Abstract
Rationale: Activation of type 2 cytokine pathways plays a central role in a large subset of subjects with asthma. Th2-high and Th2-low asthma have distinct clinical, pathologic, and molecular phenotypes and respond differently to therapy. The factors that initiate type 2 responses in some subjects with asthma are unknown.
Objectives: To determine whether expression of epithelial cytokines IL-25, IL-33, and thymic stromal lymphopoietin are associated with type 2 responses and predict response to inhaled corticosteroid (ICS) in asthma.
Methods: We analyzed pulmonary function tests, blood, and bronchoscopic biopsies from 21 healthy control subjects and 43 subjects with asthma. Subjects with asthma underwent an 8-week treatment with inhaled budesonide.
Measurements and Main Results: Epithelial expression of IL-25, but not IL-33 or thymic stromal lymphopoietin, was increased in a subset of subjects with asthma. The IL-25-high subset had greater airway hyperresponsiveness, more airway and blood eosinophils, higher serum IgE, more subepithelial thickening, and higher expression of Th2 signature genes. ICS improved FEV1 and hyperresponsiveness in the IL-25-high but not the IL-25-low subset. Plasma IL-25 levels correlated with epithelial IL-25 expression, airway eosinophilia, and beneficial responses to ICS treatment.
Conclusions: IL-25 measurements identify two subsets of subjects with distinct asthma phenotypes and different responses to ICS. Because IL-25 has a major role in triggering type 2 responses, bronchial epithelial IL-25 expression is likely a key determinant of type 2 response activation in asthma. Plasma IL-25 level reflects airway IL-25/type 2 response activation and may be useful for predicting responses to asthma therapy.
Keywords: IL-25, asthma, phenotype
At a Glance Commentary
Scientific Knowledge on the Subject
Type 2 responses play prominent roles in a subset of individuals with asthma. Epithelial cytokines can trigger type 2 responses but the contributions of specific epithelial cytokines to Th2-high asthma have not been well defined.
What This Study Adds to the Field
Our results strongly implicate epithelial IL-25 as a major driver of Th2-high, inhaled corticosteroid–responsive asthma. Plasma IL-25 level reflects airway IL-25/type 2 response activation and may be useful for predicting responses to asthma therapy.
Asthma is a heterogeneous disease that can be subclassified based on clinical, pathologic, and molecular features (1–3). Clinical phenotyping can be based on asthma severity and response to therapy (1). Pathologic phenotyping can be based on the type of inflammatory cells in airway biopsies or induced sputum (2, 4). Molecular phenotyping (or endotyping) can be based on the expression of cytokines in airway tissues (3) or induced sputum (5, 6). One major asthma endotype is Th2-high asthma. Type 2 cytokines produced by airway CD4+ T cells (Th2 cells) and by type 2 innate lymphoid cells (iLC2s) can act on resident airway cells, including epithelial and smooth muscle cells, to produce key features of asthma, including eosinophilic inflammation, airway hyperresponsiveness, and mucus overproduction. The type 2 cytokine IL-13 is necessary and sufficient for each of these abnormalities in many animal models (7, 8). Human studies indicate that IL-13 is increased in a subset of subjects with asthma, and that these Th2-high subjects have different clinical and pathologic characteristics than Th2-low subjects with asthma (3). For example, Th2-high subjects had improvements in FEV1 following inhaled corticosteroid (ICS) treatment, whereas Th2-low subjects did not. However, the mechanisms that activate the type 2 responses in subjects with Th2-high asthma are largely unknown.
Airway epithelial cells are important for allergen uptake, sensitization to allergen, and initiation of allergic inflammation (9). Epithelial-derived cytokines can trigger type 2 responses by multiple mechanisms. The epithelial cytokine thymic stromal lymphopoietin (TSLP) conditions dendritic cells and also acts directly on naive CD4+ T cells to promote Th2 cell differentiation (10, 11). The epithelial cytokines IL-25 and IL-33 act on multiple immune cells, including mast cells, basophils, and iLC2s (12–17). IL-25 (18–21), IL-33 (22), and TSLP (11) have each been reported to be both necessary and sufficient for type 2 cytokine production, eosinophilic airway inflammation, mucous metaplasia, and airway hyperresponsiveness in certain mouse models. In human studies, bronchial expression of TSLP (23), IL-25 (24, 25), and IL-33 (26) have been reported to be increased in asthma. However, it is not yet known whether differences in expression of one or more of these epithelial cytokines are associated with Th2-high/Th2-low status and response to asthma therapy.
We hypothesized that the epithelial cytokines IL-25, IL-33, and TSLP are expressed at different levels in subjects with asthma and that these differences are associated with the clinical, pathologic, and molecular heterogeneity. To test our hypothesis, we recruited 21 healthy control subjects and 43 subjects with asthma and examined epithelial cytokine expression. After determining that IL-25 expression was elevated in a subset of subjects with asthma, we compared the clinical, pathologic, and molecular phenotypes of IL-25-high and IL-25-low asthma. Because epithelial IL-25 expression is difficult to measure, we also investigated whether measurements of plasma IL-25 could be used as a surrogate.
Methods
Subjects
We recruited 21 healthy control subjects and 43 subjects with asthma. All subjects were Chinese and were recruited from Tongji Hospital. Subjects with asthma were diagnosed by a physician; had symptoms of episodic cough, wheeze, and/or breathlessness; and had accumulated dosage of methacholine provoking a 20% fall in FEV1 (PD20) less than 2.5 mg and/or greater than or equal to 12% increase in FEV1 following inhalation of 200 μg salbutamol. Physicians referred the subjects with asthma to the study personnel after they established a new diagnosis of asthma and decided to initiate the standard ICS regimen in use at Tongji Hospital. After baseline evaluation by study personnel, subjects initiated ICS prescribed by the referring physician. Healthy control subjects had no respiratory symptoms, normal spirometric value, and methacholine PD20 greater than 2.5 mg. None of the subjects had ever smoked or received ICS or oral corticosteroid or leukotriene antagonist. Written informed consent was obtained from all subjects. The ethics committee of Tongji Hospital, Huazhong University of Science and Technology, approved the study.
