 May;9:268–274. [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Migration inhibitory factor associated with delayed-type hypersensitivity. Fed Proc. 1968 Jan-Feb;27(1):13–15. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Downs C. M. Phagocytosis of coxiella burneti, phase I and phase II by peritoneal monocytes from normal and immune guinea pigs and mice. Zentralbl Bakteriol Orig. 1968 Apr;206(3):329–343. [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer B., Delfs D., McElree H. Electron microscope study of the interaction of vaccinia virus with macrophages from immunized and nonimmunized rabbits. Infect Immun. 1974 Feb;9(2):452–459. doi: 10.1128/iai.9.2.452-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Heggers J. P., Mallavia L. P., Hinrichs D. J. The cellular immune response to antigens of Coxiella burneti. Can J Microbiol. 1974 May;20(5):657–662. doi: 10.1139/m74-101. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazár J., Skultétyová E., Brezina R. Phagocytosis of Coxiella burneti by macrophages. Acta Virol. 1975 Sep;19(5):426–431. [PubMed] [Google Scholar]
- Kishimoto R. A., Veltri B. J., Canonico P. G., Shirey F. G., Walker J. S. Electron microscopic study on the interaction between normal guinea pig peritoneal macrophages and Coxiella burnetii. Infect Immun. 1976 Oct;14(4):1087–1096. doi: 10.1128/iai.14.4.1087-1096.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Walker J. S. Interaction between Coxiella burnetii and guinea pig peritoneal macrophages. Infect Immun. 1976 Aug;14(2):416–421. doi: 10.1128/iai.14.2.416-421.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T. The mediator of cellular immunity. IV. Cooperation between lymphocytes and mononuclear phagocytes. Cell Immunol. 1971 Aug;2(4):317–325. doi: 10.1016/0008-8749(71)90066-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch P. G., Canonico P. G. Characterization of monkey peripheral neutrophil granules during infection. Infect Immun. 1975 Sep;12(3):687–693. doi: 10.1128/iai.12.3.687-693.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins W. A., Zarling J. M., Rawls W. E. In vitro assessment of cellular immunity to vaccinia virus: contribution of lymphocytes and macrophages. Infect Immun. 1970 Dec;2(6):783–790. doi: 10.1128/iai.2.6.783-790.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Fiset P., Ormsbee R. A. Interaction of rickettsiae and phagocytic host cells. V. Phagocytic and opsonic interactions of phase 1 and phase 2 Coxiella burneti with normal and immune human leukocytes and antibodies. J Immunol. 1967 Oct;99(4):669–674. [PubMed] [Google Scholar]