Abstract
Cellular senescence has historically been viewed as an irreversible cell-cycle arrest mechanism that acts to protect against cancer, but recent discoveries have extended its known role to complex biological processes such as development, tissue repair, ageing and age-related disorders. New insights indicate that, unlike a static endpoint, senescence represents a series of progressive and phenotypically diverse cellular states acquired after the initial growth arrest. A deeper understanding of the molecular mechanisms underlying the multi-step progression of senescence and the development and function of acute versus chronic senescent cells may lead to new therapeutic strategies for age-related pathologies and extend healthy lifespan.
Cellular senescence is a process in which cells cease dividing and undergo distinctive phenotypic alterations, including profound chromatin and secretome changes, and tumour-suppressor activation1–6. Hayflick and Moorhead first introduced the term senescence to describe the phenomenon of irreversible growth arrest of human diploid cell strains after extensive serial passaging in culture7. Later, this particular type of senescence (replicative senescence) was causally linked to telomere attrition, a process that leads to chromosomal instability and promotes tumorigenesis, supporting the original hypothesis that senescence guards against unrestricted growth of damaged cells7,8. Subsequent studies have reinforced the importance of cellular senescence as a safeguard against cancer9. Emerging evidence indicates that the physiological relevance of cellular senescence extends beyond tumour suppression into biological processes such as embryonic development10–12, wound healing13, tissue repair14 and organismal ageing15,16. In fact, Hayflick and Moorhead initially postulated a role for replicative senescence in ageing, but until recently this theory remained untested7. The multifunctional nature of cellular senescence raises the question as to whether fundamentally different senescence mechanisms underlie these diverse biological roles. This Review focuses on this and other key emerging concepts in the senescence field, including ‘assisted’ cell cycling, multi-step senescence (or senescence progression), acute versus chronic senescence and senescence of post-mitotic cells. How these concepts relate to the role of senescent cells in ageing and age-related diseases and how the rapidly accruing new information could be exploited to clear detrimental senescent cell populations selectively to improve healthy lifespan are also discussed.
Causes and effector pathways of senescence
Research on the causes (or stresses), signalling networks and mechanisms underlying the various types of cellular senescence is still in its infancy and current insights are largely based on cell culture experiments. In addition to telomere erosion, several other tumour-associated stresses have been shown to induce a senescent growth arrest in vitro, including certain DNA lesions and reactive oxygen species (ROS)17–19. What both these stresses have in common with telomere damage is that they activate the DNA damage response (DDR), a signalling pathway in which ATM or ATR kinases block cell-cycle progression through stabilization of p53 and transcriptional activation of the cyclin-dependent kinase (Cdk) inhibitor p21. Activated oncogenes are also prominent inducers of senescence. Oncogenic Ras acts through overexpression of Cdc6 and suppression of nucleotide metabolism, causing aberrant DNA replication, formation of double stranded DNA breaks (DSBs) and activation of the DDR pathway20,21. However, senescence caused by E2F3 activation or c-Myc inhibition is DDR-independent and involves p19Arf and p16Ink4a (refs 17, 22). BRAF(V600E) is also DDR-independent and induces senescence through a metabolic mechanism involving upregulation of mitochondrial pyruvate dehydrogenase (PDH; Fig. 1)23. Several other studies underscored that senescence is closely linked to profound metabolic changes24,25. Furthermore, various tumour suppressors trigger a senescent growth arrest when inactivated, including RB, PTEN, NF1 and VHL17,26. Of these, RB inactivation engages the DDR26, whereas the others are DDR-independent and act through p19Arf and p16Ink4a. A notable species-specific difference is that senescence pathways of murine cells are more dependent on p19Arf than senescence in human cells27.
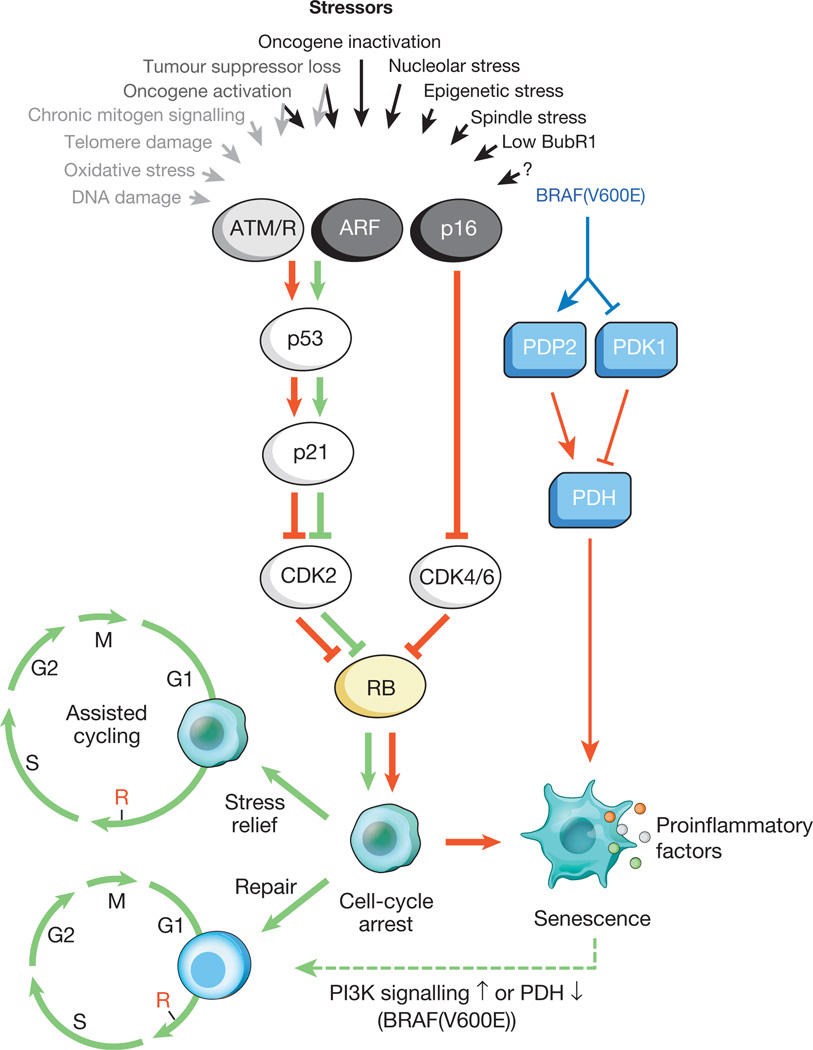
Figure 1. Senescence-inducing stimuli and main effector pathways.