Baseline Evaluation
For each subject, at study entry we recorded demographic information, collected blood and induced sputum samples, and performed spirometry and allergen skin prick testing with a panel of 14 aeroallergens. We performed bronchoscopy with brushing and endobronchial biopsies within 1 week of study entry. Biopsy techniques and methods for histology, immunohistochemistry, and gene expression analysis by quantitative polymerase chain reaction are detailed in the Methods section of the online supplement.
ICS Treatment
All subjects with asthma were placed on a standard ICS regimen (budesonide, 200 μg twice a day) by their personal physicians. Subjects began using ICS immediately after the bronchoscopy procedure and continued to use ICS throughout the 8-week study period. All subjects underwent pulmonary function testing at 4 and 8 weeks and had blood samples collected at 4 weeks.
Statistical Analysis
We analyzed data using Prism version 5 (GraphPad Software, San Diego, CA) and SPSS version 19 (SPSS Inc., Chicago, IL). For normally distributed data we calculated means ± SD and we used parametric tests (one-way analysis of variance with Tukey correction or unpaired t test) to compare across groups. For nonnormally distributed data we calculated medians (with interquartile ranges) and used nonparametric tests (Kruskal-Wallis test with Dunn intergroup comparison or Mann-Whitney test). We analyzed correlation using Spearman rank order correlation. For significance testing of PD20 and serum IgE levels, data were log transformed for normality.
Results
Subject Characteristics
Subject characteristics are summarized in Table 1. Subjects with asthma had lower FEV1 and methacholine PD20 and higher serum IgE levels and blood eosinophil numbers than healthy control subjects.
Table 1.
Overall Subject Characteristics*
| Normal Control Subjects | Subjects with Asthma | P Value | |
|---|---|---|---|
| Number | 21 | 43 | — |
| Age, yr | 35 ± 10 | 35 ± 10 | 0.75 |
| Sex, M:F (% F) | 9:12 (57) | 17:26 (60) | 0.80 |
| FEV1, % predicted | 94 (90.1–101.9) | 85 (72.3–91.2) | <0.0001 |
| Methacholine PD20, mg | 4.7 (3.5–5.5) | 0.02 (0.01–0.14) | <0.0001 |
| IgE, IU/ml | 20.5 (8.6–46.7) | 141 (39.6–289.2) | <0.0001 |
| Blood eosinophils, ×109/L | 0.07 ± 0.07 | 0.38 ± 0.21 | <0.0001 |
Definition of abbreviation: PD20 = provocative dosage required to cause a 20% decline in FEV1.
Values are presented as mean ± SD or median (interquartile range). P values are Sidak corrected for multiple testing (across the three groups).
Airway Epithelial IL-25, IL-33, and TSLP Expression
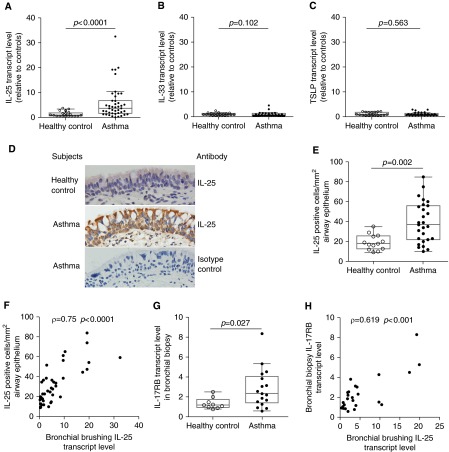
We measured the levels of IL-25, IL-33, and TSLP transcripts in bronchial brushing samples by quantitative polymerase chain reaction. IL-25 transcript levels in bronchial brushing samples of subjects with asthma were significantly higher than in healthy control subjects (Figure 1A). IL-25 expression was heterogeneous: some subjects with asthma had IL-25 levels that were within the normal range, whereas others had levels that were substantially above the normal range. There were no significant differences in IL-33 and TSLP transcript levels between subjects with asthma and healthy control subjects (Figures 1B and 1C).
Figure 1.
Bronchial epithelial expression of IL-25 and its receptor are increased in some subjects with asthma. Levels of IL-25 (A), IL-33 (B), and thymic stromal lymphopoietin (TSLP) (C) transcripts were determined by quantitative polymerase chain reaction (PCR) of RNA from bronchial brushings of healthy control subjects (n = 21) and subjects with asthma (n = 43). Values are relative to the median value for healthy control subjects. The median IL-25 cycle threshold values for healthy control subjects and asthma subjects were 24.06 and 22.14, respectively. (D) IL-25 immunohistochemistry in bronchial biopsies from healthy control subjects and subjects with asthma (original magnification ×400). (E) Quantitation of IL-25–containing cells in the epithelium of healthy control subjects (n = 13) and subjects with asthma (n = 27). (F) Correlation between bronchial brushing IL-25 transcript level and the number of IL-25–containing cells in airway epithelium for healthy control subjects (n = 13) and subjects with asthma (n = 27). (G) The transcript level of IL-25 receptor, IL-17RB, as determined by quantitative PCR of RNA from bronchial biopsies of healthy control subjects (n = 9) and subjects with asthma (n = 17). (H) Correlation between bronchial brushing IL-25 transcript level and IL-17RB transcript level in bronchial biopsies of healthy control subjects (n = 9) and subjects with asthma (n = 17). Some subjects were not included in the analyses of IL-25 protein and/or IL-17RB transcript level because bronchial biopsies from those subjects were not adequate for reverse transcriptase PCR and/or immunohistochemistry because of limitations in the number of biopsies obtained or the amount of intact tissue seen in the sections.
IL-25 immunostaining revealed that IL-25–containing cells were mainly airway epithelial cells. Consistent with our analysis of IL-25 transcript levels, only some subjects with asthma had increased numbers of IL-25–containing cells compared with healthy control subjects (Figures 1D and 1E). Moreover, the number of IL-25–containing cells was correlated with IL-25 transcript levels in bronchial brushing samples (Figure 1F). We also examined the expression of the IL-25 receptor, IL-17RB, which is known to be induced by IL-25 (24). IL-17RB transcript levels in bronchial biopsies of subjects with asthma were significantly higher than in healthy control subjects (Figure 1G). Some subjects with asthma had IL-17RB transcript levels within the normal range, whereas others had levels above the normal range. IL-17RB transcript levels in biopsies correlated with IL-25 levels in bronchial brushings from the same subjects (Figure 1H). We detected IL-17RB protein in bronchial epithelial cells and some subepithelial cells by immunohistochemistry (see Figure E1A in the online supplement). The number of IL-17RB–staining epithelial cells was higher in subjects with asthma than in healthy control subjects and the number of IL-17RB–positive cells in airway epithelium was significantly correlated with the number of IL-25–positive cells (see Figures E1B and E1C). Our data indicate that the activation of IL-25 pathway is limited to a subset of subjects with asthma.
Clinical Characteristics of IL-25-Low and IL-25-High Asthma
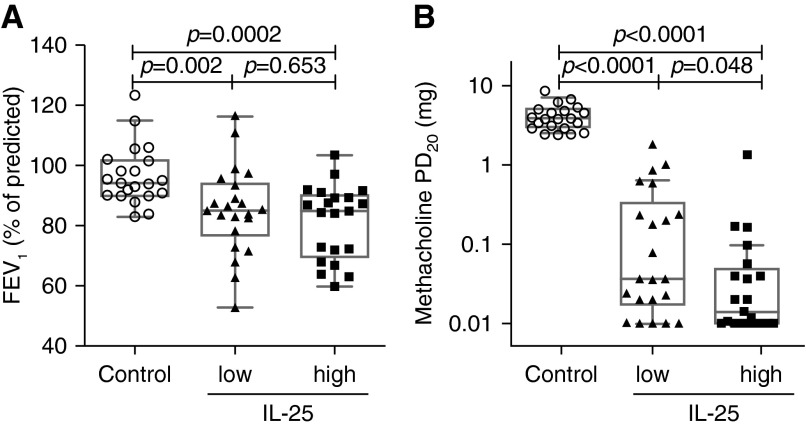
Our finding that the IL-25 pathway was only activated in a subset of subjects with asthma prompted us to hypothesize that the IL-25 pathway is involved in asthma clinical heterogeneity. We classified the subjects with asthma into IL-25-high and IL-25-low subgroups according to IL-25 transcript levels in bronchial brushing samples. We classified subjects with asthma as IL-25-low (at or below the 95th percentile for healthy control subjects) and IL-25-high (above the 95th percentile for healthy control subjects). According to this classification, 21 of 43 subjects with asthma were IL-25-high. Subjects in these two groups had similar demographic characteristics and FEV1 (Table 2 and Figure 2A). However, IL-25-high subjects with asthma had more severe airway hyperresponsiveness (as measured by methacholine PD20) (Figure 2B).
Table 2.
Subject Characteristics by IL-25 Expression*
| Normal Control Subjects | Subjects with Asthma |
P Value (Low vs. High) | ||
|---|---|---|---|---|
| IL-25 Low | IL-25 High | |||
| Number | 21 | 22 | 21 | — |
| Age, yr | 35 ± 10 | 37 ± 10 | 33 ± 11 | 0.23 |
| Sex, M:F (% F) | 9:12 (57) | 9:13 (59) | 8:13 (62) | 0.85 |
| Body mass index | 21.8 ± 3.0 | 22.5 ± 3.7 | 21.6 ± 3.2 | 0.38 |
| Age at onset | — | 32 ± 10 | 31 ± 11 | 0.68 |
| Duration of asthma | — | 3 ± 6 | 3 ± 3 | 0.58 |
| FEV1, % predicted | 94 (90.1–101.9) | 85.2 (76.9–94.1) | 84.8 (69.9–90.3) | 0.65 |
| Methacholine PD20, mg | 3.9 (3–5.1) | 0.04 (0.02–0.33) | 0.01 (0.01–0.05) | 0.048 |
| IgE, IU/ml | 20.5 (8.6–46.7) | 63.6 (24.4–152.3) | 219.4 (97.8–683) | 0.001 |
| Blood eosinophils, ×109/L | 0.07 ± 0.07 | 0.31 ± 0.2 | 0.45 ± 0.21 | 0.045 |
| Sputum eosinophils, % | 0.13 ± 0.22 | 6.34 ± 8.53 | 14.68 ± 13.62 | 0.009 |
| Biopsy eosinophils, number/mm2 | 0 (0–0.8); n = 15 | 7.8 (4.3–15); n = 18 | 23.7 (21–28); n = 15 | 0.0003 |
| Atopic, % | 2 (10) | 22 (100) | 21 (100) | — |
| Basement membrane thickness, μm | 5.19 (4.9–5.7); n = 15 | 6.2 (5.43–8.5); n = 18 | 10.3 (8.4–11.1); n = 15 | 0.0006 |
| ΔFEV1 with ICS at 4 wk, L | — | 0.12 ± 0.05 | 0.35 ± 0.11 | 0.001 |
| ΔFEV1 with ICS at 8 wk, L | — | 0.11 ± 0.09 | 0.34 ± 0.10 | 0.001 |
Definition of abbreviations: ICS = inhaled corticosteroids; PD20 = provocative dosage required to cause a 20% decline in FEV1.
Values are presented as mean ± SD or median (interquartile range) unless otherwise specified. P values are Sidak corrected for multiple testing (across the three groups). In case of missing data, the number of subjects for whom data exist is noted.
Figure 2.
Pulmonary function test results in IL-25-low and IL-25-high asthma. (A) FEV1, a measure of airway obstruction. (B) Methacholine-provocative dosage required to cause a 20% decline in FEV1 (PD20), a measure of airway hyperresponsiveness.
Pathologic Characteristics and Markers of Allergic Sensitization in IL-25-Low and IL-25-High Asthma
Eosinophilic airway inflammation and airway remodeling are common features of asthma and IL-25 can induce these abnormalities in animal models (18, 27). We found that IL-25-high subjects with asthma had more marked eosinophilic airway inflammation, as reflected by higher levels of eosinophils in both sputum and airway submucosa from airway biopsies (Figures 3A and 3B). To investigate whether IL-25 expression was associated with airway remodeling, we measured basement membrane thickness, a measure of subepithelial fibrosis, in bronchial biopsies. IL-25-high subjects with asthma had greater basement membrane thickness than IL-25-low subjects with asthma (Figure 3C). These results indicate that epithelial IL-25 expression is associated with more severe eosinophilic airway inflammation and airway remodeling in subjects with asthma.