A variety of cell-intrinsic and -extrinsic stresses can activate the cellular senescence program. These stressors engage various cellular signalling cascades but ultimately activate p53, p16Ink4a, or both. Stress types that activate p53 through DDR signalling are indicated with grey text and arrows (ROS elicit the DDR by perturbing gene transcription and DNA replication, as well as by shortening telomeres). Activated p53 induces p21, which induces a temporal cell-cycle arrest by inhibiting cyclin E–Cdk2. p16Ink4a also inhibits cell-cycle progression but does so by targeting cyclin D–Cdk4 and cyclin D–Cdk6 complexes. Both p21 and p16Ink4a act by preventing the inactivation of Rb, thus resulting in continued repression of E2F target genes required for S-phase onset. Upon severe stress (red arrows), temporally arrested cells transition into a senescent growth arrest through a mechanism that is currently incompletely understood. Cells exposed to mild damage that can be successfully repaired may resume normal cell-cycle progression. On the other hand, cells exposed to moderate stress that is chronic in nature or that leaves permanent damage may resume proliferation through reliance on stress support pathways (green arrows). This phenomenon (termed assisted cycling) is enabled by p53-mediated activation of p21. Thus, the p53–p21 pathway can either antagonize or synergize with p16Ink4a in senescence depending on the type and level of stress. BRAF(V600E) is unusual in that it establishes senescence through a metabolic effector pathway. BRAF(V600E) activates PDH by inducing PDP2 and inhibiting PDK1 expression, promoting a shift from glycolysis to oxidative phosphorylation that creates senescence-inducing redox stress. Cells undergoing senescence induce an inflammatory transcriptome regardless of the senescence inducing stress (coloured dots represent various SASP factors). Red and green connectors indicate ‘senescence-promoting’ and ‘senescence-preventing’ activities, respectively, and their thickness represents their relative importance. The dashed green connector denotes a ‘senescence-reversing’ mechanism.
Prolonged exposure to interferon-β also induces senescence, demonstrating that chronic mitogenic signalling outside the context of neoplastic transformation can stimulate senescence28. Other, less broadly studied inducers of senescence include epigenetic, nucleolar and mitotic spindle stresses (Fig. 1). For example, genome-wide chromatin decompression by exposure to histone deacetylase inhibitors triggers senescence via a p21-dependent mechanism29. A key target of epigenetic stressors that promote senescence may be the INK4a/ARF locus, which in proliferating cells is repressed by polycomb group-mediated H3K27 methylation and H2A-K119 ubiquitination30. Nucleolar stress caused by RNA polymerase I inhibitors triggers a robust p53-mediated senescence response31. Senescence can also be elicited by suboptimal expression of proteins implicated in spindle formation or mitotic checkpoint control, including human TACC3 and murine BubR1, Bub3 and Rae1, all of which engage p53 and p21 independently of the DDR, often in combination with p16Ink4a (refs 15, 32, 33). It is highly likely that additional stressors and mechanisms that drive cells into senescence will be uncovered given the rapidly evolving nature of the field. Production of proinflammatory cytokines and chemokines is emerging as a common feature of senescent cells irrespective of the senescence-inducing stressor or mechanism (Fig. 1).
Although the relative contributions of the p53–p21 and p16Ink4a–RB effector pathways to the initial growth arrest can vary depending on the type of stress, both may ultimately become engaged upon sustained senescence. For example, DNA damage initially halts cell-cycle progression through p53-mediated induction of p21, but if lesions persist, this activates p16Ink4a through p38-MAPK-mediated mitochondrial dysfunction and ROS production34,35. The extent to which effector mechanisms of in vitro senescence apply to in vivo senescence has not been tested extensively. Fat, skeletal muscle and eye of BubR1 progeroid mice have elevated levels of p19Arf, p53, p21 and p16Ink4 and are subject to precocious functional decline15,36. Genetic experiments using knockout strains for each of these tumour suppressors that dissected how senescent cells accumulate in these tissues and contribute to their deterioration, established that p16Ink4a is an effector of senescence and ageing15. However, in contrast to in vitro findings, p19Arf, p53 and p21 prevented senescence and age-related pathologies in vivo15,36. These unexpected findings led to the concept of ‘assisted’ cell cycling (analogous to assisted living) in which ageing cells, coping with an increasing burden of macromolecular damage and other chronic stresses, manage to retain their proliferative potential for a while by extending cell-cycle duration in a p21-dependent manner to provide extra time to mend cellular disabilities through engagement of compensatory mechanisms or repair (Fig. 1). Although this concept is supported by the observation that the cell-cycle time of cultured primary human cells markedly increases with passaging37, it clearly requires more validation and generalization. Inactivation of p21 improves stem cell function in intestinal crypts and bone marrow in mutant mice with short telomeres38, indicating that in situations where irreparable damage produces a sustained and robust p53 response, p21 acts to promote tissue deterioration by executing senescence.
In vitro studies of cellular senescence have traditionally been performed using a single senescence-inducing stimulus (that is, high-dose radiation or oncogenes; Fig. 1). However, in the context of organismal ageing, individual cells experience multiple cellular pressures, including various kinds of genotoxic, proteotoxic and mitotic stresses3,39. Thus, to advance our understanding of these processes, it will be imperative to examine how combinations of diverse senescence-promoting stressors impact the actions of the various downstream effector pathways and the characteristics of the resulting senescent phenotypes. Furthermore, while cellular senescence is well recognized as an in vivo tumour suppressive mechanism, its irreversibility remains a topic of debate. However, compelling new evidence indicates that BRAF(V600E) oncogene-induced senescence (OIS) can be reversed by activation of phosphatidylinositol 3-kinase (PI3K) or inhibition of PDH (Fig. 1)23,40. In addition, senescent cells have been successfully dedifferentiated into pluripotent stem cells41.
Senescence is a multi-step evolving process
Until recently, senescence was viewed as a static endpoint. However, several recent observations support the hypothesis that senescence can be a highly dynamic, multi-step process, during which the properties of senescent cells continuously evolve and diversify, much like tumorigenesis but without cell proliferation as a driver (Fig. 2)42–44. The initiating step is the transition of temporal to stable cell-cycle arrest, which typically involves prolonged inhibition of Cdk–cyclin activity by p21, p16Ink4a, or both. A change in p53 expression from intermittent to continuous may be a critical event in the transition from temporal to persistent growth arrest45.