Figure 3.
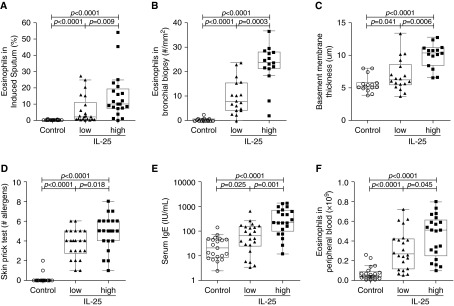
Measures of airway eosinophilic inflammation, airway remodeling, and allergy are increased in subjects with IL-25-high asthma. (A) Eosinophils as a percentage of total cells in induced sputum. (B) The number of eosinophils per square millimeter of submucosa in bronchial biopsy. (C) Reticular basement membrane thickness, a measure of subepithelial fibrosis. (D) Skin prick test using a panel of 14 aeroallergens. (E) Serum IgE. (F) Peripheral blood eosinophil count.
Skin prick tests, serum IgE level, and peripheral blood eosinophil counts are measures of allergic status. IL-25-high subjects with asthma had more positive skin tests than IL-25-low subjects with asthma (Figure 3D). We did not find any apparent relationship between IL-25 status and responses to specific allergens (see Figure E2). IL-25-high subjects also had higher serum IgE levels and peripheral blood eosinophil counts (Figures 3E and 3F).
Airway Epithelial Expression of Th2 Signature Genes and Mucins in IL-25-Low and IL-25-High Asthma
Previous studies indicate that epithelial expression of IL-13–induced genes can be used to classify Th2-high and Th2-low subjects with asthma (3, 5). To determine whether epithelial IL-25 expression is associated with this measure of Th2 status, we examined the expression of the IL-13–induced genes CLCA1, POSTN, and SERPINB2 in bronchial epithelial brushings by quantitative polymerase chain reaction. We combined these measurements to calculate a three-gene-mean for each subject as described previously (28). The three-gene-mean was higher in IL-25-high as compared with IL-25-low subjects with asthma (Figure 4A). Levels of periostin mRNA (POSTN), a potential biomarker for eosinophilic airway inflammation (29), were significantly higher in IL-25-high as compared with IL-25-low subjects with asthma (see Figure E3). Our data indicate that the IL-25 pathway is associated with more prominent epithelial type 2 responses in subjects with asthma.
Figure 4.
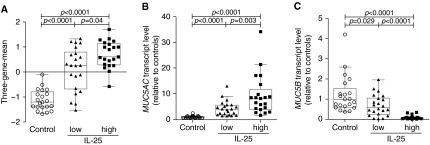
Bronchial epithelial IL-25 transcript levels are associated with the epithelial expression of Th2 signature genes and mucins. (A) The three-gene-mean derived from the transcript levels of Th2 signature gene CLCA1, POSTN, and SERPINB2 as determined by quantitative polymerase chain reaction in bronchial brushings. (B, C) Levels of transcripts for the major airway mucins MUC5AC (B) and MUC5B (C) in bronchial brushings as determined by quantitative polymerase chain reaction. Values are relative to the median value for healthy control subjects.
Mucus overproduction is another essential feature of asthma. MUC5AC and MUC5B are the major gel-forming mucins in human airway mucus (30). Both mucins are regulated by IL-13, and previous work showed that Th2-high asthma is characterized by elevated levels of MUC5AC mRNA and reduced levels of MUC5B compared with Th2-low asthma or healthy control subjects (3). We found that MUC5AC transcript levels in IL-25-high and IL-25-low subjects with asthma were significantly higher than in healthy control subjects. Moreover, IL-25-high subjects with asthma had significantly higher MUC5AC transcript levels than IL-25-low subjects with asthma (Figure 4B). In contrast, MUC5B transcript levels were significantly lower in subjects with asthma when compared with healthy control subjects, and were even lower in IL-25-high as compared with IL-25-low subjects with asthma (Figure 4C). Immunofluorescent staining for MUC5AC and MUC5B proteins in a limited number of biopsies was consistent with the differences in mucin expression detected by mRNA analysis (see Figure E4).
Distinct Responsiveness to ICS in IL-25-Low and IL-25-High Asthma
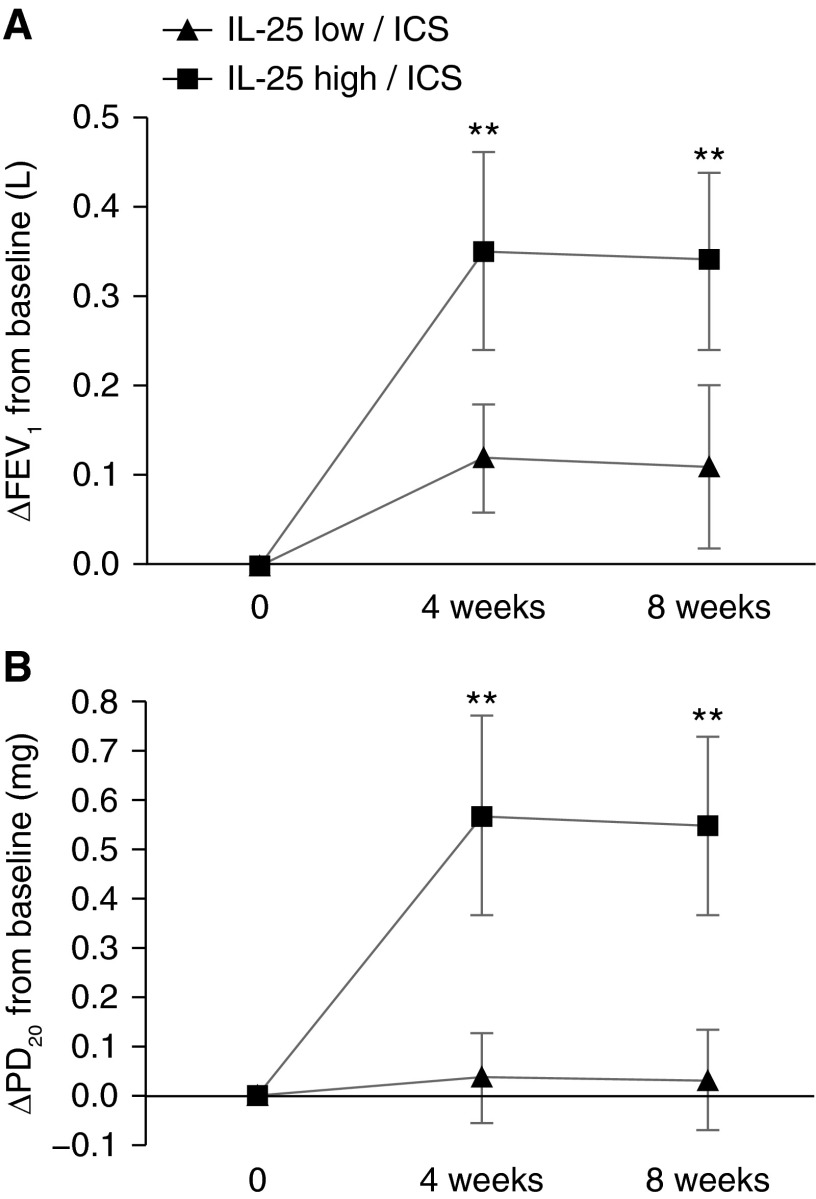
To determine whether airway epithelial IL-25 expression is associated with ICS responsiveness in subjects with asthma, we measured FEV1 and PD20 after 4 and 8 weeks of treatment with inhaled budesonide. Subjects with IL-25-high asthma had significantly more improvement in FEV1 during treatment with inhaled budesonide when compared with ICS-treated subjects with IL-25-low asthma (Figure 5A). During ICS treatment, PD20 improved in subjects with IL-25-high asthma but not in subjects with IL-25-low asthma (Figure 5B). These data indicate that the IL-25 pathway is involved in the heterogeneity of ICS responsiveness in asthma.
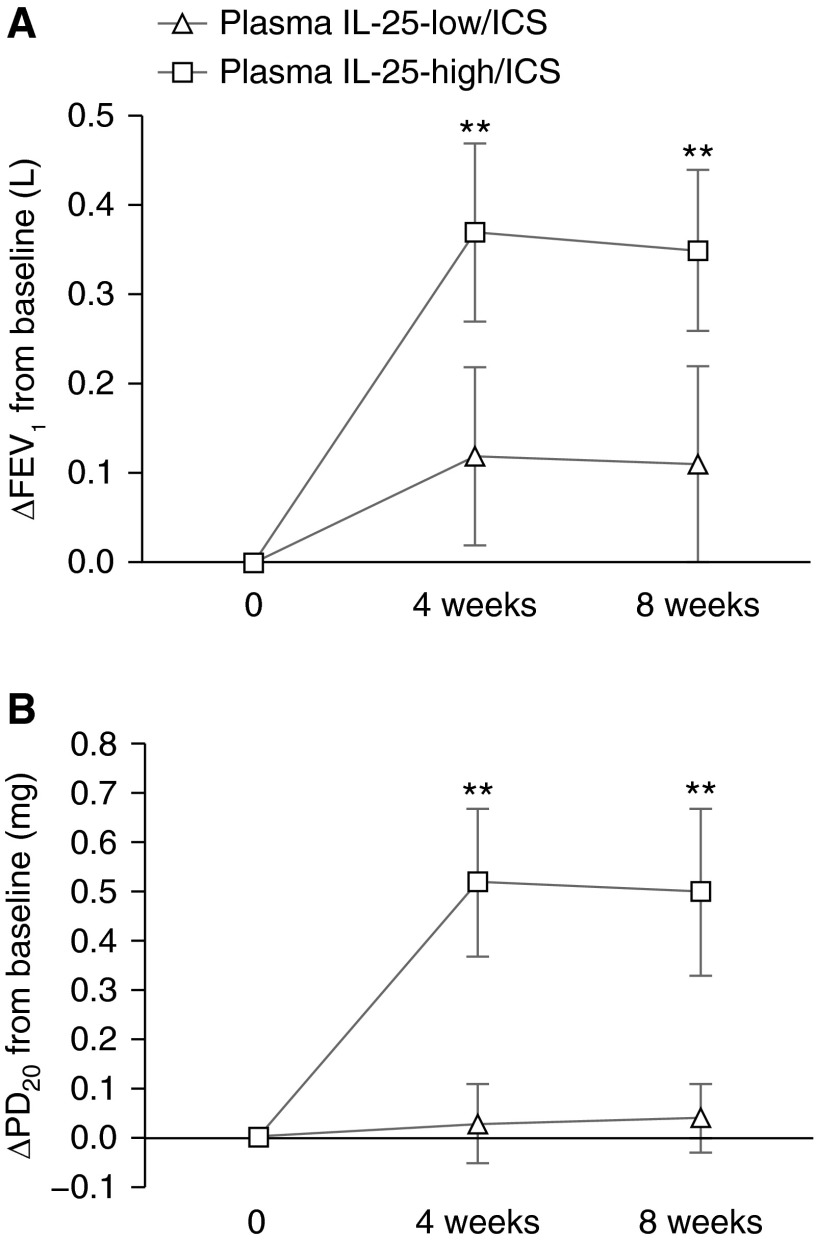
Figure 5.
Subjects with IL-25-high asthma have larger improvements in pulmonary function during inhaled corticosteroid (ICS) treatment. Twenty-two IL-25-low subjects and 21 IL-25-high subjects (as determined by epithelial IL-25 mRNA level) with asthma all received ICS treatment. FEV1 (A) and provocative dosage required to cause a 20% decline in FEV1 (PD20) (B) were measured at baseline (0) and after 4 or 8 weeks on daily inhaled budesonide (200 μg twice a day). **P < 0.01 for IL-25-low/ICS versus IL-25-high/ICS.