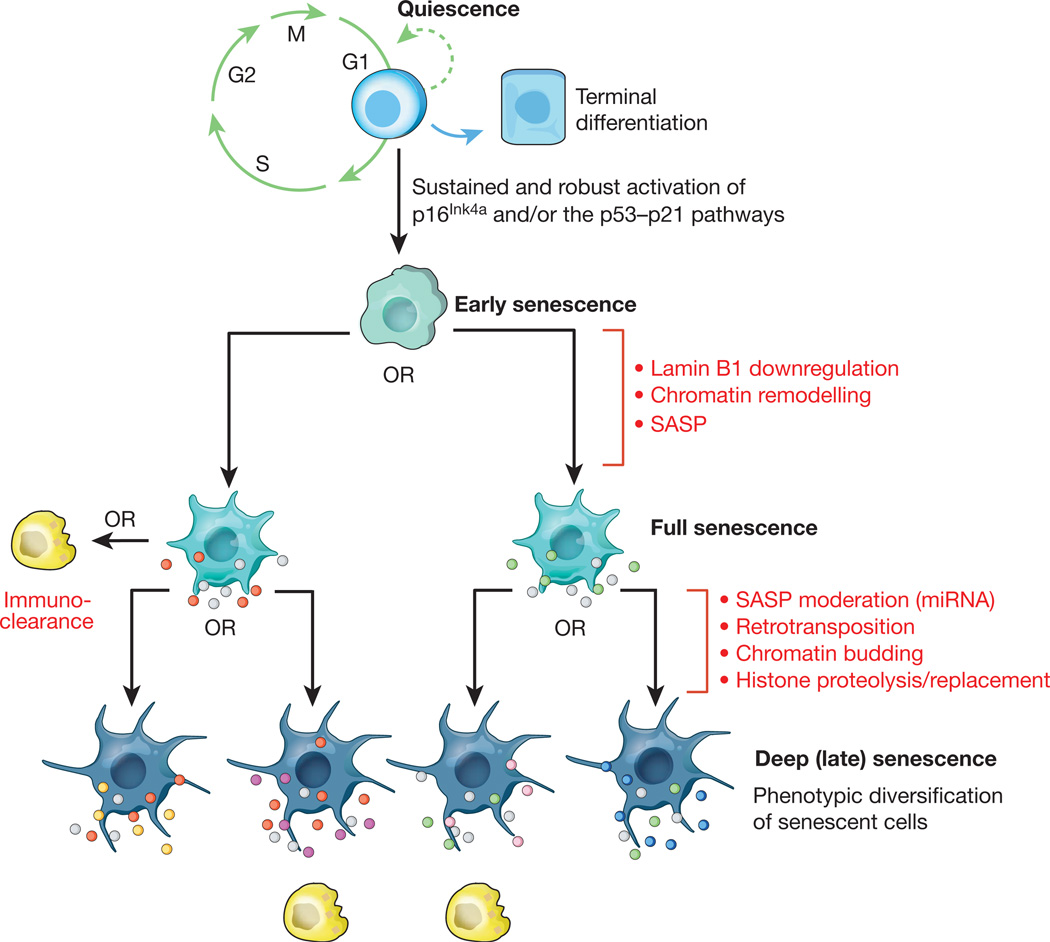
Figure 2. Hypothetical multi-step senescence model.
Mounting evidence suggests that cellular senescence is a dynamic process driven by epigenetic and genetic changes. The initial step represents the progression from a transient to a stable cell-cycle arrest through sustained activation of the p16Ink4a and/or p53–p21 pathways. The resulting early senescent cells progress to full senescence by downregulating lamin B1, thereby triggering extensive chromatin remodelling underlying the production of a SASP. Certain components of the SASP are highly conserved (grey dots), whereas others may vary depending on cell type, nature of the senescence-inducing stressor, or cell-to-cell variability in chromatin remodelling (red and green dots). Progression to deep or late senescence may be driven by additional genetic and epigenetic changes, including chromatin budding, histone proteolysis and retrotransposition, driving further transcriptional change and SASP heterogeneity (yellow, magenta, pink and blue dots). It should be emphasized that although the exact nature, number and order of the genetic and epigenetic steps occurring during senescent cell evolution are unclear, it is reasonable to assume that the entire process is prone to SASP heterogeneity. The efficiency with which immune cells (yellow) dispose of senescent cells may be dependent on the composition of the SASP. Interestingly, the proinflammatory signature of the SASP can fade due to expression of particular microRNAs late into the senescence program, thereby perhaps allowing evasion of immuno-clearance99.
For the progression to full senescence, it seems that lamin B1 downregulation triggers both global and local modifications in chromatin methylation46–48 (Fig. 2). Some mammalian cell types form regions of highly condensed chromatin called senescence-associated heterochromatin foci (SAHFs)49–51. SAHFs, which are enriched in chromatin modifications such as S83-HP1γ, HIRA, ASF1, macroH2A, H3K9me3 and γH2AX, sequester genes implicated in cell-cycle control, a phenomenon that seems to reinforce the senescence-associated growth arrest. Decondensation of (peri)centromeric satellite heterochromatin has been identified as a universal hallmark of senescence that precedes SAHF formation52. Senescence-related chromatin remodelling leads to profound transcriptional changes48,53,54. Among the assortment of upregulated genes is a prominent subset of genes that encode secreted proteins, including cytokines and chemokines with proinflammatory properties, as well as various growth factors and proteases that together alter tissue structure and function. Collectively, these factors are referred to as the senescence-associated secretory phenotype (SASP)55,56 or senescence-messaging secretome (SMS)57. The SASP is one of the key characteristics that distinguish senescent cells from quiescent, terminally differentiated, and other types of non-proliferating cells (Fig. 2). In certain cases, the SASP is dependent on persistent DNA damage signalling56, such as that created through a positive feedback loop between DDR signalling and ROS35. This loop was uncovered using human fibroblast lines in which dominant-negative TRF2 or high dose ionizing radiation induced telomere-dependent and telomere-independent DDR signalling, respectively. Both types of DDR signalling were found to cause mitochondrial dysfunction and production of ROS which led to new DNA damage and continued DDR signalling. Consistent with this, primary human fibroblasts overexpressing p16Ink4a or p21 undergo senescent growth arrest but fail to activate the DDR and do not produce a SASP58. Importantly, several senescence-inducing stimuli produce SASPs independent of DNA damage (Fig. 1), implying the existence of DDR-independent mechanisms11,12,23.
Given the intricate nature of the SASP, it is not surprising that senescent cells impact various biological processes that involve paracrine signalling, including cell proliferation, angiogenesis59, inflammation60, epithelial-to-mesenchymal transition (EMT)61, wound healing13, and other types of tissue repair14. Several SASP components, including IL-6, IL-8, WNT16B and GROα, also act in an autocrine fashion in the context of OIS, presumably to help establish a persistent growth arrest62–64. Importantly, SASP factors vary indistinct cell types and under different senescence-inducing stressors55. This plasticity within SASP composition predicts variability with respect to the biological processes impacted by different kinds of senescent cells (Fig. 2). Proinflammatory cytokines and chemokines are among the SASP components that are highly conserved across cell types and senescence-inducing stimuli55, suggesting that attracting immune cells and inducing local inflammation are common properties of senescent cells. However, accumulation of senescent cells is not always accompanied by immune cell infiltration and inflammation, as exemplified by melanocytic nevi65.