Plasma IL-25 Levels Are Associated with Epithelial IL-25 Expression and Eosinophilic Airway Inflammation
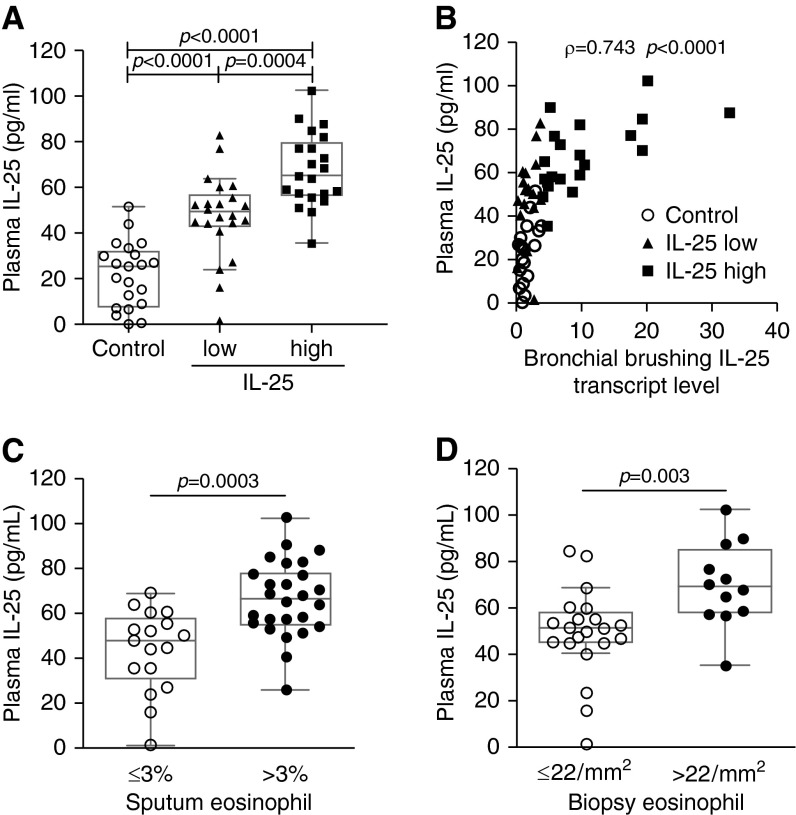
Asthma phenotyping based on bronchoscopic samples has limited application. Therefore, we examined IL-25 levels in peripheral blood by ELISA. Consistent with our findings in bronchial epithelial brushings, plasma IL-25 levels were only increased in some subjects with asthma. Subjects classified as IL-25-high based on epithelial IL-25 mRNA had significantly higher plasma IL-25 levels than subjects classified as IL-25-low (Figure 6A). Plasma IL-25 levels were correlated with the expression of IL-25 in bronchial epithelial brushings (Figure 6B). This indicates that plasma IL-25 may be a useful surrogate for airway epithelial IL-25 expression.
Figure 6.
Increased plasma IL-25 levels are associated with increased bronchial epithelial IL-25 expression and airway eosinophilia. (A) Plasma IL-25 level in peripheral blood as determined by ELISA. (B) Correlation between bronchial brushing IL-25 transcript levels and plasma IL-25 levels of all subjects, including healthy control subjects and subjects with asthma. (C) Plasma IL-25 levels in subjects with asthma with and without sputum eosinophilia. (D) Plasma levels in subjects with asthma and without submucosal eosinophilia in bronchial biopsies.
We examined the relationship between plasma IL-25 and eosinophilic airway inflammation. We used a previously described cutoff to classify the sputum eosinophil status (3% eosinophils of total sputum inflammatory cells) and bronchial eosinophil status (22 eosinophils per square millimeter submucosa) (31, 32). We found a strong association between airway eosinophilia and plasma IL-25 levels in steroid-naive asthma (Figures 6C and 6D).
Plasma IL-25 Level Is Associated with the ICS Response of Subjects with Asthma
We further explored the relationship between plasma IL-25 level and ICS response of subjects with asthma. We selected a plasma IL-25 threshold of 55 pg/ml to maximize the concordance with IL-25 status based on epithelial IL-25 mRNA levels (see Table E2). Improvement of FEV1 and PD20 was significantly greater in subjects with high plasma IL-25 (>55 pg/ml) compared with those with low plasma IL-25 (≤55 pg/ml) after 4 and 8 weeks of inhaled budesonide treatment (Figures 7A and 7B). These data indicate that plasma IL-25 level is associated with ICS responses of subjects with asthma. To evaluate the utility of plasma IL-25 as a predictor of ICS responsiveness (defined as >7.5% improvement in FEV1 after 8 weeks of ICS [33]), we performed receiver operating characteristic analysis (see Figure E5). Area under the curve for plasma IL-25 compared favorably with areas under the curve for blood eosinophil number and sputum eosinophil percentage, and was slightly lower than the area under the curve obtained using biopsy eosinophil number, which requires an invasive procedure.
Figure 7.
Subjects with elevated plasma IL-25 have larger improvements in pulmonary function during inhaled corticosteroid (ICS) treatment. Twenty plasma IL-25-low subjects (≤55 pg/ml) and 23 plasma IL-25-high subjects (>55 pg/ml) with asthma all received ICS treatment. FEV1 (A) and provocative dosage required to cause a 20% decline in FEV1 (PD20) (B) were measured at baseline (0) and after 4 or 8 weeks on daily inhaled budesonide (200 μg twice a day). **P < 0.01 for plasma IL-25-low/ICS versus plasma IL-25-high/ICS.
We also examined plasma IL-25 level in subjects with asthma after ICS treatment. We found that plasma IL-25 was significantly decreased after treatment with inhaled budesonide for 4 weeks. The decrease of plasma IL-25 was mainly observed in IL-25-high subjects. There was no significant change of plasma IL-25 in IL-25-low subjects (see Figure E6). This suggests that ICS treatment inhibits airway IL-25 expression in IL-25-high asthma.