Cultured cells usually reach senescence within several weeks after exposure to senescence-inducing stressors, but remain viable for months thereafter42. Senescent cells continue to evolve even after extended periods of culture, thereby progressing to a stage that has been termed ‘deep’ or ‘late’ senescence (Fig. 2). This phenomenon is evidenced by a dramatic increase in the transcription of transposable elements, including members of the L1, ALU and SVA transposon families, which occur several months after senescence onset42,43. These newly synthesized retrotransposon transcripts can indeed engage in active transposition and accumulate in late-senescent cell genomes. Increased retrotransposon activity is associated with senescence-associated opening of gene-poor heterochromatic regions where these elements reside42. A second process driving continued change in senescent cells is characterized by the extrusion of chromatin into the cytoplasm, resulting in the formation of cytoplasmic chromatin fragments (CCFs)44. CCFs are strongly positive for H3K27me3 and γH2AX, contain DNA, and are processed via lysosome-mediated proteolysis, resulting in overall histone loss. Both retrotransposon activation and chromatin budding are examples of continued genomic and epigenomic remodelling in senescent cells. Both processes seem random in nature and are therefore likely to drive transcriptome diversity among senescent cells, even in those resulting from a common stressor. Because transcriptional activity is a key determinant of secretome composition, it is reasonable to assume that the SASPs of these cells diversify to some degree through these changes. With the concept of senescence progression in cultured cells solidifying, it becomes essential to validate its in vivo importance.
Acute versus chronic senescence
The diverse nature of the processes in which senescence has been implicated, ranging from embryonic development10 to wound healing13, tissue repair14, cancer and ageing16, raises the question of whether the properties of the senescent cells involved in these activities are fundamentally different. If so, what would be the underlying molecular mechanisms? Valuable clues to these questions can be inferred from the apparent differences in senescence kinetics between these processes. In the context of ageing, cells chronically accumulate macromolecular damage and may become increasingly dependent on cell-cycle checkpoints and stress-relief mechanisms to retain proliferative potential (assisted cycling)15,66–68. Ultimately, more and more of these cells may stably arrest and transition into a senescent state, referred to here as chronic senescence (Fig. 3).
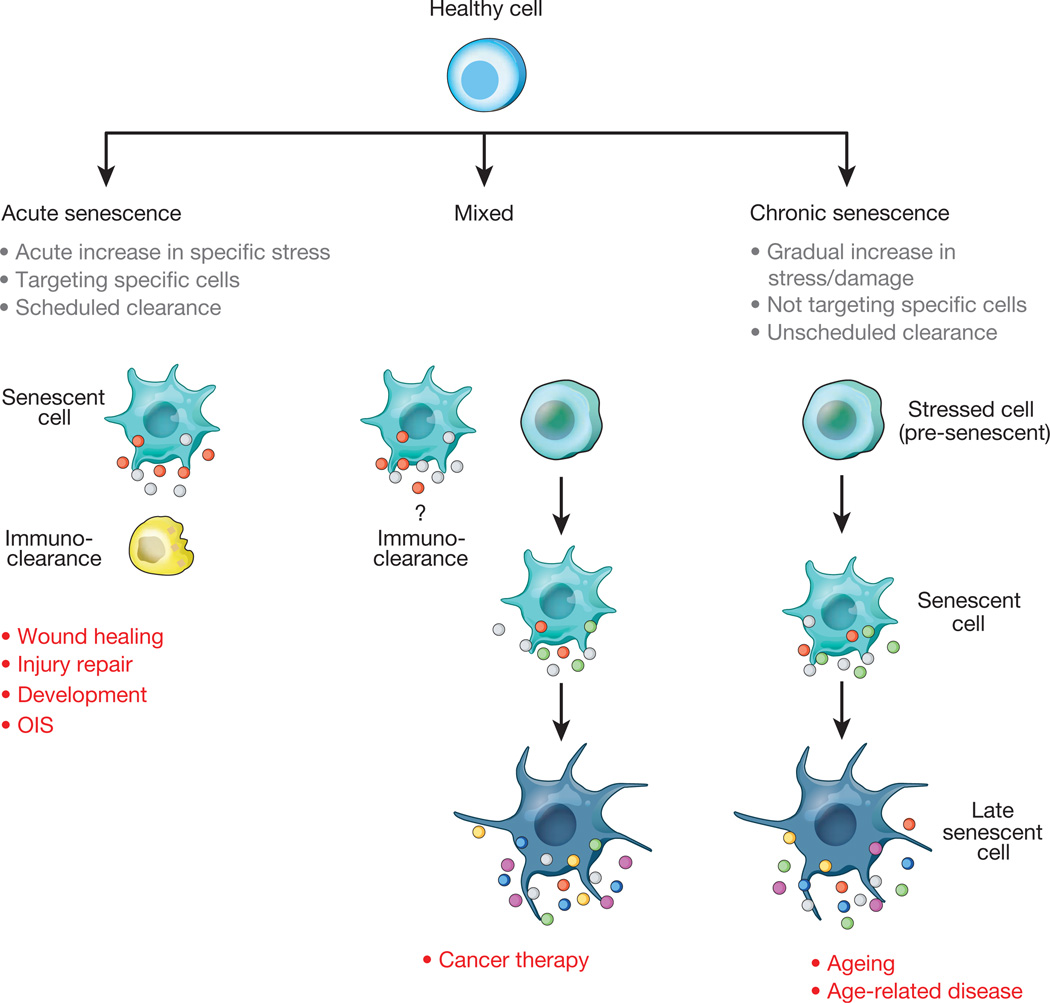
Figure 3. Acute and chronic senescent cells.
The conceptual model in which senescent cells are subdivided into two main classes based on kinetics of senescence induction and functionality. Acute senescent cells seem to mostly be part of tightly orchestrated biological processes (that is, wound healing, tissue repair, embryonic development) to halt expansion of certain cells and/or produce a SASP with defined paracrine functions. Acute senescence is induced through cell-extrinsic stimuli that target a specific population of cells in the tissue. Acute senescent cells self-organize their elimination through SASP components that attract various types of immune cells. Induction of chronic senescence occurs after periods of progressive cellular stress or macromolecular damage when tarry cycling transitions into a stable cell-cycle arrest. Chronic senescence is not programmed and does not seem to target specific cell types. Conceivably, owing to age-related immunodeficiency or production of less proinflammatory SASPs, immune cells may inefficiently eliminate chronic senescent cells, allowing continuation of multi-step senescence. Senescence induced during cancer therapy may initially be acute and later chronic in nature.