Discussion
We provide a new method for molecular phenotyping (endotyping) of asthma based on expression of the airway epithelial cytokine IL-25. We found that IL-25-high and IL-25-low subjects with asthma had distinct clinical and pathologic characteristics and distinct responses to ICS. Airway epithelial IL-25 expression correlated with Th2 signature gene expression. These results suggest that IL-25 expression is a major determinant of type 2 status (Th2-high vs. Th2-low) in asthma. Another notable finding was that plasma IL-25 levels correlated with airway epithelial IL-25 expression and were associated with ICS response in subjects with asthma. Our results indicate that measurements of plasma IL-25 are useful for asthma phenotyping and suggest that IL-25 measurements may be valuable for selecting appropriate asthma treatments.
We measured the airway epithelial expression of epithelial cytokines IL-25, IL-33, and TSLP. Each of these cytokines was reported to be elevated in prior studies of asthma (23, 24, 26). Although our study was larger than previous studies that showed increases in IL-33 (26) and TSLP (23) mRNAs in asthma, we did not detect changes in these mRNAs in our study. This likely reflects differences in study populations and not a technical limitation of our approach, because the method we used for measuring cytokine expression is highly quantitative and readily detected differences in expression of other genes. Although we did not detect differences in IL-33 and TSLP mRNAs, our data do not exclude the possibility that post-transcriptional regulation of these cytokines contributes to asthma heterogeneity in our subjects. Unlike IL-33 and TSLP, IL-25 was elevated in our group of subjects with asthma. Although there was a significant increase in IL-25 in the group of subjects with asthma, many subjects in the group had normal IL-25 levels. This is consistent with a recent report that IL-25 transcript level was increased in induced sputum from some individuals with asthma (34). None of our subjects were using oral corticosteroids or ICS or leukotriene antagonist before enrollment, which excludes the possibility that prior antiinflammatory medications accounted for differences in IL-25 expression. In summary, airway epithelial IL-25 mRNA was increased in some subjects with asthma, whereas we did not detect differences in IL-33 and TSLP mRNA levels between healthy control subjects and subjects with asthma.
IL-25 has been reported to induce iLC2s and CD4+ T cells to produce IL-4, IL-5, and IL-13 and to play a critical role in airway eosinophilia, mucus overproduction, and airway remodeling in mouse models of allergic airway disease (20, 35). To assess the relationship between IL-25 expression and asthma phenotypes, we classified subjects with asthma based on epithelial IL-25 expression and compared clinical, pathologic, and molecular characteristics of the IL-25-high and IL-25-low subgroups. Although a previous study (24) showed that the number of IL-25-immunoreactive cells in airway biopsies correlated inversely with FEV1, we found no significant correlation between epithelial IL-25 expression and FEV1 in our larger cohort. However, we did find that subjects with IL-25-high asthma had more marked airway hyperresponsiveness, airway and blood eosinophilia, IgE elevations, skin test reactivity, and subepithelial layer thickening than either IL-25-low subjects with asthma or healthy control subjects. Furthermore, hyperresponsiveness and lung function (as measured by FEV1) improved during ICS treatment in IL-25-high subjects with asthma but not in IL-25-low subjects. These differences are reminiscent of differences that have been described when comparing Th2-high and Th2-low asthma (3). To directly address whether differences in IL-25 status are associated with differences in type 2 status, we used the validated three-gene-mean metric (28), which incorporates three IL-13 responsive epithelial genes. This analysis confirmed that IL-25 status was strongly associated with type 2 status. In addition to the three genes included in the three-gene metric, we also saw differences in expression of IL-13–regulated mucin genes. MUC5AC, which is selectively increased in Th2-high asthma (3), was also increased in IL-25-high subjects and MUC5B, which is decreased in Th2-high asthma, was also decreased in IL-25-high subjects. These results demonstrate a strong association between airway type 2 responses and elevated epithelial IL-25 expression in our population. Taken together with the extensive body of evidence that shows that IL-25 initiates type 2 responses and causes eosinophilic inflammation, subepithelial fibrosis, and airway hyperresponsiveness in mouse airway disease models (18–20, 27), our results strongly suggest that increases in human airway epithelial IL-25 expression are a major contributor to airway type 2 responses and Th2-high asthma.
Although epithelial gene expression profiling is a useful research tool, it is important to develop less invasive methods for identifying endotypes, predicting response to therapy with existing agents, and identifying appropriate participants for clinical trials of novel agents. We found that plasma IL-25 correlated with bronchial epithelial IL-25 expression in our study population. Elevated plasma IL-25 levels normalized after corticosteroid inhalation, which suggests that increases in plasma IL-25 reflect increased IL-25 production in the airways. Like epithelial IL-25 mRNA, plasma IL-25 was elevated in patients with eosinophilic inflammation. More importantly, elevated plasma IL-25 identified a group of subjects with physiologic responses to ICS (increased FEV1 and decreased hyperresponsiveness), whereas we found no detectable improvement in these measures in subjects with lower plasma IL-25 levels. Plasma IL-25 measurement therefore represents a novel tool for classifying subjects with asthma. Recent studies have demonstrated the value of serum measurements of periostin, a protein that is induced by IL-13 stimulation of epithelial cells, in identifying Th2-high asthma and predicting responses to ICS (29) and to anti–IL-13 antibody (36, 37). Our work suggests the possibility that measurements of IL-25 alone or in combination with measurements of other markers, such as periostin, could help to more precisely define asthma endotypes and identify appropriate cohorts for testing treatments specifically aimed at IL-25/Th2-driven asthma.