Chronic senescence is different from a process like wound healing, by which upon wound closure, myofibroblasts suddenly undergo senescence (acute senescence) to limit excessive fibrosis at the site of injury13 (Fig. 3). Myofibroblast senescence here is induced by the extracellular matrix protein CCN1, which acts through integrin α6β1 and HSPS-mediated activation of the RAC1-dependent NADPH oxidase 1 to produce a robust and sustained accumulation of ROS13. Senescent myofibroblasts limit fibrosis by promoting the degradation of matrix components through the SASP factors they produce. Importantly, acute myofibroblast senescence also has a role in repairing damaged organs such as the liver and thus is likely to represent a more common mechanism to limit fibrosis14,69.
Similar to skin repair and recovery from liver injury, senescent cell induction is also acute and spatiotemporally controlled in uterine neo-vascularization, a developmental process that takes place at the site of embryo implantation to supply the embryo with maternal blood10. HLA-G secreted by embryonic trophoblast cells induces senescence in nearby natural killer cells, which then start to produce SASP components to promote local angiogenesis and vascular remodelling. Senescence has also been identified as a prominent mechanism for remodelling of various tissues during mouse embryogenesis, including the mesonephros, the endolymphatic sac, the apical ectodermal ridge, and the neural roof plate11,12. Developmental senescence is p21-dependent, but p53- and DDR-independent, and shares several features with OIS, including a common gene expression signature and senescence-associated β-galactosidase activity. Interestingly, OIS itself is triggered by a single defined stimulus and established with fast kinetics, which would qualify it as acute senescence. However, cells undergoing OIS are not always cleared by the immune system. For instance senescent cells in human melanocytic nevi are highly persistent.
Thus, senescence induction in tissue repair and development seems to be a scheduled or programmed process triggered by specific stimuli that target particular types of cells (Fig. 3). In contrast, during ageing-related senescence, the switch from temporal to persistent cell-cycle arrest appears unscheduled and stochastic in nature, probably involving the combined effects of distinct senescence-inducing stressors acting simultaneously on a cell. The kinetics and efficiency of senescent-cell clearance may constitute another key difference between acute and chronic senescence. During repair and embryogenesis, disposal of senescent cells seems very efficient and under strict temporal control11–14. Conversely, ageing-related senescent cells, may be more persistent due to deterioration of the immune system with ageing70,71, but further experiments are needed to refine our understanding of the relationship between senescence and the (ageing) immune system.
Senescence induced by chemotherapeutics or radiation in the context of cancer treatment may be a combination of acute and chronic senescence (Fig. 3). Acute senescence would apply to cells that generate a sustained DDR as a rapid response to overwhelming genomic damage72. In contrast, chronic senescence would pertain to cells experiencing mild genotoxic stress that can initially be managed through engagement of stress support pathways. However, as further macromolecular damage occurs over time, these cells may eventually transition from a pre-senescent state to a persistent cell cycle arrest73. An interesting untested hypothesis is that cancer therapy-induced senescence contributes to accelerated tissue and organ deterioration in cancer survivors74.
Senescence of post-mitotic cells
Most cells in mammals are post-mitotic and the question that has been raised is whether these cells can obtain key characteristics of senescent cells. Post-mitotic neurons in various parts of human and mouse brains are known to accumulate high amounts of DNA damage18. Recent research has revealed that these neurons exhibit several additional senescence-associated properties, including heterochromatinization, synthesis of proinflammatory interleukins, and high senescence-associated β-galactosidase activity75. As with mitotic cells that undergo senescence in response to sustained DNA damage, these phenotypes develop in a p21-dependent manner, further associating them with senescence (Fig. 4). Senescence-like features have also been reported for adipocytes of mice on a high-fat diet76, suggesting that post-mitotic cell senescence may be a broader phenomenon. It will be important to confirm that these terminally differentiated cells produce a SASP that negatively impacts the functionality of neighbouring cells (Fig. 4), and to explore whether differentiated cells with senescent cell properties accumulate in tissues other than brain and fat.
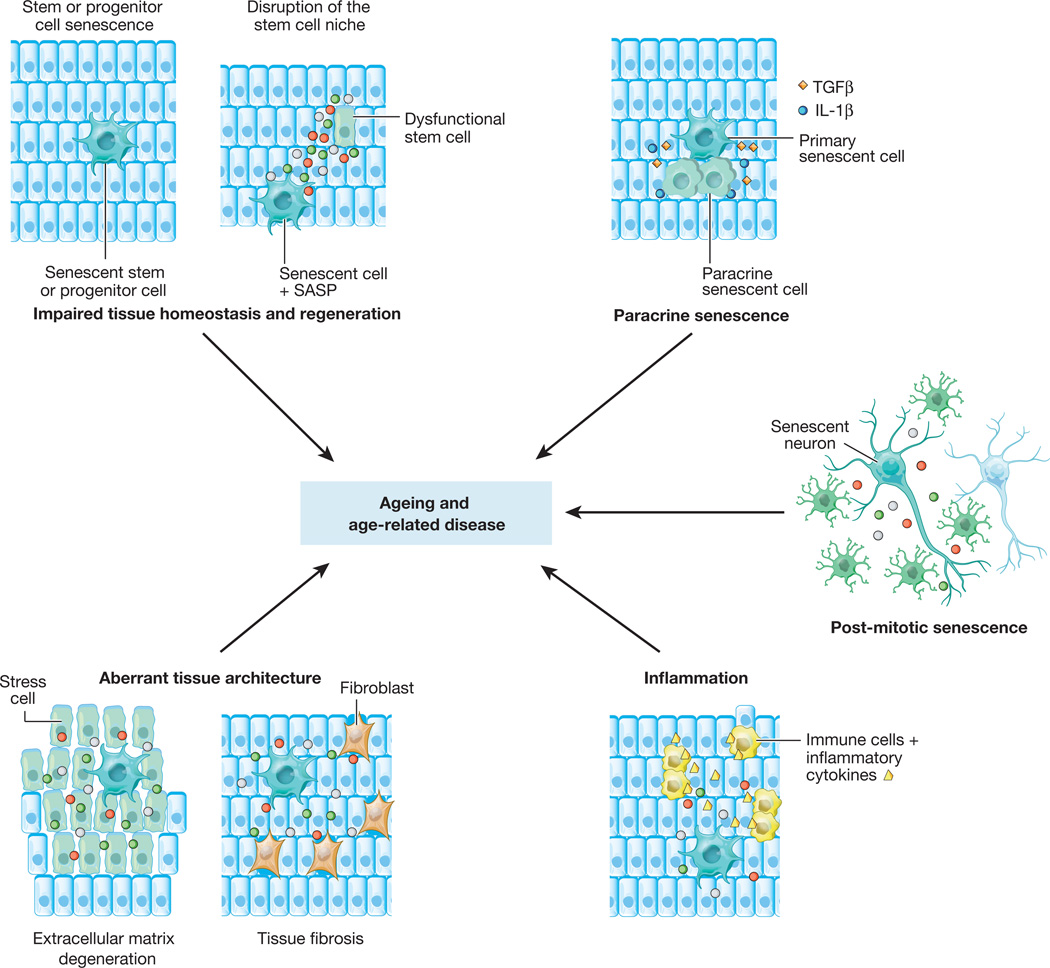
Figure 4. Mechanisms of tissue and organ deterioration by cellular senescence.
Cellular senescence is thought to contribute to age-related tissue and organ dysfunction and various chronic age-related diseases through various mechanisms. In a cell-autonomous manner, senescence acts to deplete the various pools of cycling cells in an organism, including stem and progenitor cells. In this way, senescence interferes with tissue homeostasis and regeneration, and lays the groundwork for its cell-non-autonomous detrimental actions involving the SASP. There are at least five distinct paracrine mechanisms by which senescent cells could promote tissue dysfunction, including perturbation of the stem cell niche (causing stem cell dysfunction), disruption of extracellular matrix, induction of aberrant cell differentiation (both creating abnormal tissue architecture), stimulation of sterile tissue inflammation, and induction of senescence in neighbouring cells (paracrine senescence). An emerging yet untested concept is that post-mitotic, terminally differentiated cells that develop key properties of senescent cells might contribute to ageing and age-related disease through the same set of paracrine mechanisms.
Senescence in ageing and age-related disease
The absence of senescence specific markers has hampered efforts to characterize senescent cells that accumulate in vivo in tissues and organs. The most reliable in situ detection methods that are currently available screen for multiple semi-selective senescent cell characteristics. These include, but are not limited to, high levels ofp16Ink4a, p21, macroH2A, IL-6, phosphorylated p38MAPK, DSBs, and senescence-associated β-galactosidase activity. The use of such methods has provided convincing evidence that senescent cells indeed accumulate in tissues of humans, primates, and rodents with age77–81, as well as at sites of tissue injury and remodelling10–14. Furthermore, cells with senescent cell properties can be found in the affected tissues of patients with age-related diseases such as osteoarthritis, pulmonary fibrosis, atherosclerosis, and Alzheimer’s disease1,82.
Studies on the relevance of in vivo senescence in ageing and age-associated diseases have also been complicated by the fact that key downstream effectors of senescence-inducing stressors such as p16Ink4a and p53 are tumour suppressors that, when disrupted in mice, cause death from cancer at a young age83. p53 provides additional complications to these analyses as, unlike p16Ink4a, this transcription factor also mediates apoptosis, making it difficult to assign potential ageing-related phenotypic changes in p53-null animals to the senescence program84–86. Two consecutive studies in BubR1 progeroid mice, in which p16Ink4a-positive senescent cells were targeted in different ways provided the first direct support for Hayflick and Moorhead’s early concept that senescent cells drive age-related pathologies1,7,15,16. In the first study, genetic inactivation of p16Ink4a prevented the formation of senescent cells in skeletal muscle, eye and fat, significantly attenuating the onset of age-related pathologies in these tissues15. In the subsequent study, which produced a phenocopy of genetic p16Ink4a ablation, p16Ink4a-positive senescent cells were allowed to accumulate but were consistently eliminated from weaning age onwards by the use of a transgene, termed INK-ATTAC, that selectively induced apoptosis in these cells upon administration of the synthetic drug AP20187 (ref. 16). Late-life clearance of senescent cells attenuated progression of already-established age-related disorders in skeletal muscle and fat, yet was unable to revert them.
An important question that needs to be addressed is how senescence promotes age-related tissue dysfunction (Fig. 4). One scenario is that senescence contributes to the overall decline in tissue regenerative potential that occurs with ageing. This idea is supported by the observation that progenitor cell populations in both skeletal muscle and fat tissue of BubR1 progeroid mice are highly prone to cellular senescence36. In addition to acting on stem cells in a cell-autonomous fashion by establishing a persistent growth arrest, senescence could act to disrupt the local stem-cell niche non-autonomously through the SASP87–89 (Fig. 4). Although this concept remains to be tested in vivo, the profound negative impact that the aged cellular microenvironment has on stem cell functionality is underscored by the discovery that the regenerative potential of old stem cells markedly improves when exposed to a young systemic environment via parabiotic pairing87,90.
Other SASP-based mechanisms may also contribute to tissue dysfunction. For example, proteases chronically secreted by senescent cells may perturb tissue structure and organization by cleaving membrane-bound receptors, signalling ligands, extracellular matrix proteins or other components in the tissue microenvironment55,91 (Fig. 4). In addition, other SASP components, including IL-6 and IL-8, may stimulate tissue fibrosis in certain epithelial tissues by inducing EMT61,91 (Fig. 4). Chronic tissue inflammation, which is characterized by infiltration of macrophages and lymphocytes, fibrosis and cell death, is associated with ageing and has a causal role in the development of various age-related diseases60. One idea, which remains untested, is that senescent cells that accumulate with ageing and that are present at sites of age-related pathologies promote this type of inflammation through the proinflammatory growth factors, cytokines and chemokines they secrete (Fig. 4). These may include GM-CSF, GROα, IL-1, IL-6, IL-8, macrophage inflammatory proteins (MIPs), as well as monocyte chemo-attractant proteins (MCPs)1,49,55. Together with matrix metalloproteinases, proinflammatory SASP components are thought to create a tissue microenvironment that promotes survival, proliferation and dissemination of neoplastic cells, which may explain, at least in part, why cancer rates markedly increase beyond middle age1,80,84. Finally, the SASP may intensify age-related tissue deterioration through paracrine senescence, a recently discovered mechanism by which senescent cells spread the senescence phenotype to healthy neighbouring cells through secretion of IL-1β, TGFβ and certain chemokine ligands (Fig. 4)92,93.