Our study has several limitations. First, although plasma IL-25 levels were measured before and after ICS treatment, bronchoscopy and bronchial epithelial brushing was performed only once before ICS treatment. Therefore, the direct effect of ICS treatment on bronchial epithelial IL-25 expression is unknown. Second, because plasma IL-25 level was decreased after ICS treatment in our study population, the utility of IL-25 measurements in subjects using inhaled (or systemic) corticosteroids is uncertain. Some subjects with severe asthma have persistent Th2 responses despite ICS and do benefit from anti–IL-13 antibody therapy (37), and further studies are required to determine whether IL-25 measurements are useful in severe asthma. Third, all subjects in this study were Chinese and most were allergic young adults. Additional studies are required to determine whether phenotyping of asthma based on IL-25 expression is useful in other populations of subjects with asthma. Fourth, our study was an 8-week observational study; longer clinical trials are required to determine whether IL-25 status is a useful predictor of the ability of ICS or other treatments to affect clinically important outcomes, such as rate of exacerbations. Fifth, because of the lack of drugs that specifically target IL-25, we were not able to directly determine whether increased IL-25 expression is required for development or persistence of Th2-high asthma. IL-25 antagonists are effective in mouse models of asthma (21) and our results suggest that plasma IL-25 may be useful for identifying a subset of subjects most likely to benefit from IL-25 antagonists if these become available for testing in humans. Finally, our study was not designed to identify the factors that lead to increased IL-25 expression in some subjects with asthma. IL-25 was induced following allergen challenge of subjects with allergic asthma (25) and mouse studies suggest that this effect may be at least partially caused by allergen proteases (38). Little is known about how epithelial IL-25 expression is affected by other environmental exposures, such as particulates and respiratory viruses. Our results provide additional motivation for further investigation of environmental and genetic factors that may increase IL-25 expression and drive Th2-high asthma.
Acknowledgments
Acknowledgment
The authors thank the patients who volunteered for this study; Zhenxiang Zhang, Shengdao Xiong, and Hong Huang for referring subjects with asthma for the study; Xiaoling Rao and Zhengyun Wang for bronchoscopy support; Shixin Chen, Wang Ni, and Kun Zhang for spirometry measurement; and Prescott G. Woodruff (University of California San Francisco) for advice in calculation of the three-gene-mean.
Footnotes
Supported by National Natural Science Foundation of China (grants 81170022 and 30700244), Scientific Research Foundation for the Returned Overseas Chinese Scholars of State Education Ministry grant (2008890), National Key Technology R&D Program of the 12th Five-year Development Plan (2012BAI05B01), the UCSF Sandler Asthma Basic Research Center, and the National Institutes of Health (grants AI1077439 and HL099101).
Author Contribution: G.Z. designed and conducted the clinical study, sample analyses, statistical analyses, conceived of the manuscript, and had primary responsibility for writing. D.C., Z.X., L.Y., H.S., K.Z., and X.H. performed sample collection and sample analyses. L.R.B. assisted with immunofluorescent staining. J.Z. and Y.X. contributed to clinical study design. D.J.E. assisted with statistical analyses and writing of the manuscript. All authors reviewed and approved the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201403-0505OC on August 18, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 2.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 5.Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV.Measures of gene expression in sputum cells can identify Th2-high and Th2-low subtypes of asthma J Allergy Clin Immunol 2013133388–394.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG.Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples J Allergy Clin Immunol 2011127153–160.e9 [DOI] [PubMed] [Google Scholar]
- 7.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 9.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–268. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 10.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 11.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 14.Licona-Limón P, Kim LK, Palm NW, Flavell RA. Th2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 15.Lambrecht BN, Hammad H. Asthma: the importance of dysregulated barrier immunity. Eur J Immunol. 2013;43:3125–3137. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Altemeier WA, Vandree J, Piliponsky AM, Johnson B, Appel CL, Frevert CW, Hyde DM, Ziegler SF, Smith DE, et al. Increased density of intraepithelial mast cells in patients with exercise-induced bronchoconstriction regulated through epithelially derived thymic stromal lymphopoietin and IL-33. J Allergy Clin Immunol. 2014;133:1448–1455. doi: 10.1016/j.jaci.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vercelli D, Gozdz J, von Mutius E. Innate lymphoid cells in asthma: when innate immunity comes in a Th2 flavor. Curr Opin Allergy Clin Immunol. 2014;14:29–34. doi: 10.1097/ACI.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 18.Suzukawa M, Morita H, Nambu A, Arae K, Shimura E, Shibui A, Yamaguchi S, Suzukawa K, Nakanishi W, Oboki K, et al. Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, is crucial for murine asthma. J Immunol. 2012;189:3641–3652. doi: 10.4049/jimmunol.1200461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 20.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 21.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 22.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 23.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 24.Corrigan CJ, Wang W, Meng Q, Fang C, Wu H, Reay V, Lv Z, Fan Y, An Y, Wang YH, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci USA. 2011;108:1579–1584. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, Lv Z, An Y, Wang YH, Liu YJ, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011;128:116–124. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 27.Gregory LG, Jones CP, Walker SA, Sawant D, Gowers KH, Campbell GA, McKenzie AN, Lloyd CM. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013;68:82–90. doi: 10.1136/thoraxjnl-2012-202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhakta NR, Solberg OD, Nguyen CP, Nguyen CN, Arron JR, Fahy JV, Woodruff PG. A qPCR-based metric of Th2 airway inflammation in asthma. Clin Transl Allergy. 2013;3:24. doi: 10.1186/2045-7022-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients J Allergy Clin Immunol 2012130647–654.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, et al. Muc5b is required for airway defense. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 32.Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, Wenzel SE. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. 2005;116:1249–1255. doi: 10.1016/j.jaci.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Seys SF, Grabowski M, Adriaensen W, Decraene A, Dilissen E, Vanoirbeek JA, Dupont LJ, Ceuppens JL, Bullens DM. Sputum cytokine mapping reveals an “IL-5, IL-17A, IL-25-high” pattern associated with poorly controlled asthma. Clin Exp Allergy. 2013;43:1009–1017. doi: 10.1111/cea.12125. [DOI] [PubMed] [Google Scholar]
- 35.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noonan M, Korenblat P, Mosesova S, Scheerens H, Arron JR, Zheng Y, Putnam WS, Parsey MV, Bohen SP, Matthews JG.Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids J Allergy Clin Immunol 2013132567–574.e12 [DOI] [PubMed] [Google Scholar]
- 37.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 38.Yu HS, Angkasekwinai P, Chang SH, Chung Y, Dong C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J Korean Med Sci. 2010;25:829–834. doi: 10.3346/jkms.2010.25.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]