Why senescent cells accumulate in tissues and organs with age is another key open question. One possibility is that the rate with which senescent cells are produced might increase over time. In support of this idea, several investigations have demonstrated that various stimuli that induce senescence increase with ageing32,94,95. If combined cellular stresses were to drive senescence, it would take a long time for these to accumulate. Alternatively, the efficiency with which senescent cells get eliminated may decrease with ageing. In fact, senescent cells can be killed and disposed of by immune cells, as was elegantly demonstrated in mice that undergo senescence in the context of liver fibrosis and hepatocellular carcinogenesis14,96. These observations raised the possibility that senescent cells are armed with a self-elimination program that proceeds by attracting both adaptive and innate immune cells, including T cells, macrophages and natural killer cells, through the secretion of proinflammatory cytokines and chemokines60,96,97. This program is likely to be affected in ageing humans and rodents, in which the immune system undergoes a complex series of changes in both the innate and adaptive immunity that culminate in age-associated immunodeficiency71. This may include a reduced efficiency of senescent-cell clearance. Indeed, age-related haematopoietic stem cell dysfunction compromises the immune system and may thus be an important contributor to the late-life systemic increases in senescent cells70,98. Furthermore, given that senescence may be a dynamic process rather than a static state, it is conceivable that the ability to self-eliminate through immune cells becomes compromised as senescent cells evolve.
Another important consideration is whether chronic and acute senescence can both play a role in ageing and age-related disease. Acute senescent cells are part of tightly orchestrated biological processes in which they have narrowly defined roles and a temporal presence both dictated by the composition of the SASP, limiting their ability to accumulate with ageing. In contrast, chronic senescent cells which develop after a prolonged period of gradually increasing cellular stresses are expected to exhibit high SASP heterogeneity due to a more complex and diverse spectrum of effector pathways involved in establishing this type of longer lasting senescent state. SASP heterogeneity may therefore be a mechanism to create subsets of senescent cells that are highly resistant to immune clearance and drive tissue degeneration.
Senescent-cell clearance and future directions
Studies in BubR1 progeroid mice provided proof-of-principle that clearance of senescent cells can delay age-related degenerative pathologies16. This, together with the lack of overt detrimental side effects associated with long-term clearance in BubR1 mutant mice, suggests that targeting senescent cells for destruction might be an effective therapeutic strategy for treatment of age-related diseases or improvement of healthy lifespan. Evidently, sophisticated approaches developed for selective eradication of cancer cells provide an invaluable blueprint for the development of molecular-targeted therapies against senescent cells. The problem of drug resistance that has plagued the cancer field is unlikely to apply to senolytic agents because rare residual senescent cells that remain after treatment would not be able to amplify through division.
However, although senescent-cell removal represents an attractive therapeutic avenue, there are many unknowns and potential pitfalls along this route. For example, our current knowledge about the rates and spatiotemporal patterns that drive the accumulation of senescent cells in both humans and animal models during normal ageing and in age-related diseases is limited. Another gap in knowledge relates to the degree of phenotypic heterogeneity (that is, SASP composition) between senescent cells that accumulate in vivo, not only between the acute and chronic senescent cells but also within these two classes. Also, it will be imperative to determine the impact of senescent cell clearance on the health and lifespan of normal mice, particularly now that evidence is mounting that senescence is beneficial for tissue development and repair.
Another important consideration is whether the mouse is a reliable model for recapitulating the physiological effects of senescence cell accumulation and clearance that occurs in humans. One prominent senescence-inducing stressor, telomere attrition, is specific to humans, and may be responsible for a higher baseline level of senescence in our species. If similar to telomere attrition, other senescence-inducing mechanisms were indeed more prevalent in humans than in mice, the therapeutic effect of senescent cell clearance might be even more robust than in mice. Conversely, we should consider that, in spite of its potential beneficial effects, the removal of high percentages of senescent cells could have undesirable outcomes to human health by triggering atrophy and tissue dysfunction. In addition, the most illuminating experiments in mice relied on targeting p16Ink4a-positive cells for elimination, a population that probably represents only a subset of cells undergoing senescence. Thus, the effects of clearing either p16Ink4a-negative senescent cells or, collectively, all senescent cells remain to be determined. In addition, cultured human and mouse cells differentially rely on the p53 and p16Ink4a pathways for induction of senescence27, underscoring the need for carefully validating information from mouse studies before extrapolating its results to the human situation. We should also consider that although the existence of multiple senescent-cell subtypes offers at the same time challenges and opportunities, targeting each of these populations separately might have either beneficial or detrimental effects.
The field of experimental therapeutics as it relates to senescence is a nascent yet promising area of investigation. Besides small molecules that target senescent cells, a potentially promising and straightforward bio-therapeutic approach would be to activate or reinforce the immune response against senescent cells. This approach will require a deeper understanding of the extent to which the immune system disposes of senescent cells as well as the molecular and cellular mechanisms underlying this process. Potential caveats are that immunodeficiency is not a main contributor to age-related increases in senescent cells, or that chronically senescent cells are enriched in SASP components that provide resistance to immune clearance99. Approaches exploiting the adaptive immune system to mount effective immunity against senescent cells may have limited feasibility owing to a potential lack of senescent-cell-specific antigens.
Undoubtedly, the next decade will see a tremendous expansion of data on the mechanisms, characteristics and functions of in vivo senescence, as well as the use of this information to ameliorate human age-related diseases and promote healthy lifespan.
Acknowledgements
I thank J. Campisi, J. Kirkland, R. Naylor, B. Childs, D. Baker, R. Urrutia, M. McNiven and R. Bram for helpful discussions and comments on the manuscript. I apologize to those whom I was unable to reference owing to space limitations. This work was supported by grants from the Paul Glenn Foundation and the National Institutes of Health (R01CA96985, R01CA166347 and AG41122-01P2).
Footnotes
The author declares no competing financial interests.
References
- 1.Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams PD. Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol. Cell. 2009;36:2–14. doi: 10.1016/j.molcel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Newgard CB, Sharpless NE. Coming of age: molecular drivers of aging and therapeutic opportunities. J. Clin. Invest. 2013;123:946–950. doi: 10.1172/JCI68833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. A pioneering study that introduced the term senescence to describe the phenomenon of permanent growth arrest of primary human cells after extensive serial passaging in culture.
- 8.Bodnar AG, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 9. Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. The study that established the concept of oncogene-induced senescence.
- 10.Rajagopalan S, Long EO. Cellular senescence induced byCD158d reprograms natural killer cells to promote vascular remodeling. Proc. Natl Acad. Sci. USA. 2012;109:20596–20601. doi: 10.1073/pnas.1208248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz-Espin D, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 12. Storer M, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. References 10, 11, 12 are studies demonstrating that senescence has a central role in tissue remodelling during embryogenesis.
- 13.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. References 14, 15 show that senescent cells that are produced after tissue damage act to curb fibrosis.
- 15. Baker DJ, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nature Cell Biol. 2008;10:825–836. doi: 10.1038/ncb1744. corrigendum 14, 649 (2012). A study that demonstrated a causal link between cellular senescence and age-related tissue deterioration, and the concept of assisted cycling.
- 16. Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. A study showing that clearance of p16Ink4a-positive senescent cells can delay age-related degenerative pathologies in a progeroid mouse model.
- 17.Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nature Rev. Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 18.Sedelnikova OA, et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nature Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 19.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 20.Aird KM, et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 2013;3:1252–1265. doi: 10.1016/j.celrep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 22.Lazzerini Denchi E, Attwooll C, Pasini D, Helin K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol. Cell. Biol. 2005;25:2660–2672. doi: 10.1128/MCB.25.7.2660-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplon J, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 24.Kondoh H, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 25. Dörr JR, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. References 20, 23, 25 revealed that metabolic mechanisms are causally implicated in the induction and maintenance of the senescent state.
- 26.Shamma A, et al. Rb Regulates DNA damage response and cellular senescence through E2F-dependent suppression of N-ras isoprenylation. Cancer Cell. 2009;15:255–269. doi: 10.1016/j.ccr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Moiseeva O, Mallette FA, Mukhopadhyay UK, Moores A, Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol. Biol. Cell. 2006;17:1583–1592. doi: 10.1091/mbc.E05-09-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romanov VS, et al. p21 (Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle. 2010;9:3945–3955. doi: 10.4161/cc.9.19.13160. [DOI] [PubMed] [Google Scholar]
- 30.Lapak KM, Burd CE. The molecular balancing act of p16INK4a in cancer and aging. Mol. Cancer Res. 2014:167–183. doi: 10.1158/1541-7786.MCR-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hein N, et al. In: Senescence. Nagata T, editor. Vol. 9. 2012. pp. 171–208. [Google Scholar]
- 32.Baker DJ, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nature Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt S, et al. The centrosome and mitotic spindle apparatus in cancer and senescence. Cell Cycle. 2010;9:4469–4473. doi: 10.4161/cc.9.22.13684. [DOI] [PubMed] [Google Scholar]
- 34.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passos JF, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker DJ, Weaver RL, van Deursen JM. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep. 2013;3:1164–1174. doi: 10.1016/j.celrep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grove GL, Cristofalo VJ. Characterization of the cell cycle of cultured human diploid cells: effects of aging and hydrocortisone. J. Cell. Physiol. 1977;90:415–422. doi: 10.1002/jcp.1040900305. [DOI] [PubMed] [Google Scholar]
- 38.Choudhury AR, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nature Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 39.Siegel JJ, Amon A. New insights into the troubles of aneuploidy. Annu. Rev. Cell Dev. Biol. 2012;28:189–214. doi: 10.1146/annurev-cellbio-101011-155807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vredeveld LC, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapasset L, et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–2253. doi: 10.1101/gad.173922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Cecco M, et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013;12:247–256. doi: 10.1111/acel.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, et al. Inhibition of activated pericentromeric SINE/Alu repeat transcription in senescent human adult stem cells reinstates self-renewal. Cell Cycle. 2011;10:3016–3030. doi: 10.4161/cc.10.17.17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov A, et al. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 2013;202:129–143. doi: 10.1083/jcb.201212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purvis JE, et al. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell. 2012;23:2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimi T, et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25:2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah PP, et al. Lamin B1depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27:1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funayama R, Saito M, Tanobe H, Ishikawa F. Loss of linker histone H1 in cellular senescence. J. Cell Biol. 2006;175:869–880. doi: 10.1083/jcb.200604005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narita M, et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–514. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 51.Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol. Cell. Biol. 2007;27:2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swanson EC, Manning B, Zhang H, Lawrence JB. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J. Cell Biol. 2013:929–942. doi: 10.1083/jcb.201306073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr. Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Pan KH, Cohen SN. Senescence-specific gene expression fingerprints reveal cell-type-dependent physical clustering of up-regulated chromosomal loci. Proc. Natl Acad. Sci. USA. 2003;100:3251–3256. doi: 10.1073/pnas.2627983100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:e301. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. erratum 11, 1272 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nature Rev. Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 58.Coppé JP, et al. Tumor suppressor and aging biomarkerp16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coppé JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 60.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laberge RM, Awad P, Campisi J, Desprez PY. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012;5:39–44. doi: 10.1007/s12307-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Binet R, et al. WNT16B is a new marker of cellular senescence that regulates p53 activity and the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2009;69:9183–9191. doi: 10.1158/0008-5472.CAN-09-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 64.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl Acad. Sci. USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benz G, Holzel D, Schmoeckel C. Inflammatory cellular infiltrates in melanocytic nevi. Am. J. Dermatopathol. 1991;13:538–542. doi: 10.1097/00000372-199113060-00003. [DOI] [PubMed] [Google Scholar]
- 66.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 67.Matheu A, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 68.Baker DJ, et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nature Cell Biol. 2013;15:96–102. doi: 10.1038/ncb2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging. 2010;2:627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Geiger H, Rudolph KL. Immunoaging induced by hematopoietic stem cell aging. Curr. Opin. Immunol. 2011;23:532–536. doi: 10.1016/j.coi.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Nikolich-Žugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nature Rev. Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- 73.Le ON, et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010;9:398–409. doi: 10.1111/j.1474-9726.2010.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nature Rev. Cancer. 2005;5:943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 75. Jurk D, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. A study showing that post-mitotic cells can obtain several key characteristics of senescent cells.
- 76.Minamino T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 77.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 78.Lawless C, et al. Quantitative assessment of markers for cell senescence. Exp. Gerontol. 2010;45:772–778. doi: 10.1016/j.exger.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 79.Wang C, et al. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 80.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 81.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naylor RM, Baker DJ, van Deursen JM. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin. Pharmacol. Ther. 2013;93:105–116. doi: 10.1038/clpt.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 84.Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011;21:107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodier F, Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 88.Krtolica A, et al. GROα regulates human embryonic stem cell self-renewal or adoption of a neuronal fate. Differentiation. 2011;81:222–232. doi: 10.1016/j.diff.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 91.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal–epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Acosta JC, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nelson G, et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11:345–349. doi: 10.1111/j.1474-9726.2012.00795.x. References 92, 94 show that senescent cells can induce senescence in neighbouring cells through paracrine mechanisms.
- 94.Faggioli F, Wang T, Vijg J, Montagna C. Chromosome-specific accumulation of aneuploidy in the aging mouse brain. Hum. Mol. Genet. 2012;21:5246–5253. doi: 10.1093/hmg/dds375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH. DNA damage and ageing: new-age ideas for an age-old problem. Nature Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 98.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhaumik D, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]